低负载量的双金属Au@Pt核壳催化剂催化氧化甲苯
李思汉 李小青 胡凤腾 张 超 严新焕
(浙江工业大学绿色化学合成技术国家重点实验室培育基地,杭州 310014)
0 Introduction
Volatile organic compounds(VOCs)are the major component of environmental pollution from a larger variety of sources,such as transport,industrial process and household products[1-3].VOCs are also important precursors for the formation of particulate matter(PM),ozone and photochemical smog,which greatly threaten to the environment and human health due to their toxic,mutagenic and teratogenic characteristics[4-6].The development of efficient and low-energy methods for removing VOCs is one of the important means to ease the world′s energy and environmental problems.Until now,the main approaches[7]for removing VOCs include adsorption, absorption, photocatalysis,biodegradation,pulse corona,catalytic oxidation,etc.Among these methods, catalytic oxidation is considered to be one of the most effective and commonly used methods for eliminating VOCs,which can remove VOCs with low energy consumption,no secondary pollution is found as only CO2and H2O.
Currently,gold nanoparticles (NPs)have traditionally been utilized as catalytically active centers for noble metal deposition to prepare bimetallic core-shell structures.Among the various noble metals,bimetallic Au@Ptcore-shellnanoparticlesareconsidered a typical example and have been extensively studied and used,which can increase the activity,stability and selectivity in specific chemical reactions compared to monometallic Pt or Au.The improvement of performance is attributed to the synergy of the electronic and geometric effects between the two metals[8].In addition,the bimetallic Au@Pt core-shell also can reduce the amount of Pt loading and improve the utilization of Pt compared with alloyed structures[9].
Recently,many approaches have been applied to synthesize Au@Pt core-shell nanoparticles including electrodeposition[10-11], chemical reduction[12-13]and galvanic displacement[14-15].The procedures of electrodeposition and galvanic displacement are complicated and cumbersome.The reaction often occurs under the harsh conditions,such as the use of poisonous and stubborn surfactant agents,high temperature,strong acid and alkali solvents.On the basis of economic and environmental considerations,the preparation of core-shell nanoparticles by a simple method remains still a challenge.
Herein,we developed a new and environmentally friendly method for the preparation of Au@Pt coreshell nanoparticles:the Au nanoparticles were firstly prepared by liquid-phase hydrogen reduction,then small Pt particles were deposited onto the Au nanoparticles to obtain Au@Pt core-shell nanostructures.Compared with the conventional method,this method can effectively control the particle size and crystal structure.Moreover,it can regulate the dispersion of the active components on the carrier.The low loading Au@Pt core-shell catalyst showed higher activity for toluene oxidation at low temperature.
1 Experimental
1.1 Materials
HAuCl4·4H2O and H2PtCl6·6H2O(AR)were purchased from Shanghai Tuosi Chemical Co.,Ltd.Propylene carbonate(AR)was purchased from Dongguan Youte environmental protection materials Co.,Ltd.γ-Al2O3was purchased from Shanghai Lüqiang New Material Co.,Ltd.Toluene(AR)was purchased from Aladdin.
1.2 Preparation of nanoparticles
1.2.1 Preparation of Au nanoparticles
Au nanoparticlesweresynthesized by using HAuCl4·4H2O as a precursor.The measured amount of HAuCl4·4H2O was sufficiently dissolved in 100 mL of propylene carbonate and added to the autoclave.The reaction was carried out at 40℃under hydrogen pressure of 4 MPa for 2 h to obtain Au nanoparticles solution.γ-Al2O3was added into the above solution,stirred for 24 h and calcined at 400℃for 4 h after filtrating and drying.The combined catalyst with low Au loading 0.04% (mass fraction)was obtained and recorded as Au/γ-Al2O3.
