Conduit necrosis following esophagectomy:An up-to-date literature review
Antonios Athanasiou,Mairead Hennessy,Eleftherios Spartalis,Benjamin H L Tan,Ewen A Griffiths
Abstract
Key words: Esophagectomy;Esophageal cancers;Esophagogastric anastomosis
INTRODUCTION
Esophageal cancer is one of the most common causes of cancer related mortality and morbidity worldwide[1].Despite improvements in the standardisation of surgical techniques,better case selection of patients for surgery,preoperative optimisation of nutritional status and improved intensive care unit (ICU) care,esophagectomy is still associated with significant rate of post-operative complications[2].The anastomosis between the oesophagus and the replacement conduit is challenging,with a wide variety of different surgical techniques proposed in the literature.The four main complications affecting the oesophagogastric anastomosis and the gastric conduit after the creation of neo-esophagus are anastomotic leak,anastomotic stricture,conduit ischemia and conduit necrosis[3].Gastric conduit necrosis is the severest of these complications and is considered to be life threatening with a high mortality rate[3,4].This is due to the development of widespread mediastinal sepsis,and as a result,conservative treatment is generally contraindicated.Upper gastrointestinal (GI)endoscopy is considered to be the gold standard for the diagnosis and differentiates between conduit necrosis and conduit ischemia[5].Patients with conduit necrosis require urgent fluid resuscitation,broad spectrum antibiotic and anti-fungal coverage,early surgical exploration with drainage of all infected collections and debridement of the necrotic conduit and esophageal diversion proximally[6].The purpose of this literature review is to provide the practising surgeon with an up to date literature review on this devastating complication.As such we have performed a contemporaneous systematic review and will describe and discuss the incidence of this rare complication,clinical manifestations,diagnostic strategy and management options available to help esophageal surgeons deal with this situation.
Definition
Esophageal conduit necrosis is death or ischaemia of the conduit used in the construction of the neo-esophagus,which is typically stomach,but can also occur when using the jejunum or colon for esophageal replacement.The distinction between conduit necrosis and conduit ischemia is crucial as the treatment approach is different.Fortunately,ischaemia does not always lead to conduit necrosis or anastomotic breakdown and healing can occur.The clinical range of gastric conduit ischaemia is broad and includes subclinical cases that resolve without intervention,ischemic-related anastomotic leak or stricture formation,and frank stomach necrosis.In addition,conduit ischemia may or may not be associated with anastomotic leakage.
Veeramootooet al[7]in 2009 categorised gastric conduit necrosis after esophagectomy to the following three types:Type I is consider to be Simple“anastomotic leak” without significant intramucosal necrosis.Type II is focal necrosis at the conduit tip which requires resection and refashioning of the esophago-gastric anastomosis.Type III is more extensive necrosis of the conduit requiring resection and delayed reconstruction.
Clinical manifestations
The most common clinical manifestations of conduit ischaemia and necrosis are tachycardia,tachypnoea,fever and altered mental status.There is much overlap between patients presenting with gastric conduit ischaemia/necrosis and those of an anastomotic leak.Clinical suspicion,early diagnosis and expeditious management are vital to reduce morbidity and mortality.Severe sepsis or septic shock is late signs.Signs can include saliva or GI contents exuding from the neck incision or bile within the chest drain[3].Worrying signs of potential gastric conduit necrosis include bloody or feculent nasogastric output,lactic acidosis or haemodynamically unitability such as hypotension and shock.In some patients a foul odour or “bad breath” can be apparent at the bedside and is caused by necrosis of the conduit and bacterial overgrowth in the necrotic tissue.
Diagnosis
Some surgeons routinely arrange a water soluble swallow prior to commencing oral intake,but this has some disadvantages,including the risks of aspiration,false negative results and the risk that anastomotic or conduit complications occur before the timing of the investigation[9,10].Whilst a water soluble (gastrograffin) swallow is helpful for patients with a high predicted probability of an anastomotic leak who are alert and able to sit up for the investigation,it can be largely normal even in advanced conduit ischaemia.This is because the contrast remains within the gastric lumen and not be associated with an anastomosis leak in some situations[9].In addition,contrast swallows are not appropriate for patients who are intubated and ventilated on ICU.In this scenario bedside endoscopy on the ICU,preferably performed by the operating surgeon is a better test of the viability of the gastric conduit and whether an anastomotic leak is present[11].It is for this reason that upper GI endoscopy and CT thorax with intravenous contrast (IV) and oral contrast are considered to be the “gold standard'' for the diagnosis of conduit necrosis and anastomotic leakage.
