Totalsaponinsin RubusparvifoliusL.inducelymphomacells apoptosis through upregulated Bax/Fas and downregulated Bcl-2 in vivo and in vitro
Xiao-Feng Xu*,Ru-Bin Cheng,Xue-Jin Zhang,Rui-Lan Gao
1Department of Hematology,Zhejiang Provincial Integrated Chinese and Western medicine Hospital,Hangzhou,China.2College of Pharmaceutical Science,Zhejiang Chinese Medical University,Hangzhou,China.3The First Affiliated Hospital,Zhejiang Chinese Medical University,Hangzhou,China.
Background
Rubus parvifolius L.(RP)belongs to the family Rosaceae and is mainly produced in the temperate zone of the Northern hemisphere.As early as the Xihan Dynasty of China,Erya recorded that RP were edible.Bencao Shiyi recorded the medicinal properties of the root bark and fruit of RP,which include clearing heat,cooling blood,stopping bleeding,dispersing knots,relieving pain,diuresis,and detumescence.Modern pharmacological experiments have shown that the water extract of RP has hemostatic,blood-activating,and stasis-removing effects and RP has been successfully used to treat coronary heart disease,angina pectoris,and other cardiovascular diseases[1].
Our previous studies demonstrated that total saponins of R.parvifolius L.(TSRP)can significantly induce apoptosis in human chronic myeloid leukemia K562 cells in vivo and in vitro.The mechanism involves increasing the expression of poly ADP ribose polymerase,caspase-3,and caspase-9 and inhibiting Bcl-2 expression.Additionally,we showed that TSRP can obviously inhibit the phosphorylation of Signal transducer and activator of transcription 3(STAT3)and increase the phosphorylation of adenosine monophosphate-activated protein kinase(AMPK)and c-Jun N-terminal kinase(JNK).A high concentration TSRP can significantly inhibit phosphorylation of protein kinase B(Akt)and mammalian target of rapamycin(mTOR)[2].
Lymphoma is a common tumor of the circulatory system.Although great progress has been made in treating lymphoma by the development of molecular targeted therapy and chimeric antigen receptor T-Cell immunotherapy(CAR-T),combinedchemotherapy remains the main treatment method.However,the toxicity and drug resistance of chemotherapy drugs remain as limitations.Few studies have examined the inhibitoryeffectof TSRP onlymphoma.Zheng Zhenxiao et al[3]reported that TSRP had anti-tumor activity against Hut-78 human skin T cell lymphoma.Through clinical observation,we found that oral administration of RP can reduce fever and night sweats in patients with lymphoma.Therefore,we examined the effect of TSRP on lymphoma to provide a basis for identifying the effective constituents in this traditional Chinese medicine(TCM)that inhibit the malignant proliferation of lymphoma cells.
Methods
Materials
Iscove’s modified Dulbecco’s medium (IMDM)was purchased from GIBCO(Grand Island,NY,USA).Antibodies against Bcl-2,Bax,Caspase-9,Caspase-3,Fas and β-Actin were obtained from Cell Signaling Technology(Danvers,MA,USA).All reagents were used according to the manufacturer's instructions.
Cell culture
Rajicellswereordinarily inoculated in IMDM containing 10%inactivated calf serum and 100 U/mL each of penicillin and streptomycin.The culture solutions were incubated in a constant temperature tank(37℃,5%CO2and with saturated humidity)for suspension culture.The medium was replaced every 2-3 days.Cells in the logarithmic growth phase were collected for analysis.
TSRP
TSRP was provided by the TCM Resource Engineering Lab ofZhejiang Chinese Medical University.An acetic anhydride-concentrated sulfuric acid was reacted with the available samples.The results were positive,demonstrating that the obtained substance was a triterpenoid saponin and that the purity was above 90%.TSRP was dissolved in water at a concentration of 1.0 g/L,sterilized by filtration,and stored at 4℃until use.
Establishment ofnude mice modelbearing lymphoma and grouping
Raji lymphoma cells were centrifuged for 10 min at 1000 r/min,after which the supernatant was removed.IMDM was used to prepare uniform mixtures of Raji lymphoma cells.The cell concentration was 2×107/mL, and the Raji lymphoma cells were subcutaneously transplanted into the left armpit of 3-week-old BALB/c nude mice.The dose was 0.2 mL/mouse;20 mice were used in total.Tumors formed after culture for 2 weeks(at that time,tumor nodules were observed below the skin of all mice;the nodule size was approximately 2×2 mm).Five nude mice did not develop tumors,giving a tumor formation rate of 75%.The nude mice in which tumors had formed were randomly divided into three groups,with each group consisting of 5 mice:①Control group(normal saline);②TSRP low-dose group(20 mg/kg);and③TSRP high-dose group(100 mg/kg).
