Prognostic significance of castrate testosterone levels for patients with intermediate and high risk prostate cancer
Gokhan Ozyigit,Pervin Hurmuz,Deniz Yuce,Fadil Akyol
Gokhan Ozyigit,Pervin Hurmuz,Fadil Akyol,Department of Radiation Oncology,Hacettepe University Faculty of Medicine,Ankara 06100,Turkey M.D.
Deniz Yuce,Department of Preventive Oncology,Hacettepe University Faculty of Medicine,Ankara 06100,Turkey
Abstract
Key words: Prostate cancer;Androgen deprivation therapy;Radiotherapy;Testosterone;Castration
INTRODUCTION
The use of androgen deprivation therapy (ADT) in combination with radiotherapy(RT) has been the standard treatment for treatment of patients with localized high-risk prostate cancer based on improvements in cancer-specific (CSS) and overall survival(OS) observed in multiple randomized trials[1-3].
连接锅炉至文丘里冷却器[3](可利用原惰气系统辅助冷却器,需核算换热量是否满足)工艺管路。改在完成后,锅炉产生尾气,进入冷却器冷却,通过增压风机增压后进入大舱做为覆盖气。增加两路电动控制阀用于在热介质锅炉尾气流程下游发生故障时进行阀门切换,以保证锅炉尾气可以顺利排放至大气,稳定锅炉运行。具体工艺如图1。
ADT has been accepted as the initial treatment for patients with metastatic prostate cancer or when there is elevated serum prostate-specific antigen (PSA) level during the course of disease. Surgical (bilateral orchiectomy) or medical castration [using either a gonadotropin-releasing hormone (GnRH) agonist or a GnRH antagonist ±antiandrogens] are the two methods used for this purpose. Surgical castration was considered to be the primary and a fast cost-effective modality for androgen deprivation that leads to a considerable irreversible decline in serum testosterone to the “castrate level”. Medical castration which is reversible through cessation of treatment can be achieved by either suppressing the secretion of testicular androgens or inhibiting the action of androgens at the receptor level.
Commonly used castration level of <50 ng/dL (1.7 nmol/L) was defined more than 40 years ago after surgery,when testosterone measurement techniques were limited[4].This value has been also used after ADT to define castrate level in metastatic prostate cancer[5,6]. However it was shown that the introduction of chemiluminescent immunoassays provided more accurate and sensitive method to determine serum testosterone level[7]. Oefelein et al[8]reported that with the use of chemiluminescent assay mean testosterone level was <15 ng/dL after surgical castration. Thus,using contemporary techniques castrate testosterone level was proposed to be less than 20 ng/dL (0.7 nmol/L).
Although this new cut-off value shows better results compared to 50 ng/dL currently the accepted serum castrate testosterone level is still <50 ng/dL[9,10]. In this study we evaluated the effect of two different castrate testosterone levels,<50 and <20 ng/dL,on treatment outcomes in patients with non-metastatic intermediate and high risk prostate cancers receiving definitive RT and ADT. This is the first study to evaluate the castrate levels on biochemical relapse free survival (BRFS) for nonmetastatic prostate cancer patients.
MATERIALS AND METHODS
Patient characteristics
We have a prospective treatment protocol for the definitive treatment of prostate adenocarcinoma patients which was approved by the institutional ethical review board. Details of the protocol was described and published before[11]. In this study we included subset of patients with intermediate and high risk disease according to D’Amico risk group stratification treated between April 1998 and February 2011.Intermediate risk group was defined as Gleason score (GS) of 7,pretreatment prostate-specific antigen (iPSA) >10 to 20 ng/mL,and stage T1-T2 disease. High risk group was defined as disease with extracapsular extension (stage T3a-b),PSA >20 ng/mL or GS above 7. Between April 1998 and February 2011 173 patients with median age of 69 (range,50-82 years) were treated according to the treatment protocol.
