间充质干细胞通过分解代谢糖酵解促进肺癌细胞生长
赛步青,徐嘉齐,王路娟,郑乐亮,唐敬群
(1.中南大学湘雅二医院胸外科,中国湖南长沙410013;2.中南大学基础医学院肿瘤研究所,中国湖南长沙410078)
Mesenchymal stem/stromal cells(MSCs),which reside mainly in bone marrow,are known to be recruited to tumor sites and constitute the tumor microenvironment[1].It is thought that these adult stem cells maintain a quiescent state with slow cycling in bone marrow to ensure renewal capacity[2].Bone marrow MSCs(BMSCs)have long been considered to affect cancer progression directly and indirectly.Secreted growth factors and cytokines function in a paracrine manner to activate oncogenic signaling pathways.Besides,various metabolites in cancer microenvironment contribute to the cancer progression[3].Metabolism,which was previously considered to be a consequence of cells,is now known to be causative factors in cancer cell behaviors.It has long been thought that aerobic glycolysis for ATP production is the hallmark of tumor cells because of impaired mitochondrialrespiration.During the chemotaxis,BMSCs enter into the circulation in large amounts,and ATP is required in this process.BMSCs are found to be more glycolytic than primary fibroblasts[4].The transformation of BMSCs may also rely on increase in oxidative phosphorylation for energy supply.Glycolysis reduces oxidative stress and reactive oxygen species(ROS)generation and drives stem cell differentiation.
The oxygen pressure in bone marrow is low,ranging typically from 9 mmHg to 40 mmHg[5].The MSCs in bone marrow express higher levels of glycolytic enzyme,suggesting that BMSCs rely on glycolysis.BMSCs expanded under normoxia can still use OXPHOS with a high oxygen consumption rate.However,solid tumors contain regions with mild(hypoxia)to severe oxygen deficiency(anoxia),due to the lack of blood supply to the growing tumor nodules[6].Reciprocal interaction of cancer cells and microenvironment determines the cancer progression.It needs to clarify how cancer cells and BMSCs interact and reprogram the metabolic program under the microenvironment.
A better understanding of the features of microenvironmental BMSCs raises the possibility that targeting specific metabolic features of BMSCs will help to prevent the progression of cancer in different stages.In this study,a model that allows us to investigate the dynamic change of BMSCs was established and to explore how the metabolic reprogramming of BMSCs affects cancer progression.Our study highlighted the evolution of BMSCs educated by cancer cells,and found that glycolysis of BMSCs in cancer microenvironment would determine the fate of cancer cells.
1 Materials and methods
1.1 Cells and reagents
Murine BMSCs from C57BL/6 mice were purchased from Cyagen company,China.Murine Lewis lung carcinoma(LLC)cells and BMSCs were cultured in RPMI-1640 medium supplemented with penicillin G(100 U/mL),streptomycin(100 mg/mL)and 10%fetal calf serum.Cells were grown at 37℃in a humidified atmosphere of 5%CO2and were routinely sub-cultured using 0.25%(w/V)trypsin-EDTA solution.The green fluorescent protein(GFP)-labeled BMSCs(GFP-BMSCs)and red fluorescent protein(RFP)-labeled LLC(RFP-LLC)cells were constructed by lentivirus-mediated GFP or RFP gene transduction.The lentivirus-based vector expressing GFP or RFP was purchased from Cyagen,China.Glycolysis inhibitor 2-deoxy-D-glucose(2-DG)was purchased from Sigma.
1.2 Animal experiment
Six-to eight-week-old female wild-type C57-BL/6 were used to examine the syngeneic tumor growth.Animal experiments were conducted following protocols approved by Central South University,China.All animal procedures were approved by the Institutional Animal Care and Use Committee.Cells were trypsinized with 0.25 mol/L EDTA and 0.05%trypsin(Invitrogen)on the day of transplantation and resuspended in 0.2 mL of serum-free medium.A total of 1×106of murine LLC cells with the same amount of BMSCs or without BMSCs were injected subcutaneously into syngeneic C57BL/6 mice.The animals were examined at different time points after the injection by measuring the size of subcutaneous tumors.The tumor volumes were calculated using the following standard formula:0.5×L×W2(L:length;W:width).For 2-DG treatment group,mice were injected intraperitoneally with 2-DG at 500 mg/kg.