Clear radiological evidence of anastomotic leak are more specific than the findings of conduit necrosis.However,CT is crucial for the detection of intrathoracic collections which need to be drained.CT findings include gastric and esophageal wall thickening with possible small gas bubbles in soft tissues,especially at the anastomotic level[12].Furthermore,CT sensitivity for detection of free extra-luminal contrast and mediastinal collections and contamination is high.Nonetheless,it has been observed that some patients with partial or non-full thickness gastric conduit ischemia can have a normal CT scan[12].
Routine Upper GI endoscopy within one week following esophagectomy has been proposed in the literature for the evaluation of gastric conduit and the integrity of the anastomosis[13,14].Pageet al[14],assessed the efficacy and safety of upper GI endoscopy in 100 patients within one week after esophagectomy.Their results showed that endoscopy is very safe and accurate method for the diagnosis of conduit ischaemia and necrosis and allows more individualized patient management.Tom DeMeester's group has published an endoscopic classification system for the findings of gastric conduit ischaemia and necrosis (Table2)[12].

Table1 The Esophageal Complications Consensus Group definition and classification of conduit necrosis and ischemia[8]
LITERATURE SEARCH
A systematic review was carried out according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement[15].Articles indexed in Embase and PubMed were searched from inception up to January 2019,by two authors,using the Medical Subject Headings (MeSH) for database research and text words related to:(esophagus OR esophageal) AND (esophagectomy) AND (conduit necrosis OR conduit ischemia).The process of the literature search is depicted in Figure1.Our review details the pathophysiology,predisposing factors,clinical symptoms,diagnostic approach to this problem and we will highlight treatment options for the management of this life threatening condition.
SEARCH RESULTS
Epidemiology
According to the literature,the incidence of conduit ischaemia or conduit necrosis following esophagectomy varies significantly.Much of this variation could be due to a lack of standardised definition prior to 2015.Many series report incidence of gastric tube ischemia less than 1%[16,17].Daviset al[16]report 0.5% gastric tube ischemia in series of 959 esophagectomies.Luketichet al[18]examined minimally invasive esophagectomy in a series of 1000 patients,with a 2% incidence of graft necrosis.Brielet al[19]reported a very high rate of 10.4 % of gastric conduit ischemia in a series of 230 consecutive esophagectomy.He found no statistically significant difference between gastric and colon interposition.A recent systematic review and meta-analysis showed that the rate of conduit ischemia/necrosis was 0% in 13 randomized control trials and 21% in 85 observational studies[20].
Risk factors
Identifying patients at risk of conduit ischaemia or necrosis pre-operatively is essential.This is to enable surgeons to consent patients to the increased risks of a poor outcome particularly if they have several risks factors and also to try to modify these risks prior to surgery in certain patients.Risk factors for gastric conduit ischaemia or necrosis are shown in Table3 and consist of a variety of patients related risks factors,technical/surgical factors and post-operative factors[21-26].
Several patient related risk factors are associated with conduit ischaemic and necrosis and these include diabetes mellitus,malnutrition,steroid use,hypertension,cardiac arrhythmias,reduced cardiac contractility and peripheral vascular disease[19].Lainaset al[27]reviewed 481 patients who underwent Ivor Lewis esophagectomy and on pre-operative CT imaging assessed the degree of coeliac artery calcification and stenosis.They found a strong association between pre-existing coeliac axis stenosis and subsequent conduit necrosis[27].Co-morbidities including diabetes mellitus,hypertension and peripheral arterial disease must be thoroughly reviewed and optimised prior to surgery.Patient factors such as smoking,neoadjuvant chemoradiotherapy and pre-operative weight loss were not associated with increased ischaemia[28].
