Autophagy: novel insights into therapeutic target of electroacupuncture against cerebral ischemia/reperfusion injury
Ya-Guang Huang, Wei Tao, Song-Bai Yang, Jin-Feng Wang, Zhi-Gang Mei, , , Zhi-Tao Feng,
1 Medical College of China Three Gorges University, Yichang, Hubei Province, China
2 Yichang Hospital of Traditional Chinese Medicine, Clinical Medical College of Traditional Chinese Medicine, China Three Gorges University,Yichang, Hubei Province, China
Abstract Electroacupuncture is known as an effective adjuvant therapy in ischemic cerebrovascular disease. However, its underlying mechanisms remain unclear. Studies suggest that autophagy, which is essential for cell survival and cell death, is involved in cerebral ischemia reperfusion injury and might be modulate by electroacupuncture therapy in key ways. This paper aims to provide novel insights into a therapeutic target of electroacupuncture against cerebral ischemia/reperfusion injury from the perspective of autophagy. Here we review recent studies on electroacupuncture regulation of autophagy-related markers such as UNC-51-like kinase-1 complex, Beclin1, microtubule-associated protein-1 light chain 3, p62, and autophagosomes for treating cerebral ischemia/reperfusion injury. The results of these studies show that electroacupuncture may affect the initiation of autophagy, vesicle nucleation, expansion and maturation of autophagosomes, as well as fusion and degradation of autophagolysosomes. Moreover, studies indicate that electroacupuncture probably modulates autophagy by activating the mammalian target of the rapamycin signaling pathway.This review thus indicates that autophagy is a therapeutic target of electroacupuncture treatment against ischemic cerebrovascular diseases.
Key Words: nerve regeneration; autophagy; electroacupuncture; cerebral ischemia/reperfusion injury; mTOR;LC3; Beclin1; p62; neuroprotection; neural regeneration
Introduction
Ischemic stroke, dementia, Alzheimer's disease and other ischemic cerebrovascular diseases are common entities that share a similar pathophysiology. Thrombolytic therapy is the common treatment for early ischemic cerebrovascular disease and can effectively restore the blood supply to the ischemic area. However, cerebral ischemia/reperfusion injury during thrombolytic therapy might lead to a series of pathological reactions including oxidative stress, calcium overload, glutamate excitotoxicity, in flammation, and apoptosis (Gong et al., 2014; Girbovan and Plamondon, 2015).Increasingly, studies have demonstrated that autophagy, or cellular self-digestion, is also involved in the neurological damage and cell death induced by cerebral ischemia/reperfusion injury (Li et al., 2016; Wang and An, 2016). More specifically, although autophagy plays a protective role in the early reperfusion period through cleaning damaged organelles and proteins (Su et al., 2014), during the late reperfusion period, excessive autophagy causes autophagic cell death, leading to subsequent damage of cells and tissues(Guo et al., 2014; Shui et al., 2016). Therefore, preventing and reversing cerebral ischemia/reperfusion injury through autophagy modulation is a potential novel therapeutic goal.Electroacupuncture (EA) serves as a complementary and alternative therapy for prevention and rehabilitation of ischemic cerebrovascular disease. It has been found to be potentially beneficial for reducing cerebral ischemia/reperfusion injury via benign regulation of oxidative stress(Lin et al., 2015), glutamate excitotoxicity (Sun et al., 2005),in flammation (Zhan et al., 2016), apoptosis (Liu et al., 2015;Zhang et al., 2018), and autophagy (Feng et al., 2018; Li et al.,2018). Indeed, studies (He et al., 2015; Li et al., 2016; Liu et al., 2016a; Shu et al., 2016) have shown that EA plays a key role in the whole process of autophagy, which includes the initiation of autophagy, vesicle nucleation, expansion and maturation of autophagosomes, and fusion and degradation of autophagolysosomes. Furthermore, studies (He et al.,2015; Wu et al., 2015) have indicated that the benefits could result from regulation of the mammalian target of rapamycin(mTOR)-related signaling pathway. It was reported that different EA parameters such as the selected acupoints as well as the current intensity, waveform, and duration of stimulation,produced different effects against cerebral ischemia/reperfusion injury (Shu et al., 2016; Feng et al., 2018). This article aims to provide a therapeutic target based on autophagy for EA treatment against ischemic cerebrovascular diseases.