Radiotherapy
Radiotherapy was delivered by either three dimensional conformal technique(3DCRT) before March 2009 or intensity modulated radiotherapy technique (IMRT) to a total dose of 76 Gy after March 2009 with daily fraction dose of 2 Gy. Clinical target volume (CTV) was prostate and seminal vesicles for 3DCRT. Seven 6 MV photon beams (anterior,right and left lateral,right and left anterior oblique,right and left posterior oblique) which were equally weighted were used. ICRU reference point(isocenter) dose was 73.6 Gy. For IMRT;CTV was prostate plus proximal seminal vesicle,but in case of extracapsular invasion whole seminal vesicles were included in the field. For planning target volume 5 mm is given in all directions except the rectal side where 3 mm is given. All of the patients receiving IMRT had 3 fiducials implanted to prostate one week before the planning computerized tomography and are used for image guided radiotherapy.
Androgen deprivation therapy and definition of castrate testosterone level
According to institutional treatment protocol all patients received 3 mo of neoadjuvant ADT followed by radiotherapy and additional 6 mo of ADT. ADT was delivered in the form of total androgen blockade (TAB): GnRH agonist (triptoreline,leuprolide,goserelin) plus antiandrogen (cyproterone acetate or bicalutamide).Testosterone levels were measured at each clinical follow-up visits at a single laboratory using immunoassay method (Immulite 2000,Simens,United States). The testosterone level measured during ADT was used as castrate testosterone level either at the 3rdor 6thmonths after radiotherapy,since all patients were on active adjuvant hormonal treatment during those periods. Patients were considered as having castrate level of testosterone if both measurements taken at the 3rdor 6thmonth of follow-up after RT were under desired value of 20 ng/dL or 50 ng/dL depending on subset analysis. Threshold value for testosterone recovery time was calculated from the first measurement of testosterone to the date when it is above 50 ng/dL.
Follow up
Patients were seen in every 3 mo for the first 2 years,4 months for the 3rdand 4thyear every 6 mo thereafter. In each visit,total serum PSA,free PSA and total testosterone levels were measured. ASTRO Phoenix definition (nadir PSA+2 ng/dL) was used to define biochemical relapse.
Statistical analysis