不失一般性,以下也考虑(6)式。为了完成BCST任务,Alice 和Bob合作引入两个辅助粒子s、t,这两个粒子处于初始态|00〉st,并使之与|ω〉34态构成复合系统|ω〉34|00〉st。然后,分别施行以粒子s、t为目标粒子,以粒子3、4为受控粒子的两个受控非门运算,量子态|ω〉34|00〉st变成
Performing a surgical resection without compromising blood supply is essential for safe esophageal reconstruction.The blood supply to the upper abdominal viscera is derived from the coeliac axis.Coeliac axis stenosis due to median arcuate ligament syndrome or atherosclerosis may impair the viability of the gastric conduit used in esophageal chest reconstruction[27].In most cases,the conduit of choice is the stomach,deriving its blood supply primarily from the right gastro epiploic artery[29].Injury to the conduit during abdominal dissection or at the time of repositioning within the thorax or neck can be detrimental[30].Tension on the anastomosis or venous obstruction can inhibit healing at the anastomosis.This could be due to extrinsic compression at the thoracic inlet,and is considered to contribute to an increased rate of failure in cervical anastomoses[30].Extrinsic compression at the hiatus can also occurdue to tightness,oedema or acute diaphragmatic hernia.In addition to blood flow,maintaining adequate tissue oxygenation to the anastomosis is vital for anastomosis healing.
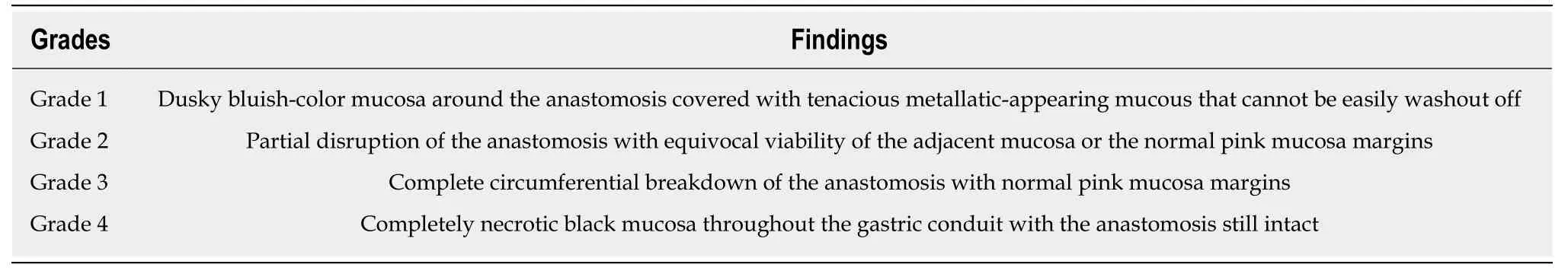
Table2 Endoscopic classification system for the findings of gastric conduit ischaemia and necrosis[12]
The effect of thoracic epidural analgesia on the perfusion of the gastric conduit is controversial.The majority of units use thoracic epidural analgesia for post-operative analgesia,especially after open trans-thoracic procedures.One small study suggested that the epidural,perhaps due to the sympathetic block,improved blood supply to the gastric conduit[31].In another small study,Al-Rawiet al[32]showed by using laser Doppler flowmetry that thoracic epidural bupivacaine decreases significantly the arterial blood pressure and cardiac output,presumably due to block of cardiac sympathetics.This study has also shown that arterial pressure has a greater impact on the tip of the gastric conduit than on the pyloric end of the gastric tube.
Ischaemic pre-conditioning
Ischaemic pre-conditioning of the gastric conduit prior to esophagectomy in order to reduce conduit necrosis and anastomotic leak rates was first described by Urschelet al[33]in 1997.Pre-conditioning can be performed radiologically or surgically.Laparoscopic ligation or radiological embolization of the left gastric artery,left gastroepiploic artery and short gastric arteries can reduce significantly the gastric blood supply[34].Nevertheless,the correct timing,appropriate technique and benefits of pre-conditioning remain controversial
Gastric conduit necrosis following esophagectomy is thought to have an increased association with minimally invasive techniques,especially in the early stages of the learning curve.Crenshawet al[35]found that extracorporeal stapling of the gastric conduit-led to a significant reduction in the incidence of gastric conduit failures when compared with the intracorporeal stapling technique.A retrospective analysis by Ramageet al[36]including 155 patients after minimally invasive esophagectomies(MIOs),showed 2.6% of conduit necrosis.The authors concluded that conduit necrosis is strongly related to the learning curve.Prophylactic measures such as ischaemic preconditioning become less relevant as the operating surgeon's experience increases.On the other hand,tension free and correct position of gastric tube,preservation of gastroepiploic arcade without injuries and sufficient defect of the diaphragmatic hiatus are crucial factors[36].