Relevant studies were retrieved from online electronic databases including, PubMed, Embase, Web of Science, and China National Knowledge Infrastructure. Search terms consisted of three groups: autophagy, interventions (acupuncture, electroacupuncture, and EA), and diseases (ischemic stroke, cerebral ischemia, and cerebral ischemia reperfusion injury). All the articles in this review were published by August 2018 and were available in full text.
Process and Molecular Mechanism of Autophagy
Autophagy is an evolutionarily conserved self-degradative process that involves the break down and recycling of longlived proteins, lipids, and organelles, and is essential for cellular homeostasis and survival (Parzych and Klionsky,2014; Lu et al., 2018). Generally, there are three types of autophagy: macroautophagy, microautophagy, and chaperone mediated autophagy (Glick et al., 2010), among which, macroautophagy is most widely studied in relation to cerebral ischemia/reperfusion injury. The progress of macroautophagy (hereafter called autophagy) has several stages, including initiation, vesicle nucleation, expansion and maturation of autophagosomes, and fusion and degradation of autophagolysosomes (Hale et al., 2013; Chen et al., 2014; Huang et al., 2015b). The whole process is defined as autophagic flux (Lu and Hu, 2016) and involves a series of molecules(Figure 1).
Autophagy can be induced by ischemia, hypoxia, and stress responses during cerebral ischemia/reperfusion injury(Wang et al., 2018). In most cases, the initiation of autophagy is modulated by mammalian target of rapamycin complex 1 (mTORC1) (Rabanal-Ruiz et al., 2017). Additionally,the ULK1 complex—consisting of UNC-51-like kinase-1(ULK1) or ULK2, focal adhesion kinase family-interacting protein of 200 kDa (FIP200), and autophagy-related-gene(Atg) 13—also plays a key role at this stage (Ganley et al.,2009). Under nutrient-rich conditions, mTORC1 inhibits the initiation of autophagy by activating the ULK1 complex and phosphorylating ULK1 and Atg13. Under nutrient-de ficient conditions, mTORC1 dissociates from the ULK1 complex to induce autophagy (Rabanal-Ruiz et al., 2017).
Following initiation, a double-membrane vesicle is formed around the organelles and proteins that are to be degraded.At this stage, the class-III phosphatidylinositol 3-kinase(PI3K) complex—consisting of PI3K (mammalian homolog of yeast Vps34), Beclin1 (a homolog of Atg6), and p150 (a homolog of Vps15)—plays an essential role. Moreover, Atg1 and Atg9 are also required for vesicle nucleation (Sun et al.,2009). Further bending and extending of the double-membrane help the formation of mature autophagosomes, and the item to be degraded is wrapped in the double-membrane. At this stage, two ubiquitin-like conjugating systems,namely, the Atg12-Atg5-Atg16-like 1 (Atg16L1) complex(Kim et al., 2015; Chandra et al., 2016) and microtubule-associated protein-1 light-chain 3 (LC3) (a homolog of Atg8)phosphatidylethanolamine conjugate are required (Satoo et al., 2009; Yang et al., 2017).
The fusion of matured autophagosomes and lysosomes facilitates the formation of autophagolysosomes through acidification. After which, the encapsulated mitochondria, endoplasmic reticulum, or other organelles or proteins in the autophagolysosomes are degraded by lysosomal enzymes,and the degraded product is released into the cells for recycling. SNAREs, small GTPase Rab7, and lysosome-associated membrane protein (LAMP)-1 and LAMP-2 are required for the fusion (Hubert et al., 2016). The autophagosome membrane-protein p62 and the degradation of cargoes sequestered by autophagolysosomes are involved in the final step (Katsuragi et al., 2015; Liu et al., 2016b).