Analysis of tumor inhibition by TSRP in nude mice bearing lymphoma
For the TSRP low-dose and high-dose groups,100µL was intragastrically administrated for each mouse every day at concentrations of 4 and 20 mg/mL,respectively.For the control group,the same volume of normal saline was intragastrically administered to each mouse every day.The activities(including eating,properties of stool and urine,mental status,etc.)of the mice were observed every day.Tumor size was measured every three day.The longer diameter a and shorter diameter b(mm)were measured with a Vernier caliper and tumor volume was calculated as follows:V=π/6×ab2(mm3)[4],after which the tumor growth curve was drawn.After intragastric administration of TSRP or saline for 3 weeks,the mice were sacrificed by cervical dislocation.The tumor was removed and weighed,and the tumor inhibition rate was calculated as follows:tumor inhibition rate=(1-tumor weight in the treatment group/tumor weight in the control group)×%.
TUNELanalysis
Paraffin sections containing the tumor tissue were prepared.After antibody labeling of the tissue using the TUNEL method,50-100 μL freshly prepared 3,3-Diaminobenzidine (DAB) solution as the chromogenic substrate was added to each section,and color changes were observed under a microscope.After color development,the section was rinsed with distilled water and restained with hematoxylin.Neutral gel was used to mount the section and the section was observed under a fluorescence microscope.
Immunohistochemistry analysis
Dewaxing, antigen retrieval, primary antibody incubation, and secondary antibody incubation(horseradish peroxidase-labeled)were conducted for the sections.DAB solution wasadded asthe chromogenic substrate.If the result is positive,the color was brown yellow.After restaining the sections with hematoxylin,neutral gum was used to mount the sections.
MTT assay
Lymphoma cells were cultured under normal oxygen and low oxygen conditions.Single cell suspensions were prepared with the culture solution containing 10% fetalcalf serum.Next,103-104cellswere inoculated into each well of a 96-well plate;the volume of each well was 200µL.Raji cells were treated with 0,100,200,or 400µg/mL TSRP.After culturing the cells for 48 h,20µL MTT solution was added to each well(5 mg/mL,prepared with PBS,pH 7.4)and incubation was continued for 4 h.The supernatant in the wells was carefully suctioned and discarded,after which 150µL dimethyl sulfoxide was added to each well.The samples were incubated with shaking for 10 min to dissolve the crystals.Absorption at a wavelength of 490 nm was measured in each well with an enzyme-linked immune assay reader.
DAPI staining
Based on the results of our previous experiment[2],0,100,200,and 400µg/mL TSRP were used.Raji cells in the logarithmic growth phase were cultured.The cells were treated with TSRP for 24 h,rinsed with cold PBS twice,and were fixed for 30 min with ethanol at-20℃.The fixed Raji cells were rinsed with PBS,stained for 15 min with 10 μg/mL DAPI at 25℃,and then rinsed with PBS three times.Raji cells were observed under a fluorescence microscope,and cells containing concentrated chromatin or cracked nuclei were considered as apoptotic.
Western blot analysis
Raji cells were treated with 0,100,200,and 400µg/mL TSRP individually.Total protein was extracted,and the sample concentration was measured.For sodium dodecyl sulfate-polyacrylamide gel electrophoresis,20µg sample was added to each well of the gel.After electrophoresis,the proteins were transferred to a nitrocellulose membrane using a semidry transfer cell.Next,Tris buffered saline Tween(TBST)containing 5%nonfat milk was added for overnight incubation at 4℃ to block nonspecific binding.After blocking,membranes were incubated with primary antibodies against Bcl-2,Bax,Fas,Caspase-9 and Caspase-3 and β-Actin for 2 h,and the membrane was rinsed with PBS containing 0.05%Tween 20 and filtered three times.The secondary antibody was added,followed by incubation for 1 h and washing with TBST three times.The membrane was placed into DAB substrate solution and reacted for 3 min.The reaction was stopped by adding water,and then an imager was used for radiography.