To try to counteract the association between gastric conduit necrosis and minimally invasive esophagectomy,several authors have suggested that gastric ischaemic preconditioning may reduce ischaemic complications.This takes the form of dividing either the left gastric artery or short gastric vessels a week or so prior to planned oesophagectomy.The hypothesis is that the conduit has time to get “pre-conditioned”and reduces the risk of conduit necrosis.Berrisfordet al[21],reviewed 77 consecutive patients who underwent a total MIO.Their results suggest that ischaemic conditioning of the stomach prior to MIO is safe and there is a trend to reduced morbidity related to gastric-conduit ischaemia[21].Wajedet al[37]advocate laparoscopic ischaemic conditioning by ligation of the left gastric vessels 2 weeks prior to MIO in addition to extracorporeal stapling to reduce the incidence of conduit necrosis,particularly in three stage surgery.A small randomized study by the same group did not identify any clinical benefits with ischaemic pre-conditioning[38].Table4 shows the most important studies in humans regarding gastric ischaemic pre-conditioning prior to esophagectomy[37-43].
Intra-operative assessment of the gastric conduit
Visual intra-operative clinical assessment of the conduit perfusion is not particularly accurate as it relies on the subjective assessment of the colour and viability of the conduit and rate of bleeding at the anastomotic edge.Newer more modern and less subjective ways to assess tissue perfusion have been developed to aid in intraoperative assessment of gastric conduit viability.These techniques include fluorescence angiography,laser Doppler flowmetry and spectrophotometry,transmucosal oxygen saturation measurement,hydrogen clearance,visible light spectroscopy,intra-operative endoscopy,and laser-induced fluorescence of indocyanine green (ICG)[44-57].

Figure1 Schematic diagram of procedural methodology.
The most common intra-operative devices used are Doppler ultrasound and ICG fluorescence imaging,mainly because they provide adequate visualization of the vessel networks of the gastric wall[47-56].ICG fluorescence imaging is considered to be a potential technique for higher sensitivity,especially after the encouraging results in different tumours,mainly in colorectal and liver cancer[58].Table5 illustrates the most important studies of ICG fluorescence imaging in esophageal cancer[47-56].The vast majority of the published articles conclude that ICG fluorescence is useful for the prediction of the risk of anastomotic leak and also can be used for intraoperative modifications with better placement of the anastomosis and resection of the ischaemic area of the fundus[47-56].A randomised trial in this area specifically assessing the use of ICG assessment during esophagectomy is greatly required.
MANAGEMENT OPTIONS
Intra-operative conduit ischaemia
Acute intraoperative conduit ischaemic is a challenging scenario for esophagogastric surgeons.Should the conduit look hypo-perfused due to inadequate blood flow,recognising it in a timely fashion is essential if the conduit is to be saved.The hiatus should be checked to ensure it is not too tight and there is no impingement on the gastroepiploic vessels.Additionally,the gastric conduit should be checked to ensure it is not twisted.If the situation develops during the neck phase of the procedure,then the thoracic inlet should be checked for tightness.This can be released to some extent by resection of the sternal head and the manubrium;which is mandatory should the graft be positioned at this level substernally.If there is any doubt as to the viability of the conduit,anastomosis should not be performed.If it looks like the gastric conduit is unsalvageable then it should be resected and a cervical esophagostomy formed,with subsequent plans for a delayed reconstruction instituted.Delayed reconstructioncan be performed with colon or small bowel,depending on the clinical situation.