Role of Autophagy in Cerebral Ischemia/Reperfusion Injury
Previous studies have demonstrated that a series of complicated pathological processes, including oxidative stress (Rodrigo et al., 2013), endoplasmic reticulum stress (Xin et al.,2014), excitotoxicity (Hou and MacManus, 2002), calcium overload (Halestrap, 2006), in flammatory response (Ahmad et al., 2014), apoptosis (Lopez-Neblina et al., 2005), and autophagy (Xu and Zhang, 2011) are involved in the mechanisms underlying cerebral ischemia/reperfusion injury.Research based on autophagy has revealed a complex relationship between autophagy and other pathological changes;autophagy can be elicited by oxidative stress (Filomeni et al., 2015), endoplasmic reticulum stress (Meng et al., 2015),and excitotoxicity (Puyal et al., 2012), and these pathological changes can be secondarily reversed by activated autophagy.However, the role of autophagy in cerebral ischemia/reperfusion injury remains controversial. Studies have suggested that autophagy can be a double-edged sword in terms of its role in cerebral ischemia/reperfusion injury (Wei et al.,2012; Chen et al., 2014). During the first few hours of reperfusion, autophagy plays a protective role in cerebral ischemia/reperfusion injury by degrading damaged organelles and misfolded proteins, and by producing amino acids and nucleotides for recycling (Yan et al., 2011; Xia et al., 2013).However, if still active in the later stages of reperfusion, autophagy can lead to over-degradation of undamaged organelles and proteins, finally leading to autophagic cell death and secondary damage to cells and tissues (Gao et al., 2012;Wu et al., 2015). Therefore, autophagy regulation is thought to be a potential therapy target for cerebral ischemia/reperfusion injury. Recent studies have indicated that some traditional Chinese medicines, particularly acupuncture, might act against cerebral ischemia/reperfusion injury by modulating autophagy (Yang et al., 2016; Ting et al., 2017; Huang et al., 2018). Indeed, this has become a hot topic in the research community.
Electroacupuncture for Cerebral Ischemia/Reperfusion Injury via Modulating Autophagy
Acupuncture, one of the most important components of traditional Chinese medicine, has been used to prevent and rehabilitate diseases for more than 3000 years (Zhuang et al., 2013; Yu et al., 2018). With its remarkable effects as an adjuvant therapy, acupuncture now has been generally acknowledged worldwide as an alternative medicine. As early as 1979, acupuncture needles were approved by the U.S.Food and Drug Administration as a Class III (investigational) medical device and their clinical application was approved for licensed practitioners (Hammerschlag, 2000). In the same year, the World Health Organization approved 43 kinds of diseases and disorders such as nausea and vomiting,pain, and the rehabilitation of ischemic stroke as treatable with acupuncture. Moreover, evidence from laboratories indicated that EA, a clinical therapy combining electrical stimulation and needle insertion, could dramatically restore blood supply, reduce the volume of cerebral infarct,ameliorate learning and memory impairment, and reduce neurological deficits in rats with cerebral ischemia/reperfusion injury (Feng et al., 2018; Li et al., 2018). The beneficial efficacy was considered to be achieved through modulation of autophagy-related markers.

Figure 1 Process and molecular mechanisms of autophagy in cerebral ischemia/reperfusion injury (CIRI).
Electroacupuncture modulates the initiation of autophagy
AMP-activated protein kinase (AMPK)—an intracellular energy sensor—is activated by a rise in the AMP/ATP ratio that occurs following the fall in ATP levels during cerebral ischemia/reperfusion injury (Manwani and McCullough,2013). Further, AMPK inhibits the activation of mTOR through phosphorylating TSC2, which is a negative regulatory protein of mTOR. Inactivated mTOR rapidly dephosphorylates Atg13 and ULK1, leading to the formation of the ULK1 complex and eventually accelerating the initiation of autophagy (Alers et al., 2012; Gallagher et al., 2016).