Statistical analysis
Statistical analysis was conducted with SPSS17.0 statistical software(SPSS,Inc.,Chicago,IL,USA).Data from each group were expressed as the average value±standard deviation and then evaluated by one-way ANOVA.P<0.05 indicated that the results were statistically significant
Results
Inhibition effect of TSRP on transplanted tumor of nude mice bearing lymphoma cells
After intragastric administration with different concentrations of TSRP for 3 weeks,the weights of the excised tumors in the low-dose and high-dose TSRP groupswere0.86±0.01and0.21±0.03g,respectively,and wereobviously lowerin mass compared to those from the control group(1.34±0.50 g).Based on the formula for determining the tumor inhibition rate:tumor inhibition rate=(1- tumor weight of the treatment group/tumor weight of the control group)×%.The tumor inhibition rates for the high-dose and low-doseTSRP groupsin mice transplanted with tumors were 90.84%and 27.96%,respectively.These values were significantly different compared to the control group(Both P<0.001,Table 1).
Change in size oftransplanted tumor after treatment with TSRP
As the intragastric administration time increased,tumor size changed in the low-dose and high-dose TSRP groups compared to those in the control group,showing significant differences.This reveals that TSRP strongly inhibits the growth of Raji cells in vivo(Table 2).
TUNEL analysis of apoptosis of transplanted tumor cells
Apoptotic cells were stained for fluorescence analysis undera laserconfocalmicroscope (×200).The high-dose group contained more apoptotic cells than the control group,and apoptotic cells presented a central distribution.Using Leica Confocal software,the fluorescence intensity of the three groups of lymphoma apoptotic cells was quantitatively analyzed.The results showed that the fluorescence intensity of the control group was 10.49±2.32,while these values in the 20 and 100 mg/kg TSRP groups were 26.14±2.42 and 82.43±7.81,respectively.Compared to the controlgroup,these differenceswere significant(Both P<0.001,Figure 1).