Table3 Risk factors for gastric conduit necrosis
Oezceliket al[59]published a series of 554 patients who underwent esophagectomy with gastric pullup and described an interesting strategy.In 37 patients (7%),the combination of an ischemic graft and substantial comorbid conditions prompted a delayed neck anastomosis.To avoid a high risk anastomosis in these patients,the gastric conduit was brought up and secured in the neck,and a cervical esophagostomy was constructed.Subsequently,a delayed esophagogastric anastomosis was performed through neck incision.Outcomes were analysed at a median of 22 mo.None of the patients has developed conduit ischemia or necrosis.The authors reported well-perfused conduits at the time of reconstruction without anastomotic leak,sepsis or wound infections post-operatively.They concluded that delayed reconstruction is strongly recommended for patients with significant comorbidities and for patients with inadequate blood supply of the conduit during the esophagectomy[59].
Post-operative conduit ischaemia /necrosis
Patients with mild ischaemic changes with a small anastomotic leak may be managed successfully with an esophageal stent,naso-gastric drainage and enteral feeding[60].Lianget al[61]reported two patients who were salvaged using a temporary removable self-expandable metal stent (SEMS) placed endoscopically and concomitant chest washout.However,both patients had type II conduit necrosis which is most likely to have successful outcome using SEMS.The aforementioned management is considered to be effective only in a very cautiously selected patients and it is recommended only if there is minor gastric conduit necrosis,without inflammation of the mediastinum and if the patient remains clinically stable.Patients without an associated anastomotic leak should be closely monitored.
In selected cases with minor areas of peri-anastomotic ischaemia or conduit necrosis in a stable patient with no major comorbidity,the anastomosis may be suitable for re-fashioning or repaired over a T-tube.If this is not the case,it is safer to completely take down the anastomosis at the second thoracotomy with debridement of necrotic tissue,wide drainage,proximal diversion with an end cervical esophagostomy and replacement of remaining stomach within the abdomen[3].Nutritional access with a feeding jejunostomy should be obtained if this has not already been achieved.
Delayed reconstruction after conduit necrosis
Reconstruction can be performed in a few ways following the take down of the necrotic conduit.
Colonic reconstruction
Esophageal replacement by colonic interposition is an uncommon procedure.These reconstructions can be pedicled or free grafts with or without venous or arterial supercharging.When the colon is used as the conduit,graft necrosis after neck anastomosis has been reported as high as 16%[62].This is likely exacerbated by colonic bacterial contamination,particularly if the patient has not been prepped.This is usually fatal if not recognised and treated early.Fisheret al[63]sought to identify the frequency of this operation in England,identify techniques and associated problems from the two largest centres performing this procedure.Fifty-two percent preferred to use the left colon with 81% preferring a substernal placement.All patients had early satiety,20 described dysphagia and 18 regularly took anti-reflux medication.Colon interposition results in an acceptable long-term health-related quality of life.Fewcentres regularly perform this operation,and centralizing to high-volume centres may lead to better outcomes[63].Supercharged colon interposition is an alternative surgical technique for the reduction of ischemic related morbidity[64,65].In addition to the abdominal blood supply,the venous drainage and arterial supply is augmented with additional microvascular anastomoses in the neck (branches of the carotid artery or jugular veins depending on anatomy).Small series have showed low rates of leak,bowel ischaemia and graft loss.In a retrospective series by Fujita and co-workers[64],24 patients underwent reconstruction without supercharged colon interposition and were compared with 29 patients with supercharged colon.The vast majority of patients underwent thoracic esophagectomy.The results revealed that the group who received supercharged colon had a significantly lower rate of conduit necrosis and anastomotic leak.These techniques require the skills of a microvascular surgeon.