1.3 RNA-Seq
C57BL/6 mice were subcutaneously injected with RFP-LLC cells with or without GFP-BMSCs.About 30 days later,the RFP-LLC cells and GFPBMSCs were collected from the tumor sites by flow cytometry cell sorting and subjected to RNA sequencing analysis.The RNA sequencing was conducted by BGI,China,including the sample quality control,library preparation and sequencing.Each library was sequenced at a depth of 10 G clean reads on the BGISEQ500 platform.The RNA sequencing data have been submitted to the Gene Expression Omnibus and could be accessed by the accession numbers GSE120349 and GSE120456.
1.4 Pathway-Act-Network
Pathway-Act-Network was constructed by Cytoscape software(v2.8.0;http://www.cytoscape.org).The differentially expressed genes enriched in significant pathways were analyzed by KEGG.
2 Results
2.1 BMSCs promote cancer cell growth in vivo
Firstly,the effects of BMSCs on tumor cell growth in a syngeneic LLC mouse model were determined.RFP-LLC cells were injected subcutaneously into C57BL/6 mice with or without GFPBMSCs.When the tumors grew(9 days after inoculation),the tumor volumes were measured every 3 days.Thirty days after inoculation,the tumors were excised.The result revealed that allograft tumors were significantly larger when the RFP-LLC cells were co-transplanted with BMSCs,compared to those after injection with RFP-LLC cells alone(Figs.1A and 1B,P<0.05,Student’s t-test).It demonstrated that BMSCs promoted cancer cell growth.
2.2 Metabolic reprogramming of BMSCs determines the fate of cancer cells
In order to uncover the mechanism of how BMSCs promote cancer growth,the different transcriptomic signatures were compared by RNA sequencing.The RFP-LLC cells and GFP-BMSCs were subcutaneously injected into C57BL/6 mice.About 30 days later,GFP-BMSCs and RFP-LLC cells from the primary inoculation cancer site were collected by flow cytometric sorting technology.The RFP-LLC cells that were subcutaneously injected alone were isolated by flow cytometric sorting technology as a control.To investigate the effect of cancer cells on the gene expressions of BMSCs,MSCs collected from bone marrow were also used as a control.Gene expression profiling was performed on BMSCs or LLC cells collected from 3 samples in each group.Compared to control BMSCs,intratumoral BMSCs had 1 820 upregulated and 1 147 downregulated genes.The upregulated genes in BMSCs were enriched in glycolysis/glyconeogenesis,fructose and mannose metabolism,glutathione metabolism,et al(Fig.2A).On the other side,compared to the upregulated genes in LLC cells in the LLC cell injection alone group,those in the coinjection group were enriched in cytokine-cytokine receptor,osteoclast differentiation,cancer gene related path-ways,including TNF signaling,NF-kappa B signaling,et al(Fig.2A).Then the Pathway-Act-Network according to differentially expressed molecule-enriched pathways identified by KEGG database was built.The results revealed that the core predicted pathways in the network of BMSCs were metabolic pathways,MAPK signaling pathway and HIF-1 signaling pathway.However,the metabolic pathways were suppressed in LLC cells in cancer microenvironment(Figs.2B and 2C).Levels of transcripts including HK1,PFK1,PGM,LDH,the indicators of glycolysis,increased.Accordingly,increases in SLC2A1,SLC16A3 and SLC16A4 were observed in BMSCs collected from primary sites(Fig.2D).This finding suggested that glycolysis is the predominant ATP generator for BMSCs in primary cancer sites,and that metabolic reprogramming of BMSCs may determine the fate of cancer cells.
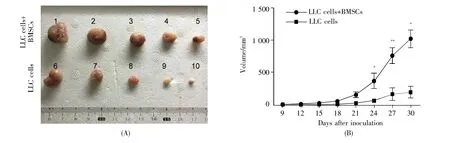
Fig.1 BMSCs promoted cancer cell growth in vivoRFP-LLC cells were injected subcutaneously into C57BL/6 mice with or without GFP-BMSCs.(A)Image of excised tumors.Thirty days after inoculation,the tumors were excised.Ten mice were divided into 2 groups(n=5 in each group).The tumors numbered 1~5 were excised from mice co-injected with LLC cells and BMSCs,and those numbered 6~10 were from mice injected with LLC cells alone;(B)Tumor growth curve.After inoculation of cells,the tumor volumes in mice of the 2 groups were measured every 3 days.Data were analyzed with Student’s t-test,*P<0.05,**P<0.01.