In one study, EA (dense disperse wave; frequency, 1—20 Hz; peak voltage, 6 V; intensity, 0.2 mA) was applied to the Quchi (LI11) and Zusanli (ST36) acupoints 30 min/day for 3 days, starting 24 hours after reperfusion in a Sprague-Dawley rat middle cerebral artery-occlusion model. The result was a significant reduction of neurological deficits and cerebral infarct volume, significantly down-regulated protein expression levels for ULK1 and Atg13, and up-regulated protein expression levels for mTORC1. Thus, it effectively protected the brain from cerebral ischemia/reperfusion injury (Liu et al., 2016a). In another study, EA pretreatment(dense disperse wave; frequency, 2—15 Hz; intensity, 1 mA)was applied at the Baihui (GV20) acupoint in male C57BL6 mice with cerebral ischemia/reperfusion injury 30 min/day for 5 days. The results revealed that the Neurological Severity Score was significantly higher in the ischemia/reperfusion (I/R) group than in the sham group. Importantly, EA pretreatment markedly decreased the Neurological Severity Score. Moreover, the protein expression levels of mTOR and phosphorylated mTOR (p-mTOR) increased in the I/R group compared with the sham group, and EA pretreatment further promoted the expression of these proteins in the hippocampus (Zhou et al., 2016).
It has also reported that p53 plays a positive role in the activation of autophagy (White, 2016). EA pretreatment(dense disperse wave; frequency, 2—15 Hz; intensity, 1 mA)at the GV20 acupoint was applied to male Sprague-Dawley rats with cerebral ischemia/reperfusion injury 30 min/day,5 days/week, for 2 weeks. The results were increased protein expression levels of phosphorylated p53 (p-p53), p53, and LC3-II in the I/R group compared with the sham group,while pretreatment with EA significantly attenuated these increases in (Li et al., 2018).
Electroacupuncture in vesicle nucleation
The nucleation of vesicles is mainly mediated by class-III PI3K, Beclin1, Atg1, Atg9, and p150, among which, Beclin1 plays a crucial role (Funderburk et al., 2010; Mei et al.,2016). In one study (Shu et al., 2016), after establishing a male Sprague-Dawley rat model of cerebral ischemia/reperfusion injury by middle cerebral artery occlusion surgery,EA treatment (frequency, 2—20 Hz) was applied continuously for 30 minutes, once/day to the Shuigou (GV26) acupoint at different times after reperfusion. The results showed that EA stimulation at 24 and 72 hours after reperfusion greatly decreased cerebral infarct volume. Additionally, the protein cortical expression levels of Beclin1 decreased in the EA group compared with the I/R group, and there was a statistically significant difference between the EA and I/R 24-hours groups. Similar results were also reported in other studies.One showed that pretreatment with EA at GV20 (frequency,2—15 Hz; intensity, 1 mA) in male Sprague-Dawley rats 30 min/day for 5 days before middle cerebral artery occlusion remarkably down-regulated Beclin 1 protein expression levels (Wu et al., 2015).
However, another study showed the opposite result. After establishing a Sprague-Dawley rat model of cerebral ischemia/reperfusion injury by suture occlusion of the left middle cerebral artery, EA stimulation (dense disperse wave;frequency, 1—20 Hz; peak voltage, 6 V) at Shenting (GV24)and GV20 acupoints was administered 30 min/day for 7days beginning 2 hours after reperfusion. The results revealed that latency on a water-maze test markedly decreased in the EA group compared with the I/R group. Further, protein and mRNA expression levels of Beclin-1 in the hippocampus and left cerebral cortex markedly increased in the I/R group compared with the sham group, while treatment with EA for 10 days further up-regulated Beclin1 expression (Feng et al., 2018).