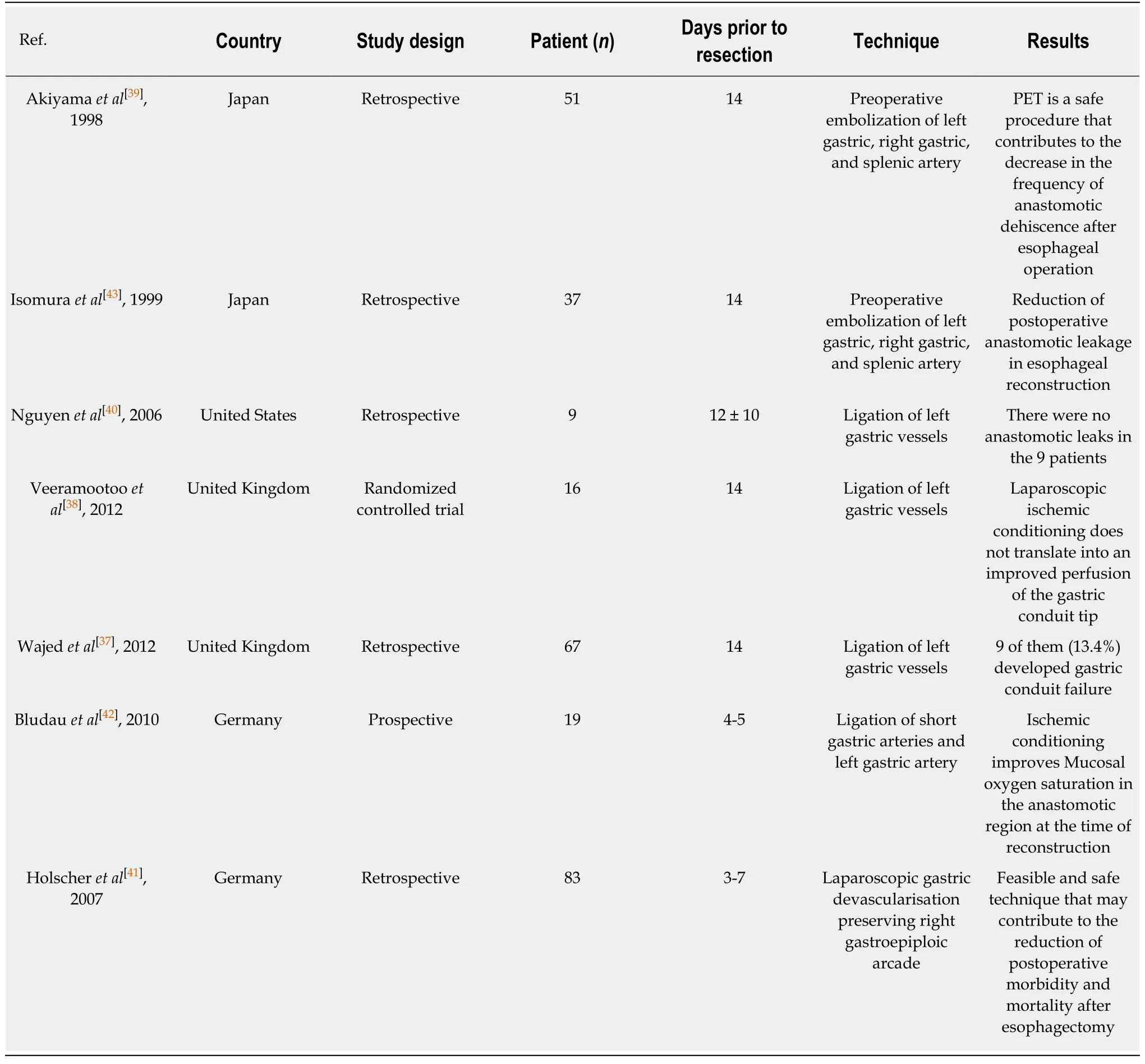
Table4 Published Series of gastric ischaemic pre-conditioning prior to oesophagectomy in humans
According to the literature,colon interposition due to gastric conduit necrosis is recommended only after esophageal diversion and delayed neck reconstruction[66,67].Esophageal diversion is considered to be a staged damage-control operation in combination with mediastinal drainage,nutritional supplementationviaa feeding jejunostomy and antimicrobial therapy.
Jejunal reconstruction
Jejunal interposition with or without vascular supercharging is an alternative option for esophageal reconstruction[68].Advantages of this technique include the lack ofneed for pre-operative bowel preparation,it is relatively easy to mobilise,sizable mesenteric blood vessels,comparable size to esophagus,bowel anastomoses with low leak rates and active peristalsis.Disadvantages included that it is a rare form of esophageal replacement and only a few centres have experience of its use.Depending on circumstances its use will usually require the help of a micro-vascular surgeon to either supercharge or free graft the blood supply in the neck vessels.Augmentation of pedicle blood supply increases the length of conduit and restores more blood flow,which allows a neck anastomosis of the jejunum to be performed in the left neck[69].Asciotiet al[68],reviewed retrospectively 26 patients who underwent reconstruction with supercharged pedicled jejunum and found a 19.2% had cervical anastomotic leak,7.7% of graft loss and there were no mortalities.All patients underwent oesophageal reconstruction with a neck anastomosis.Use of jejunum is contraindicated in Crohn's disease,short bowel syndrome and short fatty mesentery.Jejunal conduit necrosis is usually due to technical errors,poor vascular supply,venous thrombosis and perioperative hypotension[70].According to the literature,jejunal interposition after gastric or colon conduit necrosis is indicated only after esophageal diversion and delayed neck reconstruction[64,65].