2.3 Blocking glycolysis reduces the growth of cancer cells
As glycolysis was the predominant ATP generator for BMSCs in primary cancer sites,we then investigated if blocking glycolysis could inhibit the growth of cancer cells.LLC cells were injected subcutaneously into C57BL/6 mice with BMSCs.Fifteen days after inoculation,the mice which received mixed LLC cells and BMSCs were injected intraperitoneally with the glycolysis inhibitor 2-DG or PBS control.The tumor volumes were measured every 3 days.Thirty days after transplantation,the tu-mors were excised.The result revealed that 2-DG obviously inhibited the growth of tumor(Fig.3).
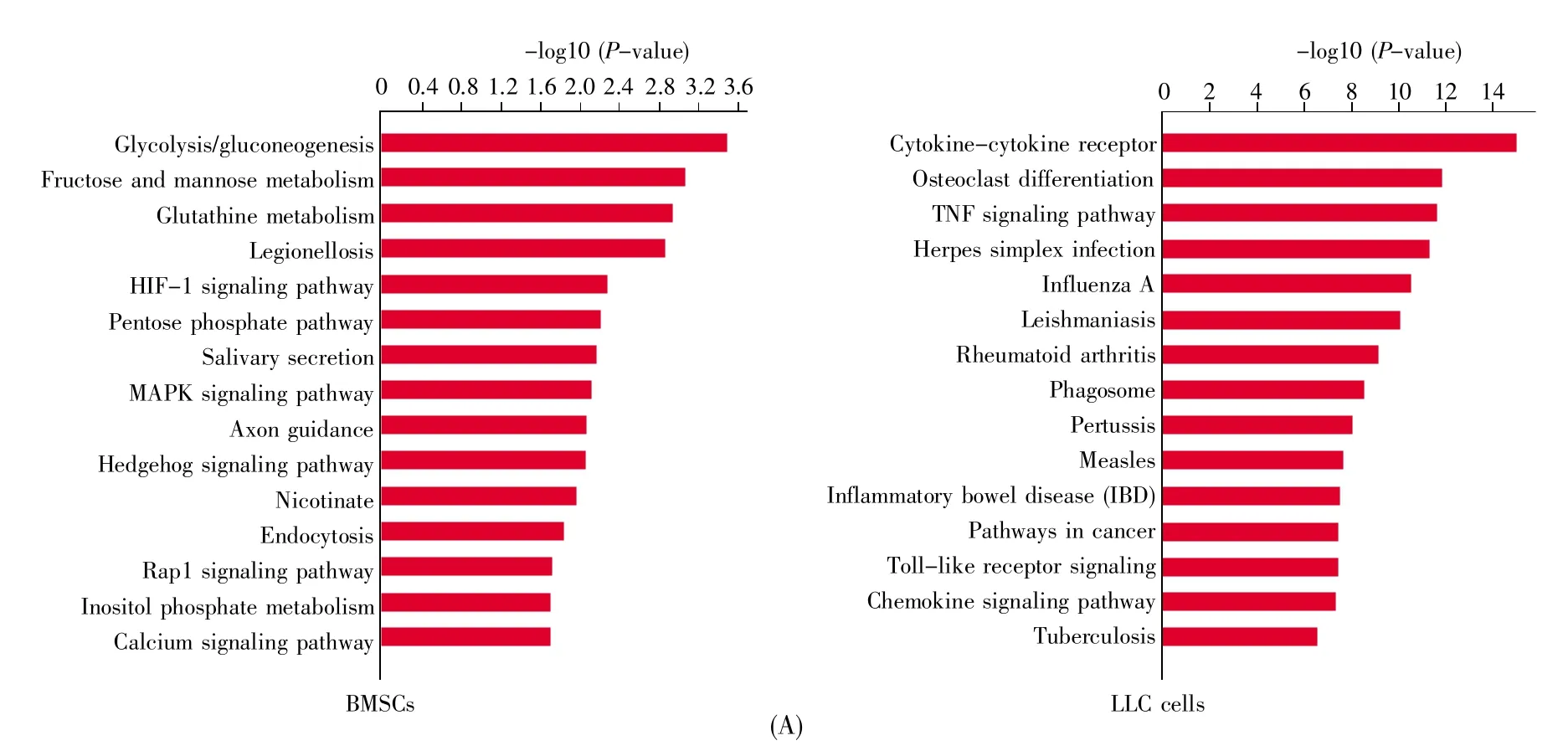

Fig.2 Transcriptomic profiles of BMSCs and LLC cells in cancer microenvironmentC57BL/6 mice were subcutaneously injected with RFP-LLC cells with or without GFP-BMSCs.About 30 days later,GFP-BMSCs and RFP-LLC cells were collected from the primary inoculation cancer site by flow cytometric sorting technology.Different transcriptomic signatures of BMSCs and LLC cells in microenvironment were compared by RNA sequencing.Gene expression profiling was performed on BMSCs or LLC cells collected from 3 samples in each group.(A)KEGG analysis of BMSCs and LLC cells in cancer microenvironment.The differentially expressed genes were obtained by comparing the transcriptomic profiles between control BMSCs or LLCs and BMSCs and LLCs in cancer microenvironment.The RFP-LLC cells that were subcutaneously injected alone and were isolated by flow cytometric sorting technology were used as a control.The MSCs that were isolated from bone marrow were also used as a control;(B)Pathway-Act-Network based on enriched pathways.The Pathway-Act-Network was built according to differentially expressed molecule-enriched pathways identified by KEGG database.A node represents a signaling pathway.Red indicates that the signaling pathway is activated,while green indicates that the signaling pathway is suppressed.Yellow indicates that signaling pathway is activated in some samples and suppressed in the other samples;(C)Pathway-Act-Network based on metabolic pathways.Red indicates that the signaling pathway is activated,while green indicates that the signaling pathway is suppressed;(D)FPKM of glycolysis-related genes in BMSCs by RNA sequencing.Student’s t-test,*P<0.05;**P<0.01;***P<0.001.