SPSS 21.0 (IBM Inc.,Armonk,NY,United States) version was used for the statistical analysis. The value of P <0.05 was used to determine statistical significance. Time to last follow-up and biochemical failure was calculated starting from the final date of RT. Kaplan-Meier test was used to estimate survival probabilities and Cox regression was used for hazard rates. Receiver operating characteristic curve (ROC) analyses was used to compare two cutoff values on BRFS.
RESULTS
Median follow up duration was 125 mo (10.4 years). Median initial PSA level was 14.2 ng/dL (range,2-100 ng/dL) and median Gleason score was 7 (range,3-9). The clinical and treatment characteristics of the patients are shown in Table1. All patients received 9 months of planned ADT. Ninety-six patients (56%) had castrate testosterone level of <20 ng/dL and 139 patients (80%) had castrate testosterone level<50 ng/dL. Median testosterone recovery time after TAB cessation was 6 months(range,6-30 mo).
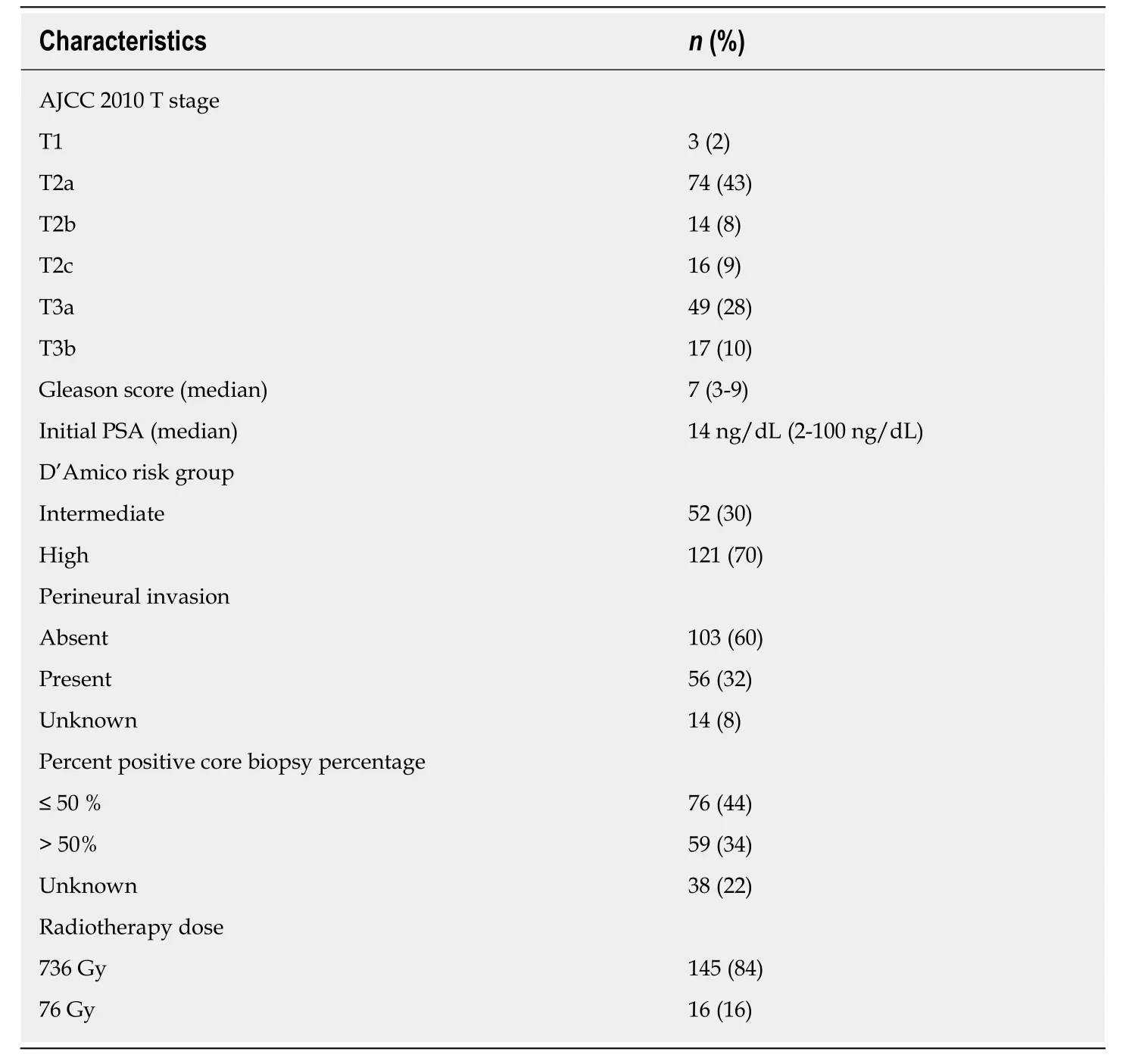
Table1 The clinical and treatment characteristics of the patients (n = 173)
Biochemical relapse free survival
When castrate testosterone level of <20 ng/dL is used 5 and 10 years BRFS rates were 90% and 83%,respectively (P = 0.001) (Figure1). Patients with castrate testosterone <50 ng/dL have 5 and 10 year BRFS rates of 86% and 76%,respectively (P = 0.006)(Figure2). Thus,both cutoff values are valid at predicting BRFS.
Patients with castrate testosterone value <20 ng/dL have significantly better BRFS compared to other patient groups (P = 0.003). Figure3 shows patients with castrate testosterone value of >50 ng/dL have better BRFS in the first five year compared to patients with castrate testosterone value between 20-50 ng/dL. However,in long term patients with testosterone 20-50 ng/dL have better BRFS than patients with BRFS >50 ng/dL. Thus,it seems that using cutoff castrate testosterone value of <20 ng/dL has better predictive value for estimation of BRFS in the follow up.
Multivariate analysis for independent predictors of BRFS was presented in Table2.Accordingly,BFFS was found to be independent from the baseline patient characteristics including D'Amico risk group,AJCC 2010 tumor stage,and Gleason Score and LHRH type.
When we compare two cutoff values using receiver operating characteristic curve(ROC) analyses,it was found that using 20 ng/dL is better than 50 ng/dL in predicting the BRFS (AUC = 0.63 vs 0.58,respectively) (Figure4,Table3).