Table5 Clinical studies for the evaluation of ischemic gastric conditioning using indocyanine green fluorescence imaging
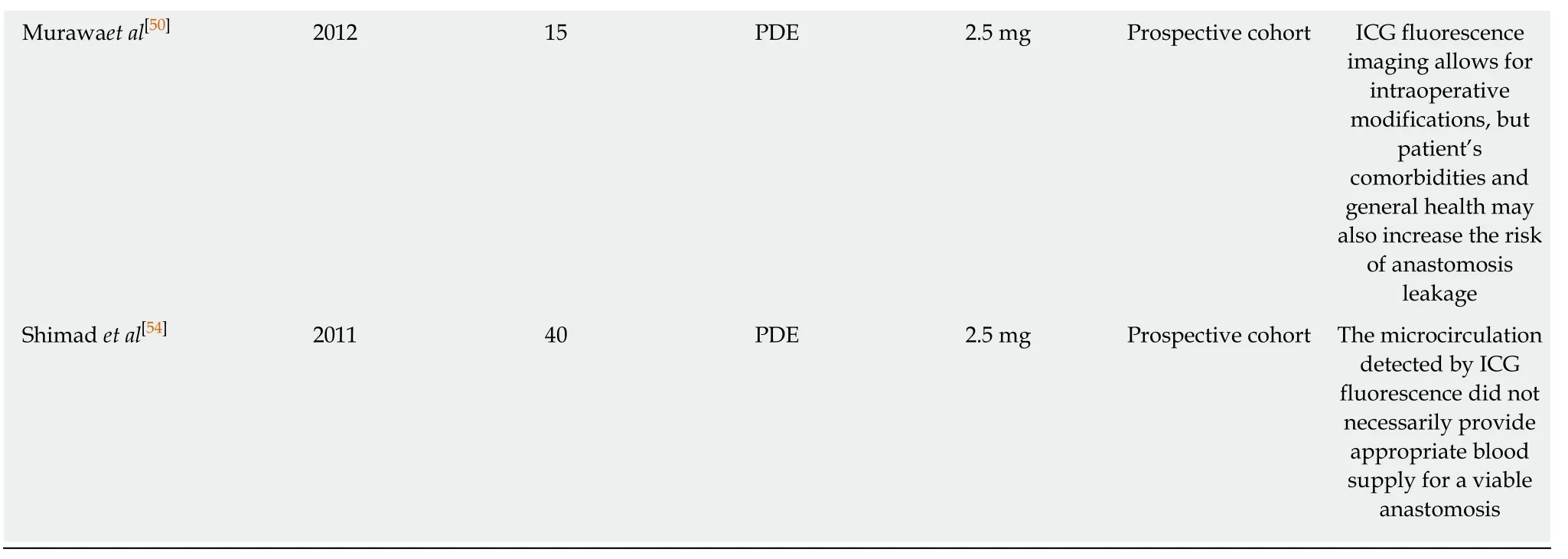
ICG:Indocyanine green;ER:Esophageal resection;PDE:Hamamatsu Photonics K.K,Hamamatsu,Japan.