1.2.2 Preparation of Au@Pt core-shell nanoparticles
The calculated amount(nAu∶nPt=1:0,3∶1,2∶1,1∶1,1 ∶2,1 ∶3,0 ∶1)of H2PtCl6·6H2O was added in the above-prepared Au nanoparticles solution.Then the mixed solution was added to the autoclave with 4 MPa H2.The solution was vigorously stirred for 3 h at 40℃to obtain Au@Pt core-shell nanoparticles.To deposit the sol on the support,a certain amount of the pretreated γ-Al2O3carrier was added to the abovenanoparticle solution.After stirring and impregnating 24 h,the sample was filtered,dried and then calcined in the muffle furnace at 400℃for 4 h to obtain combined core-shell Au@Pt catalysts (the loading amount was shown in Table 1).
1.3 Catalyst characterization
The XRD patterns of the materials were performed on a Rigaku D/Max-2500 X-ray diffractometer,which used a Cu Kα radiation(λ=0.154 nm)in the 2θ scan range(40 kV and 100 mA)from 10°to 80°with a step of 0.05°.Transmission electron microscopy(TEM)was taken on a JEOL JEM-1200EX with an accelerating voltage of 60 kV equipped with energy dispersive spectroscopy(EDS).The X-ray photoelectron spectrometer(XPS)was carried on non-monochromatic Al Kα(1 486.6 eV)radiation.The N2adsorption-desorption experiments were measured at liquid nitrogen temperature(77 K).The specific surface area of the material wascalculated from the desorption isotherm by Brunauer-Emmett-Teller(BET)theory.The different H2reduction temperature and hydrogen consumption of different oxidation state substances was measured using H2-TPR (temperature programmed reduction)method with online thermal conductivity detector(TCD)recording the temperature dependence of H2concentration.
1.4 Catalytic activity measurements
The catalytic activity ofthe catalystwas evaluated in the self-made atmospheric fixed bed catalytic reaction device.The catalyst (0.5 g)was placed in the middle of the reaction tube. The saturated toluene vapor of 0℃was introduced into the reaction tube by bubbling method.In the feed stream,the concentration(volume fraction)was 1×10-3and gas hourly space velocity (GHSV)of VOCs was 18 000~54 000 mL·g-1·h-1.The toluene and oxide content in the tail gas was monitored online by gas chromatography with a flame ionization detector(FID)and a TCD to determine toluene conversion and CO2selectivity at different temperatures,and the temperature at which toluene achieved 98%conversion was recorded as T98.
The conversion of toluene and selectivity of CO2are calculated as follows:
where φC7H8,inand φC7H8,outrepresent the volume fraction of toluene before and after reaction,respectively;φCO2and φCOrepresent the volume fraction of CO2and CO.
2 Results and discussion
2.1 Catalyst load test
The metal loading of different Au-Pt molar ratio catalysts were determined by ICP-AES (Table 1).It can be seen that the actual loading is slightly lower than the theoretical calculation,indicating that a loss of some metal might occur during the synthesis or calcination step.However,it has little influence on the catalyst within the margin of error,which can be neglected basically.Therefore,the actual atomic ratio of the catalyst can be replaced by the theoretical value.