Another study reported that anti-apoptotic Bcl-2 family members—such as Bcl-2—interact with the Bcl-2-homology 3 motif of Beclin1 to suppress the formation of the Vps34-Beclin1 complex, thereby inhibiting the nucleation of vesicles (Kang et al., 2011). One study showed applied EA (dispersed wavelength; frequency, 1—20 Hz; intensity, 6 mA) at GV20 and GV24 acupoints 30 min/day for 14 days in Sprague-Dawley rats beginning 24 hours after middle cerebral artery-occlusion surgery. They found a reduction in the average escape latency over the 6-day testing period and up-regulation of Bcl-2 mRNA expression levels (Liu et al.,2015).
Electroacupuncture modulates the expansion and maturation of autophagosomes
A series of molecules have been reported to be involved in the expansion and maturation of autophagosomes, among which, LC3 plays a crucial role in promoting their formation(Huang and Liu, 2015). The carboxyl (C)-terminal amino acids of LC3 are cleaved by Atg4 to expose the glycine residue, transforming it into LC3-I, which is soluble. Soluble LC3 is converted to the membrane-bound form LC3-II via conjugation with phosphatidylethanolamine and the assistance of the E1-like enzyme Atg7 and the E2-like enzyme Atg3 (Huang et al., 2015a). LC3-II is attached to the surface of the vesicle membrane to promote the extension of the double-layer membrane, which is eventually sealed to form the mature autophagosome. Thus, this protein is an autophagosomal marker.
A middle cerebral artery-occlusion model was established in male Sprague-Dawley rats to induce cerebral ischemia for 2 hours followed by reperfusion. Continuous EA stimulation at LI11 and ST36 acupoints (dense disperse wave; frequency, 1—20 Hz; peak voltage, 6 V) was applied 30 min/day for 3 days to rats with cerebral ischemia/reperfusion injury.The results were that EA reduced the cerebral infarct volume that was induced by cerebral ischemia/reperfusion injury.In addition, a western blot assay showed that EA inhibited the conversion of LC3B-I to LC3B-II and greatly down-regulated the protein expression levels of Atg4 (He et al., 2015).Similar results were also reported in other studies. One study revealed that EA treatment at GV26 in Sprague-Dawley rats dramatically down-regulated the expression of LC3-II and the LC3-II/LC3-I ratio (Shu et al., 2016). Another study administered EA treatment at LI11 and ST36 acupoints (density wave; frequency, 1—20 Hz; peak voltage, 6 V) 30 min/day beginning 24 hours after reperfusion in Sprague-Dawley rats with cerebral ischemia/reperfusion injury. They found that EA treatment for 3 days significantly decreased the LC3BII/LC3B-I ratio in the rats (Liu et al., 2016a). Additionally,pretreatment with EA at GV20 also reduced cerebral infarct volume and the LC3-II/LC3-I ratio in Sprague-Dawley rats with cerebral ischemia/reperfusion injury (Wu et al., 2015).
Electroacupuncture modulates how autophagosomes fuse with lysosomes
Autophagolysosomes—which are formed when mature autophagosomes fuse with lysosomes—are considered one of the most accurate markers of autophagy. The structure and quantity of autophagosomes and autophagolysosomes can be observed by electron microscopy. Mature autophagosomes are double-membrane limited vacuoles containing undegraded cytoplasm such as ER membranes and mitochondria but no lysosomal proteins (Ylä-Anttila et al., 2009;Ma et al., 2012). Accumulation of autophagosomes indicates either the activation of autophagy or a blockage steps downstream of autophagy, such as inefficient fusion and decreased lysosomal degradation.
One study reported that in rats with cerebral ischemia/reperfusion injury, the numbers of lysosomes and autophagolysosomes increased in the striatum around ischemic areas, chromatins were edge-aggregated, and mitochondrial spines were disordered. EA stimulation applied to LI11 and ST36 for 3 days greatly reduced the numbers of lysosomes and autophagolysosomes, and also ameliorated the damaged mitochondria ultrastructure (Liu et al., 2016c).Another study reported that numerous autophagosomes and lysosomes that had engulfed cytoplasmic materials were observed in Sprague-Dawley rats with middle cerebral artery occlusion (ischemia for 2 hours followed by reperfusion for 12 hours), while quantitative analysis via electron microscopy images showed that EA pretreatment at GV20 remarkably reduced the numbers of autophagosomes (Wu et al.,2015). Furthermore, Liu et al. (2016a) also found that the numbers of autophagosomes, autolysosomes, and lysosomes markedly increased in Sprague-Dawley rats with cerebral ischemia/reperfusion injury, while EA stimulation at LI11 and ST36 for 3 days reversed this increase.