Expression of Bcl-2,Bax,and Fas of transplanted tumor determine by immunohistochemistry analysis
The results showed that Bcl-2 expression of Raji cells from the tumor treated with TSRP was clearly reduced compared to in the control group,while the Bax expression was clearly increased compared to that in the control group.This indicates that TSRP induces Raji cell apoptosis by reducing Bcl-2 expression and increasing Bax expression. Additionally, Fas expression in cells treated with TSRP was clearly reduced compared to that in the control group,demonstrating that TSRP induces Raji cell apoptosis also by reducing Fas expression(Figure 2).
Inhibitory effect of TSRP on Raji cell proliferation with MTT analysis
MTT analysis was conducted to determine whether 100-400 µg TSRP inhibited Rajicellgrowth dose-dependently.At a concentration of 400 μg/mL,the absorbance was 0.366±0.031 compared to 0.780±0.052 in the control group(P<0.001 Table 3).
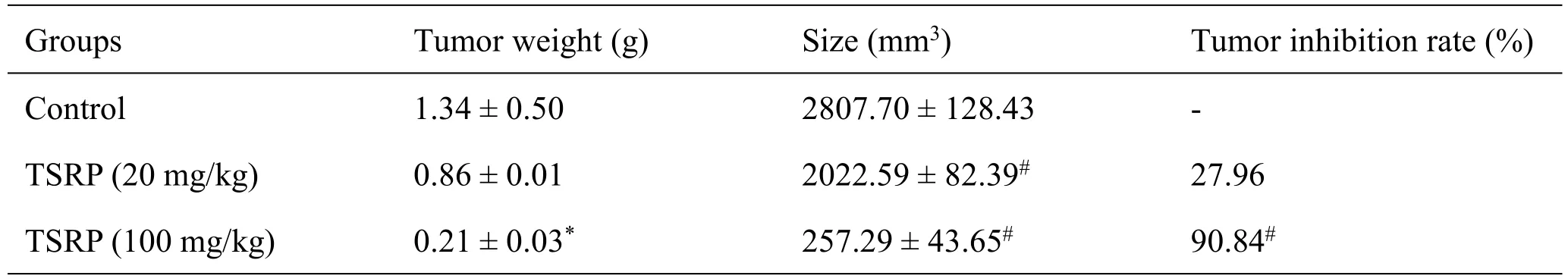
Table 1 Comparison of the tumor weight,size and tumor inhibition rate of each group after intragastric administration with TSRP
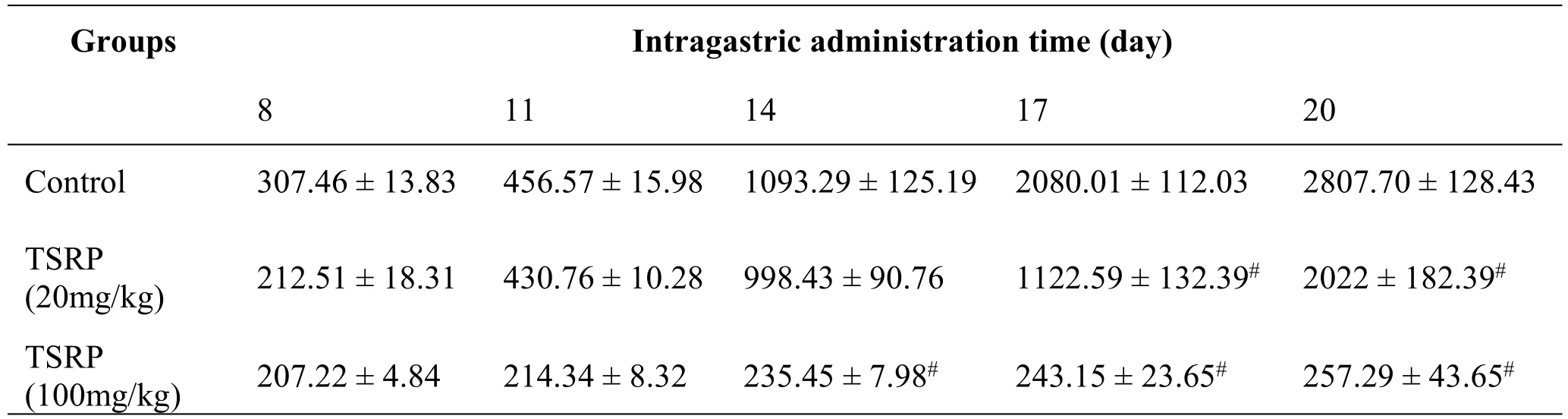
Table 2 Tumor size change of the nude mice after intragastric administration(mm3)
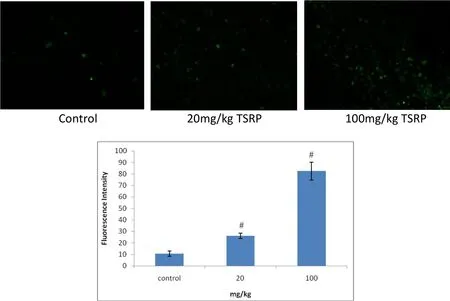
Figure 1 Comparing Raji cell apoptosis of the transplanted tumor of each group with TUNEL analysis
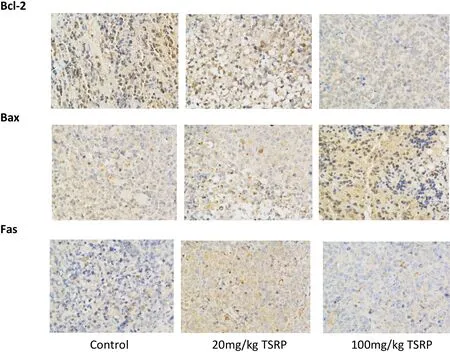
Figure 2 Expression of Bcl-2,Bax and Fas of the transplanted tumor of each group(×200)
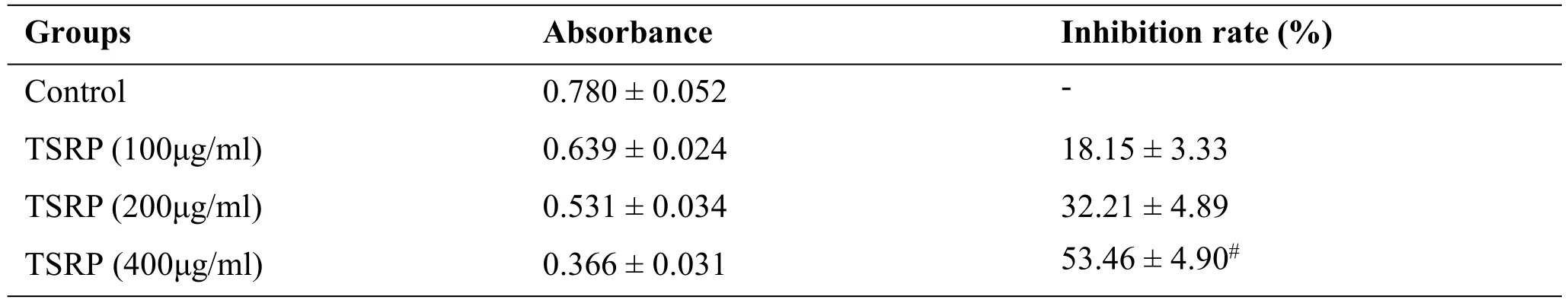
Table 3 Effects of TSRPat different concentrations with MTT
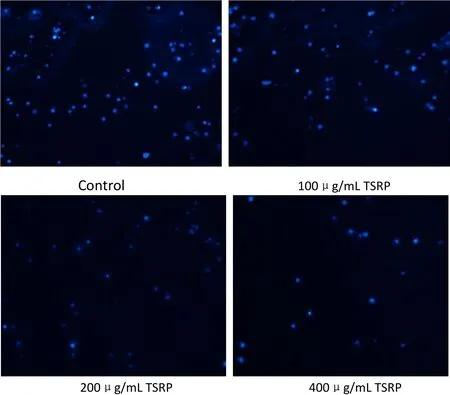
Figure 3 Morphology comparison after TSRP induces Raji cell apoptosis with DAPI analysis(fluorescence microscope×200).