DISCUSSION
Testosterone is known to promote the growth of prostate cancer cells,thus reducing circulating testosterone to castrate levels is the primary aim of treatment in advanced prostate cancer[4]. Historically,the recommended castrate threshold was below 50 ng/dL,and this value is still used in guidelines and clinical trials[5]. However,recent studies show better outcomes when threshold was below 20 ng/dL and the optimum serum castrate levels of testosterone to be achieved with ADT are still debated[8]. In this study we evaluated the effect of use of two different cutoff values on BRFS for patients with intermediate and high risk prostate cancer treated with modern RT techniques plus ADT. We found that using 20 ng/dL is better than 50 ng/dL in predicting the BRFS. To the best of our knowledge,current study is the first demonstrating the prognostic significance of castrate testosterone level in nonmetastatic prostate cancer patients receiving radiotherapy and hormonal treatment with the longest follow up period.
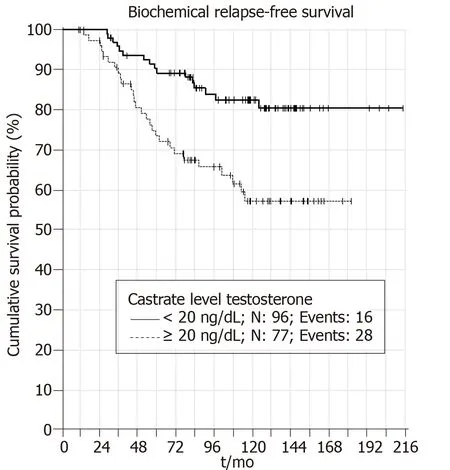
Figure1 Five and 10 years biochemical relapse free survival rates when castrate testosterone level of <20 ng/dL is used (P = 0.001).
There are some studies that have reported metastatic prostate cancer patients undergoing ADT have superior survival and time to progression if lower castrate levels of testosterone (20 ng/dL) are achieved[9,12,13]. Perachino et al[12]retrospectively reviewed 129 patients with a metastatic bony-only prostate cancer previously untreated with ADT who received 3 months of goserelin. With a mean follow-up of 47.5 mo,the risk of death was significantly correlated with Gleason score,6-month PSA level and the 6thmonth serum testosterone level. It was concluded that lowering the testosterone level as much as possible should be the goal of ADT in patients with metastatic prostate cancer. Bertaglia et al[13]assessed the relationship between serum testosterone levels after 6 months of ADT and treatment outcomes in 153 patients (54 with metastatic disease and 99 with biochemical failure) with prostate cancer. They showed that testosterone levels <50 ng/dL failed to be associated with time to treatment failure and overall survival. However,a cutoff of <20 ng/dL was associated with a nonsignificant lower risk of progression and a significant lower risk of death. Using ROC analyses it was concluded that testosterone value of 30 ng/dL offered the best overall sensitivity and specificity for prediction of death. However,these studies are retrospective studies included mixed population of patients with either metastatic/recurrent disease or non-metastatic disease but not receiving local RT.
However current literature seeking for the answer of the same question for nonmetastatic disease is small and heterogeneous. PR-7 trial randomly assigned patients having biochemical failure after radiation therapy or surgery plus radiation therapy to continuous or intermittent ADT. Klotz et al[14]evaluated the relationship between testosterone levels and cause-specific survival (CSS) and time to androgenindependent progression in 626 patients of the continuous ADT arm of the PR-7 trial.It was concluded that nadir serum testosterone less than 20 ng/dL within the first year of ADT correlates with better CSS and duration of response to ADT in men being treated for biochemical failure undergoing continuous ADT.
A recent study by Bryant et al[15]examined the association of sub-castrate testosterone nadir with PSA response and long-term clinical outcomes in 764 US veterans with intermediate or high-risk localized prostate cancer treated with ADT and definitive radiotherapy from 2000-2015. Patients were categorized into testosterone nadir groups based on the minimum testosterone measurement during continuous ADT (<20 ng/dL vs 20-49 ng/dL). With a median follow up of 5 years the results showed that compared to the <20 ng/dL group,the 20-49 ng/dL group showed higher 10-year biochemical recurrence rates (28.1% vs 18.3%,P = 0.016) and metastasis rates (12.9% vs 7.8%,P = 0.01).