Electroacupuncture modulates the degradation of autophagolysosomes
p62—an adapter protein—plays a critical role in degradation(Komatsu and Ichimura, 2010). Serving as a bridge between ubiquitinated proteins and LC3, p62 binds with ubiquitinated proteins at the C-terminal and interacts with LC3-II at the N-terminal to form a complex that is finally degraded within autophagolysosomes (Long et al., 2017).
Li et al. (2016) reported that EA stimulation influenced this degradation. They first established a middle cerebral artery occlusion model in Sprague-Dawley rats in which they induced cerebral ischemia for 2 hours followed by reperfusion for 6, 24, or 72 hours. EA stimulation was applied to GV26 (continuous wave; frequency, 2 Hz; intensity, 1 mA)for 30 min/day before sacrifice. The results revealed that EA treatment ameliorated neurological deficits. In addition, the protein expression level of p62 was markedly lower 6 hours and 24 hours after reperfusion, but there was no significant decrease 72 hours after reperfusion. Treatment with EA 6 hours and 24 hours after reperfusion noticeably attenuated the decrease in p62 levels. No significant differences in p62 levels were found between the groups at 72 hours.
Electroacupuncture Modulates the Signaling Pathways that Control Autophagy
An increasing number of studies have demonstrated that autophagy is involved in cerebral ischemia/reperfusion injury through a variety of signaling pathways (Guo, 2017; He et al.,2017; Yu et al., 2017). However, the modulation of autophagy-related signaling pathways by EA treatment has not yet been completely elucidated. Previous studies have indicated that EA treatment probably modulates autophagy via the PI3K-mTOR signaling pathway (He et al., 2015) (Figure 2).
The PI3K-mTOR signaling pathway is known to be a classical autophagy pathway. The PI3K family is classified into three subgroups: class-I, II, and III PI3K. Generally, class-I PI3K activates and participates in the conversion of phosphatidylinositol (4,5)-bisphosphate (PIP2) lipids to phosphatidylinositol (3,4,5)-trisphosphate (PIP3) (Hemmings and Restuccia, 2012). PIP3 serves as a second messenger to recruit proteins bearing PH domains. Among these proteins,protein kinase B (AKT) plays a pivotal role in class-I PI3K signaling networks. AKT binds to the PIP3-rich region on the plasma membrane, allowing 3-phosphoinositide-dependent protein kinase 1 (PDK1) to phosphorylate Thr308 and Ser473 in the “activation loop”, leading to fully active AKT(Yu et al., 2015). TSC2 is directly phosphorylated by AKT,which destabilizes TSC2 and disrupts its interaction with TSC1, relieving the inhibition of Rheb, thus making mTOR activation possible and finally inhibiting the activation of autophagy (Inoki et al., 2002; Wataya-Kaneda, 2015).
In one study, EA was applied to LI11 and ST36 acupoints(dense disperse wave; frequency, 1—20 Hz; peak voltage,6 V) for 30 min/day in Sprague-Dawley rats with cerebral ischemia/reperfusion injury. Expression levels of PI3K and mTOR proteins in cerebral cortex significantly decreased in the I/R group compared with the sham group, and EA treatment for 3 days markedly attenuated these decreases (He et al., 2015). In another study, EA pretreatment (frequency,2—15 Hz; intensity, 1 mA) for 30 min/day was performed at GV20 in Sprague-Dawley rats with cerebral ischemia/reperfusion injury. The study results indicated that cerebral infarct volume and LC3-II protein expression levels in cerebral cortex were remarkably higher in the I/R group compared with the sham group. EA pretreatment for 5 days markedly reduced cerebral infarct volume and down-regulated LC3-II expression. Further analysis indicated that the protein expression levels of phosphorylated AKT (p-AKT)at Thr308 and phosphorylated mTOR (p-mTOR) were noticeably higher in the cortices of the I/R group than in the sham group. EA pretreatment further promoted the expression of p-AKT and p-mTOR (Wu et al., 2015).