TSRP induction of Raji cell apoptosis observed by DAPI staining
The nucleus of the control group had a regular and round shape and showed uniform staining.As the TSRP concentration increased,the nucleus became cracked to form round bodies of different sizes wrapped by the cell membrane(known as apoptotic bodies).Apoptosis was clearly observed(Figure 3).
Expression of Bcl-2,Bax,Fas,Caspase-9,and Caspase-3 evaluated by western blot analysis
Addition of TSRP significantly inhibited Bcl-2 activity and increased Bax activity in Rajicellsin a dose-dependent manner.This revealed that TSRP induces apoptosis by inhibiting Bcl-2 and increasing Bax expression.TSRP also inhibited Fas expression in a dose-dependent manner.Regarding the pathway by which TSRP induces Raji cell apoptosis in vitro,we found that Caspase-9 and Caspase-3 expression was also increased with increasing TSRP concentrations(Figure 4,Table 4).This suggests that TSRP induces Raji cell apoptosis through the chondriosome pathway.
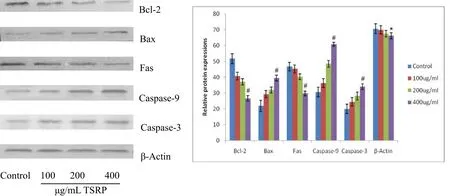
Figure 4 Effect of TSRP at different concentrations on activity of Raji cell apoptosis controlling proteins Bcl-2,Bax,Fas,Casepase 9 and Casepase 3
Discussion
Presently, lymphoma is treated by traditional chemotherapy, targeted drug combined with chemotherapy,or radiotherapy[5-8].However,these treatments have numerous serious side effects such as immune system and hemopoietic system damage.Thus,TCM may be valuable for inhibiting lymphoma cell proliferation and inducing the apoptosis of these cells.Herba RP is a plant in the genus Rubus L.and has fairly standard features of the genus;its components can remove pathogenic heat from the blood,arrest bleeding,remove obstructions and relieve pain,induce diuresis,and reduce edema,among other effects.Previous studies have mainly focused on cardiovascular effects of this plant,but its effects on tumors remain unclear.In preliminary studies,we showed that[2]TSRP significantly induced K562 cell apoptosis in vivo and in vitro,and the mechanism was related to increased expression of PARP,Caspase-3,and Caspase-9 and inhibition of the expression of Bcl-2.We also found that TSRP clearly inhibited the phosphorylation level of STATS in vitro and increased the phosphorylation levels of AMPK and JNK.A high-dose TSRP can significantly inhibit the phosphorylation level of Akt and mTOR.Therefore,it is necessary to explore the anti-lymphoma effects of TSRP.In this study,we found that administration of TSRP(at concentrations of 20 and 100 mg/kg)to nude mice inhibited the transplanted tumor.In vitro,an MTT assay showed that100-400 μg/mL TSRP had inhibitory effects on Raji cells in a dose-dependent manner.This reveals that TSRP has fairly strong inhibitory effects on lymphoma cell growth in vivo and in vitro.