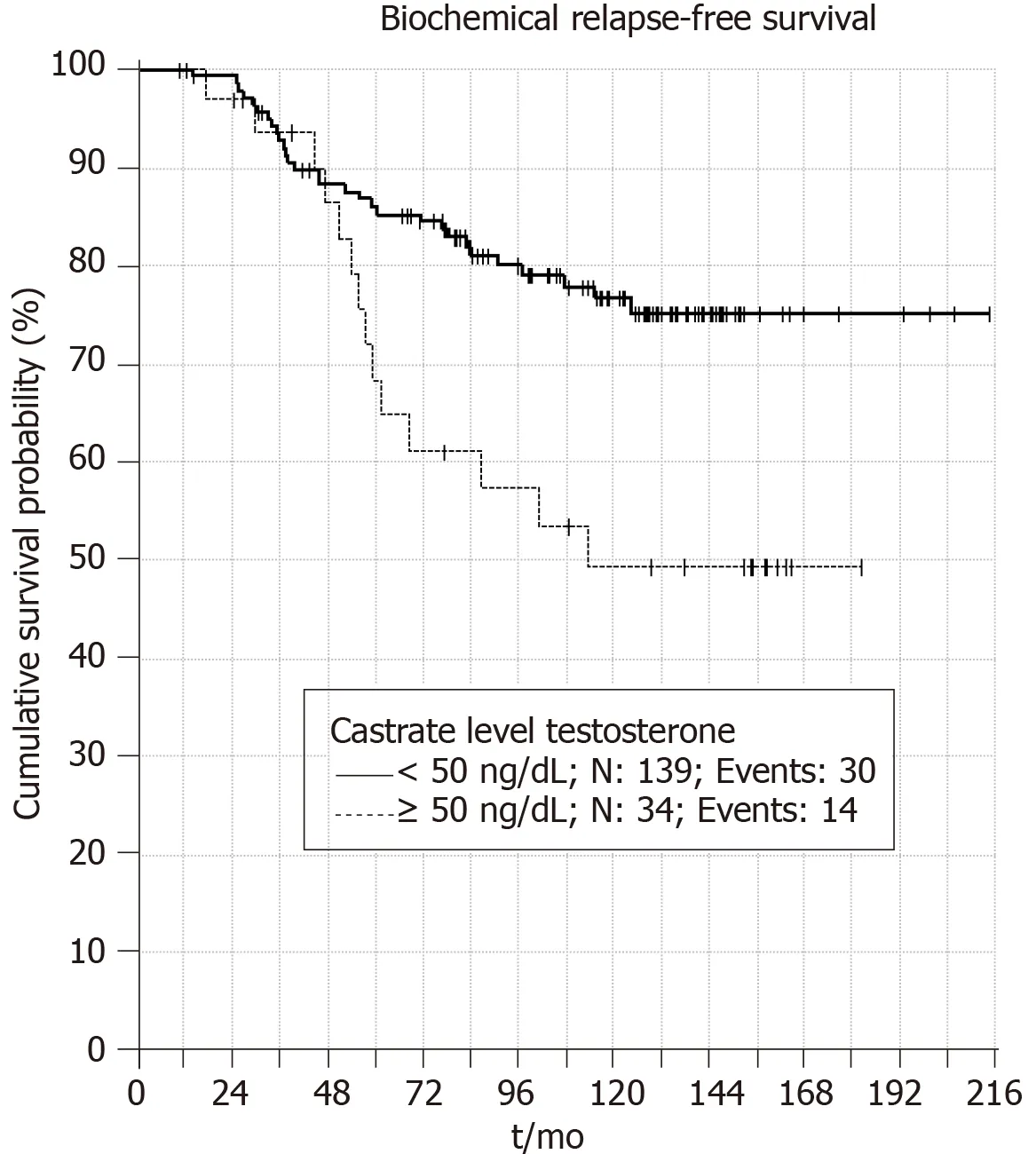
Figure2 Five and 10 years biochemical relapse free survival rates when castrate testosterone level of <50 ng/dL is used (P = 0.006).
On the contrary,Nabid et al[16]evaluated the testosterone level at the end of ADT to predict the treatment outcomes in intermediate and high risk prostate cancer based on the data of 796 from two randomized trials (PCS III ClinicalTrials.gov,No.NCT00223145 and PCS IV,ClinicalTrials.gov,No. NCT00223171). All patients received ADT and definitive RT. Castration was defined as testosterone level below 1.7 nmol/L (50 ng/dL) and outcomes were compared between the 3 groups;≤ 0.7 nmol/L,0.7-1.7,≥ 1.7 nmol/mL. The results were presented in abstract form. With a median follow up of 9 years the results revealed no difference in treatment outcomes between the groups.