Conclusion
As a crucial pathological change in cerebral ischemia/reperfusion injury, autophagy plays a double-edged role. During the first few hours of reperfusion, autophagy helps maintain cell survival through clearing damaged organelles. However,excessive autophagy at later stages leads to autophagic cell death (Yan et al., 2011; Gao et al., 2012; Xia et al., 2013).Therefore, autophagy may be a potential therapeutic target for ischemic cerebrovascular diseases. EA, an important alternative therapy, can effectively reduce the risk of recurring ischemic cerebrovascular disease and disability. In recent years, results from animal experiments have demonstrated that EA exerts an influence on the whole process of autophagic flux by regulating the expression of autophagy-related markers such as the ULK1 complex, Beclin1, LC3 and p62. Other studies have indicated that EA treatment might regulate autophagy via the PI3K-AKT-mTOR signaling pathway, thereby playing a neuroprotective role in cerebral ischemia/reperfusion injury (Table 1).
This review suggests that the regulation of autophagy for EA treatment against cerebral ischemia/reperfusion injury depends on the timing of ischemia and reperfusion, as well as the EA parameters such as selected acupoints, current intensity, waveform, and duration. The duration of ischemia is generally 1.5 hours or 2 hours, and reperfusion usually occurs 6, 12, 24, or 72 hours afterward. Recent studies indicate that after cerebral ischemia for 2 hours, EA treatment within 2 hours of reperfusion may combat cerebral ischemia/reperfusion injury by promoting autophagy (Feng et al., 2016).However, EA treatment more than 24 hours after reperfusion might work against cerebral ischemia/reperfusion injury by suppressing autophagy (Liu et al., 2016b). Therefore,we propose that the timing of ischemia and reperfusion plays a key role in determining the mechanisms through which EA regulates autophagy.
EA is a form of acupuncture in which a small electric current is passed between pairs of acupuncture needles. It is characterized as being safe without toxic side effects and is being accepted by more and more countries and regions.However, the acupoints for EA treatment of cerebral ischemia/reperfusion injury are still controversial. The commonly selected acupoints are GV20, GV24, GV26, LI11,and ST36 (Figure 3). Moreover, the effects on the nervous system and brain injury depend on how many acupoints are used; one or many. Therefore, to better understand the mechanisms through EA treatment regulates autophagy in cerebral ischemia/reperfusion injury, more attention should be paid to the selection of acupoints.
In summary, the mechanisms of regulating autophagy via EA treatment in cerebral ischemia/reperfusion injury needs to be further studied. Experiments on the role of autophagy in cerebral ischemia/reperfusion injury, the EA parameters,and selected acupoints will help provide the best EA therapy for modulating autophagy in the treatment of ischemic cerebrovascular diseases.