Myocutaneous flap reconstruction
An alternative method for the management of conduit necrosis using muscle flaps has been proposed in the literature.These are more applicable for dealing with partial defects in the gastric conduit in the neck,but can be used in selected cases for salvage of completely circumferential defects.Myocutaneous flap is utilized usually in order to reconstruct gastric conduit defect due to conduit necrosis as well as to cover anastomotic defect.However,is not recommended for complete oesophageal neck reconstruction by formation of tube-style tissue due to high rate of complications including stenosis and necrosis[71].Sternocleidomastoid,pectoralis major and trapezius flaps have all been reported to cover tissue defects in the neck.However,much of the published literature is from individual case reports.Furthermore,fasciocutaneous free flaps,for example anterolateral thigh and radial forearm have also been reported which can replace the oesophagus in the neck area with the skin side tubularised.A single pedicled pectoralis major myocutaneous for cervicaloesophageal reconstruction is the most commonly used flap due to the fact that there is no need for free tissue transfer and also because it is easier to harvest the muscle due to its location to the chest[72].This flap has a lower rate of flap failure compared with free flaps for cervical-oesophageal reconstruction[73].
Morbidity and mortality
Mortality of gastric conduit necrosis has been reported to be as high as 90%[4].Esophageal conduit necrosis is an uncommon but disastrous complication of esophageal surgery.Postoperative conduit ischemia is reported internationally.Average rates of ischemic complications for stomach,colon,and jejunum are 3.2%,5.1%,and 4.2%,respectively[4].
Daviset al[16]showed that gastric conduit reconstruction has less rate of anastomotic leak and conduit ischemia in compare with colon reconstruction.Mooreheadet al[17],in their review of 760 esophagectomy patients in whom the stomach,colon,or jejunum was used for chest or neck reconstruction,showed that gastric conduit had the lowest rate of ischemia (1%),followed by small bowel (11%),while colon had the highest rate(13.3%).Moreover,Brielet al[19]compared colon and gastric conduit after two or three stage esophagectomy and they reported 10% colon conduit ischemia and 7% gastric conduit ischemia.
CONCLUSION
In spite of recent advances in esophageal cancer surgery,the management of conduit necrosis is extremely challenging.Management options include conservative treatment and more aggressive treatments such as stent insertion,surgical debridement and repair of the esophagus using jejunum,colon or a musculocutaneous flap.Conservative management includes close clinical monitoring of patient symptoms and signs including heart rate,blood pressure,temperature,respiratory rate and oxygen saturations in addition to blood results and the administration of broad spectrum antibiotics.Identifying and acting upon any deterioration is vital.While there are several interventional options,deciding upon the most appropriate for each individual patient,is challenging for the most experienced surgeon.All interventional options are high risk.The most effective treatment method remains controversial.
The literature available for review is limited and so surgeons should endeavour to report all cases of esophageal necrosis,their management whether successful or unsuccessful.Several multi-institutional databases are in current use.For example,Esodata (https://www.esodata.org) under the auspices of ISDE - International Society for the Diseases of Esophagus - have developed a web portal for creating and sharing expert views and current knowledge on complications and outcomes esophageal surgery.In addition,the oesophagogastric anastomotic leak audit(http://www.ogaa.org.uk/) aims to collect data of anastomotic complications from esophagectomy,including conduit necrosis,from a large group of international esophageal units to define the accurate incidence and outcome of this problem.It is only with prospective and standardised data from these multi-centre registries that we can help address the void of high quality literature of this important topic.It is hoped that once we have standardised data of many patients with this devastating condition,that the precise management strategy to obtain best outcomes will become clear.
As with most complex surgical procedures,high volume surgeons and high volume centres have significantly higher success rates with esophageal resections[74,75].Knowledge of the potential complications,identifying them in a timely fashion and managing them appropriately is essential.The management of this problem should be individualised to the specific patient depending on severity of ischaemia and clinical features.The description in 1942 by Churchill and Sweet[76]of their early successes with esophago-enteric anastomoses due to “unusual attention to detail” and technical“exactitude” remain a cornerstone for future advances by surgeons involved in esophageal resection and replacement.
 World Journal of Gastrointestinal Surgery2019年3期
World Journal of Gastrointestinal Surgery2019年3期
- World Journal of Gastrointestinal Surgery的其它文章
- Liver preservation prior to transplantation:Past,present,and future
- Classification and guidelines of hemorrhoidal disease:Present and future
- Laparoscopic celiac plexus ganglioneuroma resection:A video case report
- Single incision laparoscopic fundoplication:A systematic review of the literature
- Learning curve of enhanced recovery after surgery program in open colorectal surgery
- Management of pancreatic head adenocarcinoma:From where to where?