The cell death process triggered by the in vivo and in vitro factors in the cell is known as cell apoptosis.Alterations in the normal apoptosis procedure in the organism can result in many diseases such as tumors[9].In vivo,the TUNEL method showed that apoptotic cells also increased with increasing TSRP concentrations.Compared to the control group,the difference was significant.Therefore,TSRP in vivo inhibits lymphoma cell growth through apoptosis.DAPI analysis suggested that the cell nucleus was cracked to result in the formation of apoptotic bodies of different sizes as the TSRP concentration increased in vitro,showing obvious apoptosis.Therefore,TSRP clearly induced Raji cell apoptosis in vivo and in vitro.
The endogenous apoptosis pathway(chondriosome pathway)originates from pressure inside of a cell.For example,rays,toxic drugs,a lack of growth factors,and other factors result in Caspase-9 activation,trigger the caspase cascade reaction,and cause cell apoptosis[10-12].Caspase-3 is the most important molecule involved in apoptosis in the caspase family,and it is related to morphology changes such as chromatin agglutination,karyopyknosis,and karyorrhexis during apoptosis[13].In early stages of apoptosis,Caspase-3 receives a signal from upstream caspase molecules and becomes activated;the relevant cytoplasm and karyon substrate is disrupted to result in cell apoptosis[14-15].According to western blot analysis,the expression of Caspase-9 and Caspase-3 also increased with increasing TSRP concentrations in a dose-dependent manner.This indicates that TSRP in vitro induces apoptosis through the chondriosome pathway.Further in vivo experiments are required to confirm these results.
Bcl-2 and Bax are two important members of the Bcl-2 gene family and have contrasting functions:inhibiting cell apoptosis and promoting cell apoptosis,respectively.Theformation ofBax homodimers induces apoptosis.Bcl-2 protein inhibits apoptosis mainly by combining with Bax to form heterodimers[16-18].The ratio of Bcl-2 to Bax also determines the formation of such dimmers to finely control cell fate[19-22].The Fas/CD95 system is a signal transduction system that mediates cell apoptosis and has been widely studied;this system belongs to the family of the tumor necrosis factor receptor and nerve growth factor receptor,which mediates cell death and is known as the death domain;it activates the Fas death signal and causes the tumor cell to undergo apoptosis[23-24].
Our results showed that the rate of positive Bcl-2 expression in cells treated with TSRP in vivo was obviously reduced compared to that in the control group.Additionally,the Bax expression was obviously increased compared to in the control group.This reveals that TSRP induces Raji cell apoptosis by reducing Bcl-2 expression and increasing Bax expression.We also found that Fas-positive expression in cells treated with TSRP was clearly reduced compared to that in the control group,suggesting that TSRP induces Raji cell apoptosis by reducing Fas expression.In vitro experiments showed the same results.Therefore,TSRP induces Raji cell apoptosis likely by altering the Bcl-2/Bax ratio and Fas signal pathway.
It remains unclear whether Bcl-2 affects apoptosis controlled by the Fas system.In some cells,Bcl-2 can inhibit CD95 and induce apoptosis.However,in other cells,there is no correlation between Bcl-2 and CD95.This will be examined in our future studies.Our results revealed a correlation between Bcl-2 and Fas expression.
Conclusion
TSRP induces Raji cell apoptosis by inhibiting Bcl-2,increasing Bax expression, and reducing Fas expression in vivo and in vitro.Additionally,TSRP has some effectson the chondriosome pathway of apoptosis in vitro.
1. Zheng YL,Hu CL.The experimental study of focal cerebral ischemia treated by Rubus parvifolius.Res Tradit Chin Med 2002,18:37.
2. Ge YQ,Xu XF,Yang B,et al.Saponins from Rubus parvifolius L.induce apoptosis in human chronic myeloid leukemia cells through AMPK activation and STAT3 inhibition.Asian Pac J Cancer Prev 2014,15:5455-5461.
3. Zheng ZW,Zhang LJ,Huang QL,et al.Antitumor effect of total saponins of Rubus parvifolius L.on three cutaneous tumors in vitro. Chin J Dermatovenereol Integr Tradit West Med 2007,6:67-69.
4. Evans BD,Smith IE,Shorthouse AJ,et al.A comparison of the response of human lung carcinoma xenografts to vindesine and vincristine.Br J Caner 1982,45:466-468.
5. Zhao ZJ,Chen Y,Francisco NM,et al.The application of CAR-T cell therapy in hematological malignancies: advantages and challenges.Acta Pharm Sin 2018,8:539-551.