2.2 Morphology and structure of the catalyst
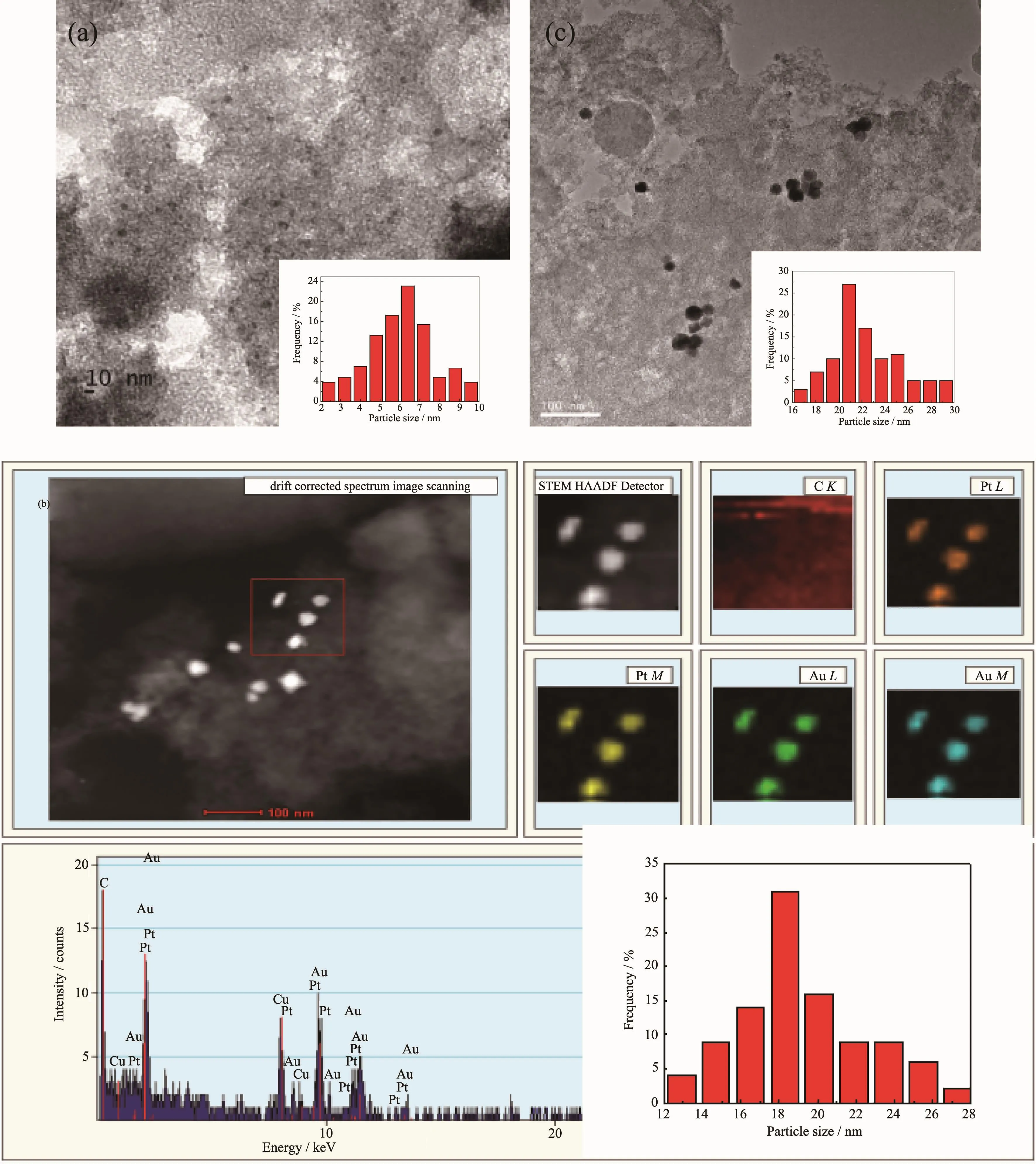
Fig.1 TEM image of Au nanoparticles(a),elemental mapping of bimetallic Au1Pt2nanoparticles(b),TEM image of bimetallic Au1Pt2nanoparticles after reaction(c)and the corresponding particle size distributions
Fig.1 shows the TEM images and particle size distributions of Au and Au-Pt nanoparticles.The monometallic Au nanoparticles were uniformly dispersed with wide particle size distribution in a range of 3.75~10.61 nm and the average size was 6.57 nm (Fig.1a).Due to the effect of propylene carbonate as dispersant and stabilizer,nanoparticles are directly adsorbed on the surface of the support,which effectively inhibits the growth of nanoparticles and hinders the penetration of nanoparticles into the pores of the support[16].Thus Au nanoparticles have smaller size of the active phase and finer dispersion.The bimetallic Au-Pt nanoparticles were significantly larger than the monometallic Au.The sizes(Fig.1b)were between 12.5 and 29.1 nm,and the average size was 18.3 nm.This indicates that Au nanoparticles gradually grow,which results from the deposition of Pt on the surface of Au or the agglomeration of Au(Pt)nanoparticles.As can be seen from Fig.1c,the catalyst after reaction showed partial agglomeration and the overall particle size increased,but the amplitude of variation was not significant,demonstrating that the catalyst remain stable and is consistent with XRD characterization.However,the TEM images of bimetallic Au-Pt do not allow us to distinguish between Au and Pt particles because Au and Pt exhibit almost the same imaging contrastand similar crystal structure[17].From the elemental mapping images of bimetallic Au-Pt NPs(Fig.1b),Au-L(M)and Pt-L(M)which are represented by different colors corresponded to each other,that is,the regions with Au must have the presence of Pt.This indicates that Pt grows on the surface of Au instead of forming its own nucleus or that Au and Pt are physically mixed together,which may form core-shell or alloy structure.