Table 1 Effect of acupuncture on autophagy in cerebral ischemia and reperfusion
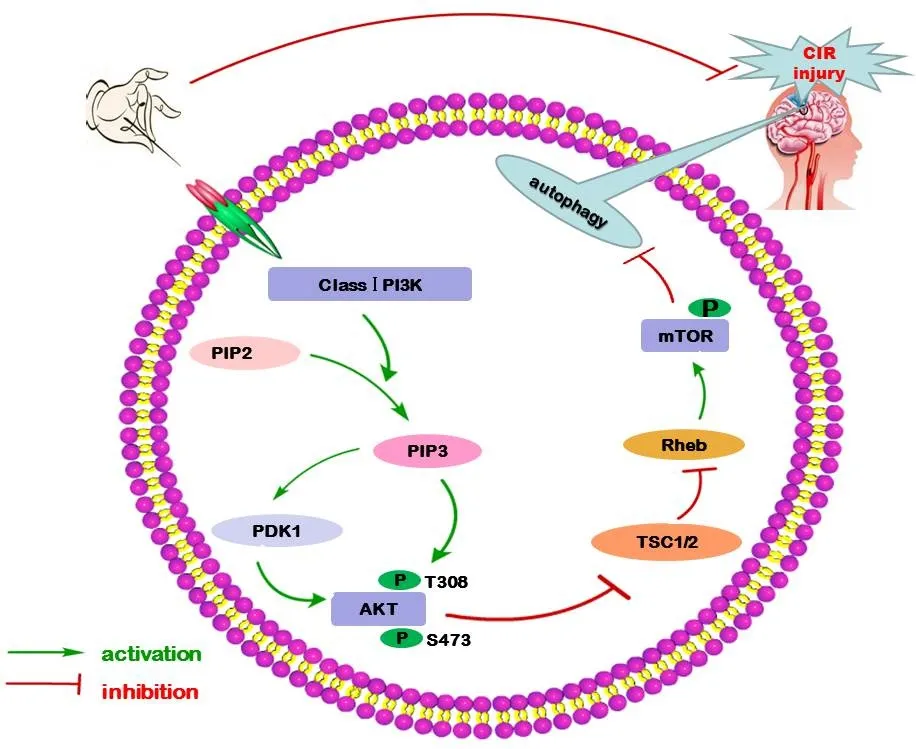
Figure 2 Modulation of autophagy the signaling pathway by electroacupuncture (EA) for treating cerebral ischemia/reperfusion(CIR) injury.
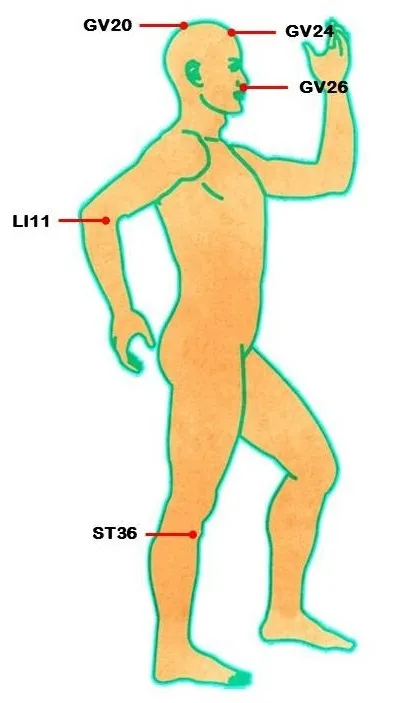
Figure 3 Acupoints reported in literatures (He et al., 2015; Wu et al.,2015, 2017; Feng et al., 2016, 2018; Li et al., 2016, 2018; Liu et al.,2016a, c; Shu et al., 2016; Zhou et al., 2016) to which acupuncture was applied to treat cerebral ischemia/reperfusion injury.
Author contributions:Review conception, partial manuscript drafting and revising: ZGM and ZTF; literature review, data collection and manuscript drafting: YGH; provision of some positive suggestions for the English language: WT and JFW; provision of valuable critical revisions:SBY. All authors approved the final version of the manuscript.
Conflicts of interest:All authors declare that they have no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Muscle secretion of toxic factors,regulated by miR126-5p, facilitates motor neuron degeneration in amyotrophic lateral sclerosis
- Comparative study of microarray and experimental data on Schwann cells in peripheral nerve degeneration and regeneration: big data analysis
- Busting the myth: more good than harm in transgenic cells
- Characteristics and advantages of adenoassociated virus vector-mediated gene therapy for neurodegenerative diseases
- Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in in flammation, cell death and nociception
- Nicotinamide adenine dinucleotide phosphate oxidase activation and neuronal death after ischemic stroke