6. Wang ZG,Wu ZQ,Liu Y,et al.New development in CAR-T cell therapy.J Hematol Oncol 2017,10:53
7. Gravelle P,BurroniB,PéricartS,etal.MechanismsofPD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma:asummary ofimmunohistochemicalstudies.Oncotarget 2017,8:44960-44975.
8. Ilcus C,Bagacean C,Tempescul A,et al.Immune checkpoint blockade:the role of PD-1-PD-L axis in lymphoid malignancies. Onco Targets Ther 2017,10:2349-2363.
9. Pérez-Garijo A, Steller H.Spreading the word:non-autonomouseffects ofapoptosis during development, regeneration and disease.Development 2015,142:3253-3262.
10.Hosen N,Park CY,Tatsumi N,et al.CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia.Proc Natl Acad Sci USA 2007,104:11008-11013.
11.Brown EJ,Frazier WA.Integrin-associated protein(CD47)and its ligands.Trends Cell Bio 2001,11:130-135.
12.12.Wang Y,Krivtsov AV,Sinha AU,et al.The Wnt/b-Catenin Pathway IsRequired forthe Developmentleukemiastem cellsin AML.Science 2010,327:1650-1653.
13.Koch U,Wilson A,Cobas M,et al.Simultaneous loss of beta-and gamma-catenin does not perturb hematopoiesis or lymphopoiesis.Blood 2008,111:160-164.
14.Yilmaz OH,Valdez R,Theisen BK,et al.Pten dependence distinguisheshaematopoietic stem cells from leukaemia-initiating cells.Nature 2006,441:475-482.
15.Kharas MG,Okabe R,Ganis JJ,etal.Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice.Blood 2010,115:1406-1415.
16.Kang Q,Zou H,Yang X,et al.Characterization and prognostic significance of mortalin, Bcl-2 and Bax in intrahepatic cholangiocarcinoma. Oncol Lett 2018, 15:2161-2168.
17.Lindqvist LM,Heinlein M,Huang DCS,et al.Prosurvival Bcl-2 family members affect autophagy only indirectly,by inhibiting Bax and Bak.ProcNatl AcadSciUSA 2014,111:8512-8517.
18.Wang ZX,Fang JF,Xiao JZ.Correlation of the expression of inflammatory factors with expression of apoptosis-related genes Baxand Bcl-2,in burned rats.Exp Ther Med 2019,17:1790-1796.
19.Gustafsson AB, Gottlieb RA. Bcl2-family members and apoptosis,taken to heart.Am J Physiol Cell Physiol 2007,292:45-51.
20.Okada Y,Shimizu T,Maeno E,etal.Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death.J Mem brBiol 2006,209:21-29.
21.21.Shimizu T,Numata T,Okada Y. A role of reactive oxygens peciesin apoptotic activation of volume-sensitive Cl(-)channel.Proc Natl Acad Sci USA,2004,101(17):6770-6773.
22.Kutuk O,Basaga H.Bcl2-protein family:implications in vascular apoptosis and atherosclerosis.Apoptosis 2006,11:1661-1675.
23.Liang Y,Nylander KD,Yan C,et al.Role of caspase-3-dependent Bcl-2 cleavage in potentiation of apoptosis by Bcl-2. Mol Pharmacol 2002,61:142-149.
24.Montes M,Coiras M,Becerra S,etal.FunctionalConsequences forApoptosis by Transcription Elongation Regulator 1(TCERG1)-Mediated Bcl-x and Fas/CD95 Altern ative Splicing.PLoS One 2015,10:e0139812.
25.Tauber SC,Harms K,Falkenburger B,et al.Modulation of HippocampalNeuroplasticity by Fas/CD95 Regulatory Protein 2(Faim2)in the Course of Bacterial Meningitis.J Neuropathol Exp Neurol 2014,73:2-13.
 Traditional Medicine Research2019年2期
Traditional Medicine Research2019年2期
- Traditional Medicine Research的其它文章
- Complementary and alternative medicine for cancer treatment:magic or fraud?
- Annual advances of integrative pharmacology in 2018
- Advances in research on the anticancer mechanism of the natural compound cucurbitacin from Cucurbitaceae plants:a review
- What are the challenges facing cancer therapy base on the dissipative structure theory
- The effect of long-term traditional Chinese medicine treatment on disease-free survival of postoperative stage I-III lung cancer patients:a retrospective cohort study