Comparison of current study with the similar studies are shown in Table4.Duration of hormonal treatment and the RT dose might be the limitations of the study. Institutional treatment protocol included patients prospectively starting from 1998 thus most of the patients received 3DCRT. However,the dose at the ICRU reference point is still 73.6 Gy. The cohort in this study received 9 months of ADT in the form of total androgen blockade. It is known that after GnRH analogues testosterone 95% of testosterone production is eliminated. Adding anti androgens to GnRH in metastatic disease showed improved survival in metastatic prostate cancer but its role in non-metastatic cases are still debatable due to the side effects of the treatment[17,18]. At the beginning of our study in 1998,the long-term results of RTOG 92-02 and EORTC-22961 were not available. Therefore,ADT duration had been planned as 9 months. We revised ADT duration to 2 years and continue with GnRH analogues only after initial 28 d of antiandrogens in our institutional protocol after the final results of RTOG-9202 and EORTC-22961[19,20].
In current study serum testosterone was measured using modern immunoassay method. With a median follow up time of 125 mo,our treatment outcomes are in consistent with the literature supporting the use of lower castrate testosterone level. It seems that lowering testosterone levels below 20 ng/dL should be achieved for better treatment results. This might be achieved either by using long term ADT,TAB or novel antiandrogen treatments. Compared to similar studies previously described our patient have a homogenous treatment protocol and follow up duration is longer. All of the patients were treated according to our institutional treatment protocol and all received the planned treatment. Radiotherapy techniques and fields are described in detail compared to studies described above that has no irradiation data. All the follow ups were carried out at a single center using the same lab. Thus our patients seem to have more homogenous treatment and follow up.
In conclusion,we demonstrated that castrate testosterone level of less than 20 ng/dl achieved after primary RT plus ADT is associated with better BRFS. Using castrate cut off value of 20 ng/dL is better in estimating the BRFS compared to 50 ng/dL. Further studies using current standard of care of high dose IMRT and longer ADT duration might support these findings.

Table2 Multivariate analysis for independent predictors of biochemical failure free survival

Table3 Receiver operating characteristic curve analysis for two cut-off values of castrate testosterone levels
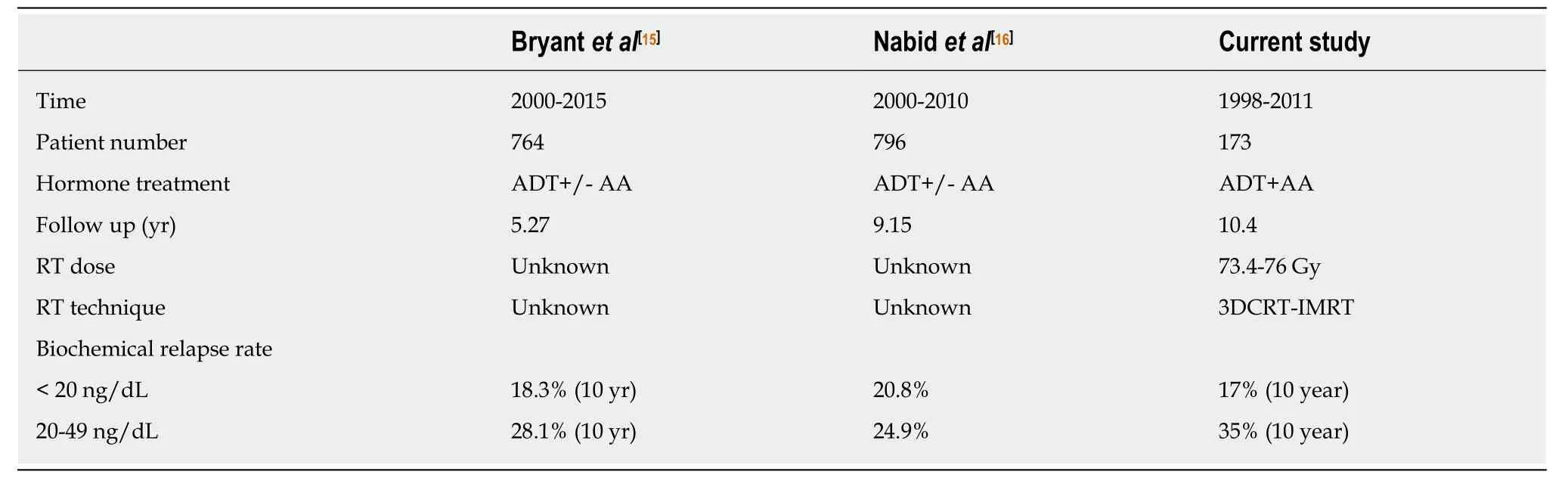
Table4 Comparison of studies analyzing the effect of castrate testosterone levels in intermediate and high risk prostate cancer patients receiving androgen deprivation treatment and definitive radiotherapy

Figure3 Biochemical relapse free survival rates for patients with different castrate testosterone cut-off values (P = 0.003).