Fig.3 Blocking glycolysis reduced the growth of cancer cellsLLC cells were injected subcutaneously into C57BL/6 mice with BMSCs.Fifteen days after inoculation,the mice received mixed LLC cells and BMSCs were injected intraperitoneally with glycolysis inhibitor 2-DG or PBS control.(A)Image of excised tumors.Thirty days after inoculation,the tumors were excised.Fourteen mice were divided into 2 groups(n=7 in each group).Tumors numbered 1~7 were from mice injected with PBS and numbered 8~14 were from mice injected with 2-DG;(B)Tumor growth curve.After inoculation with cells,the tumor volumes were measured every 3 days.Fourteen mice were divided into 2 groups(n=7 in each group).Data were analyzed with Student’s t-test,*P<0.05,**P<0.01.
3 Discussion
The present work provided an evidence of how BMSCs improved cancer cell growth.We found an interaction between BMSCs and cancer cells.Both BMSCs and cancer cells showed spatial evolution during cancer progression.What’s more,in cancer microenvironment,the growth of lung cancer cells was dependent on glycolysis of BMSCs.
In order to grow fast,cancer cells need energy and have to build blocks for biosynthesis.Metabolic reprogramming is considered a hallmark of cancer.Early studies demonstrated that aerobic glycolysis was specific to cancer cells due to damaged mitochondria.The Warburg effect refers to the fact that cancer cells prefer to produce ATP by glycolysis instead of oxidative phosphorylation(OXPHOS).However,recent evidence suggests that most tumor cells have functional mitochondria and are able to carry out oxidative phosphorylation to meet the requirement of macromolecular synthesis.Although the Warburg effect has been considered to be a hallmark of cancer cells,in some cancers,OXPHOS in cancer cells can also be activated[1,7].A possible explanation is that the surrounding stromal cells increase glycolysis,whose products can be utilized by oxidative phosphorylation in cancer cells,which is termed the“reverse Warburg effect”[8].In this study,a model was established that allows us to investigate the metabolic states of BMSCs and cancer cells in vivo.The result showed that in cancer microen vironment,BMSCs displayed switched metabolic states.The upregulated genes in intratumoral BMSCs were enriched in glycolysis/glyconeogenesis,fructose and mannose metabolism,glutathione metabolism,et al.However,the metabolic pathways were suppressed in LLC cells in cancer microenvironment.Levels of transcripts involved in glycolysis increased.We concluded that BMSCs increase glycolysis,whose products can be utilized by cancer cells.Metabolic reprogramming of BMSCs in cancer microenvironment would determine the fate of cancer cells.
Competing interests
The authors have declared that no competing interests exist.