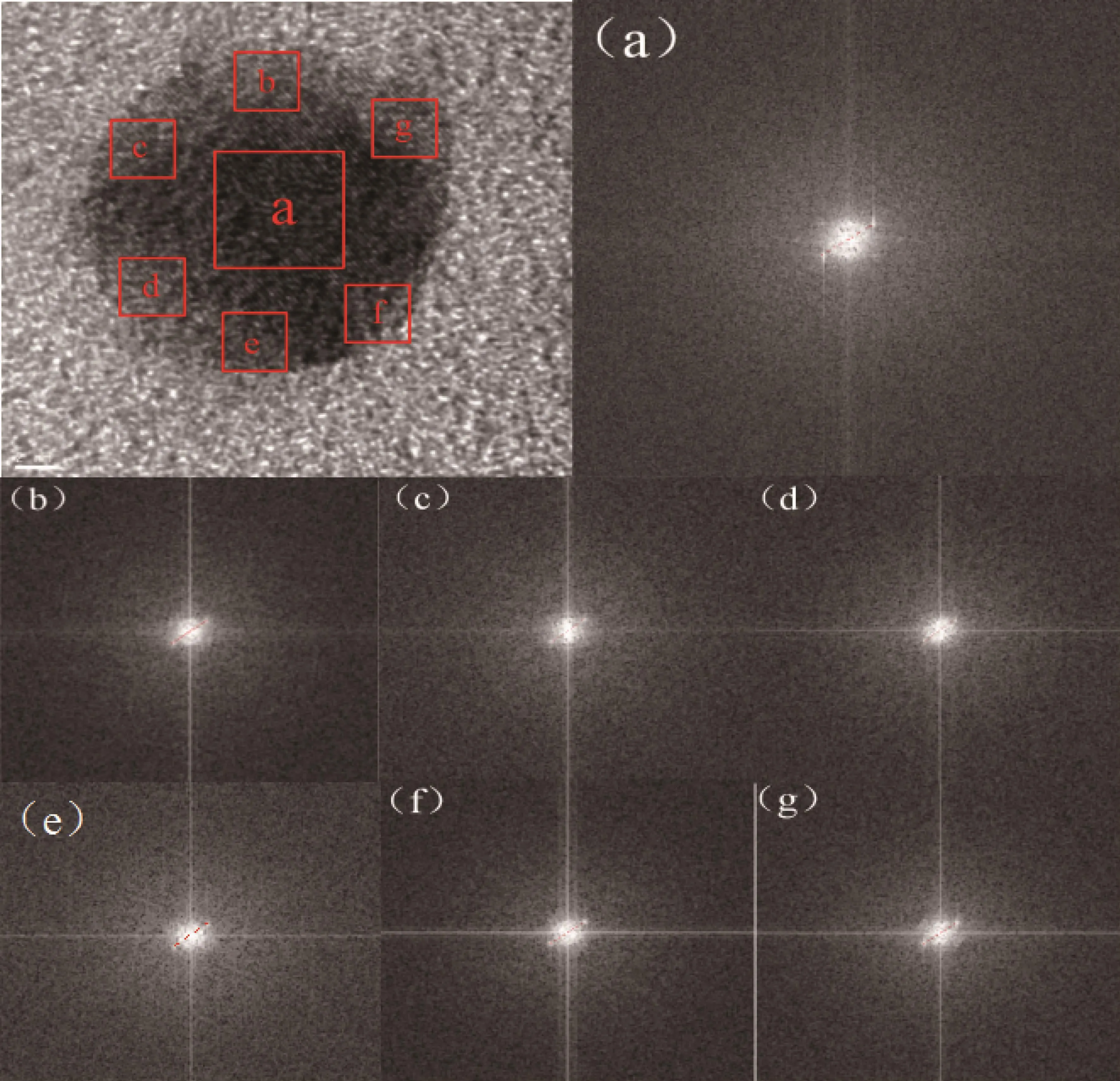
Fig.2 HRTEM image of Au-Pt nanoparticles corresponding to the first point in Fig.1b and FFT patterns of each area(a~g)
High-resolution TEM (HRTEM)images of Au-Pt nanoparticles are shown in Fig.2.In order to verify the core-shell structure of nanoparticles,we performed Fourier transform on the high-resolution structures to obtain the corresponding diffraction patterns.The lattice spacing was calculated by using digital photomicrography software.From the diffraction pattern(Fig.2),the lattice spacing at the center position of the nanoparticles(1#)was 0.235 nm for the(111)lattice planes of fcc metallic Au[18].The lattice spacing of the diffraction fringes in the b~g region(Fig.2)was 0.224,0.225,0.226,0.225,0.224 and 0.225 nm for the(111)lattice planes of fcc metallic Pt[19].The HRTEM image of the Au-Pt nanoparticles revealed that the d-spacings of adjacent fringes for metal cores and metal surrounding were(0.235±0.002)nm and(0.225±0.002)nm[20],respectively,corresponding to the(111)planes of facecentered cubic Au and Pt,which indicates that bimetallic Au@Pt NPs are core/shell structures.In order to verify the universality of this conclusion,we selected two more points (Fig.S1 and S2),and the lattice spacings of a~g and b~g regions was shown in Table S1,which matches well with the(111)planes of the Au and Pt,respectively.We can draw the same conclusion that the nanoparticle must be core-shell structure,which accords with elemental mapping of Au@Pt nanoparticles.