Figure4 Receiver operating characteristic curve analyses comparing 2 cut-off castrate testosterone values.
ARTICLE HIGHLIGHTS
Research background
Historically,testosterone level of <50 ng/dL has been used to define castrate level after surgery or after androgen deprivation treatment in metastatic prostate cancer (PC). However,recent studies show better outcomes when threshold was below 20 ng/dL. In this study we evaluate the effect of two different castrate testosterone levels on biochemical relapse free survival in patients with non-metastatic intermediate and high risk PC receiving definitive modern radiotherapy and androgen deprivation treatment.
Research motivation
Current literature seeking for the answer of the castrate testosterone level question for nonmetastatic disease is small and heterogeneous.
Research objectives
The aim of the study was to evaluate the effect of two different castrate testosterone levels,<50 and <20 ng/dL,on treatment outcomes in patients with non-metastatic intermediate and high risk prostate cancers receiving definitive RT and ADT. This is the first study to evaluate the castrate levels on biochemical relapse free survival for non-metastatic prostate cancer patients.
Research methods
Between April 1998 and February 2011;173 patients wi th intermediate and high risk disease were treated. Radiotherapy was delivered by either three-dimensional-conformal technique to a total dose of 73.4 Gy at the ICRU reference point or intensity modulated radiotherapy technique to a total dose of 76 Gy. All the patients received 3 mo of neoadjuvant ADT followed by RT and additional 6 mo of ADT. ASTRO Phoenix definition was used to define biochemical relapse.
Research results
Median follow up duration was 125 months. Ninety-six patients (56%) had castrate testosterone level <20 ng/dL and 139 patients (80%) had castrate testosterone level <50 ng/dL. Both values are valid at predicting BRFS. However,patients with testosterone <20 ng/dL have significantly better BRFS compared to other groups (P = 0.003). When we compare two values,it was found that using 20 ng/dL is better than 50 ng/dL in predicting the BRFS (AUC = 0.63 vs 0.58,respectively).
Research conclusions
In current study serum testosterone was measured using modern immunoassay method. With a median follow up time of 125 mo,our treatment outcomes are in consistent with the literature supporting the use of lower castrate testosterone level. It seems that lowering testosterone levels below 20 ng/dL should be achieved for better treatment results. This might be achieved either by using long term ADT,total androgen blockage or novel antiandrogen treatments. Compared to similar studies previously described our patient have a homogenous treatment protocol and follow up duration is longer. All of the patients were treated according to our institutional treatment protocol and all received the planned treatment. All the follow ups were carried out at a single center using the same lab. Thus,all patients seem to have more homogenous treatment and follow up.
Research perspectives
In this study we demonstrated that castrate testosterone level of less than 20 ng/dl achieved after primary RT plus ADT is associated with better BRFS. Using castrate cut off value of 20 ng/dL is better in estimating the BRFS compared to 50 ng/dL. Further studies using current standard of care of high dose IMRT and longer ADT duration might support these findings.
 World Journal of Clinical Oncology2019年8期
World Journal of Clinical Oncology2019年8期
- World Journal of Clinical Oncology的其它文章
- Emergence of neural regulatory mechanisms in carcinogenesis
- Wilms tumor with dilated cardiomyopathy: A case report