The XRD patterns of γ-Al2O3,supported catalysts and the standard diffraction peaks of Au and Pt are shown in Fig.3.The diffraction pattern for monometallic Pt had several peaks at 39.8°,46.2°and 67.4°,corresponding to(111),(200)and(220)planes(PDF No.89-7382),respectively.Wide-angle X-ray diffraction showed broad peaks at 38.2°,44.4°,64.6°,77.5°,which are characteristic of(111),(200),(220)and (311)planes of monometallic Au (PDF No.89-3697),respectively.The XRD patterns of the bimetallic Au@Pt core-shell catalysts matched quite well with the(111),(200),(220)and(311)peaks in the Au pattern and nodiffraction peaksofPtcan be observed.This indicates that only a monolayer of Pt is deposited on Au withoutforming individualPt particles[21]or there exist small size Pt particles(1~2 nm,which can be undetected by XRD technique)or the loading of Pt on the Au surface is very low[22],otherwise the Au and Pt peaks of physical mixture would appear in the XRD pattern.From Fig.3,it can be seen that the XRD peak of Au (220)and(311)tended to be flat with the increase of Pt content in the bimetallic catalysts.The Au@Pt particle size on the support become smaller with increasing Pt content.Among the prepared Au@Pt/γ-Al2O3catalyst,the maximum dispersion was obtained by Au1@Pt2catalyst,which leads to high catalytic activity.As can be seen from the XRD pattern of Au1@Pt2/γ-Al2O3catalyst after reaction,the number of diffraction peaks did not change and the structure did not change significantly,indicating that the catalyst has good stability.
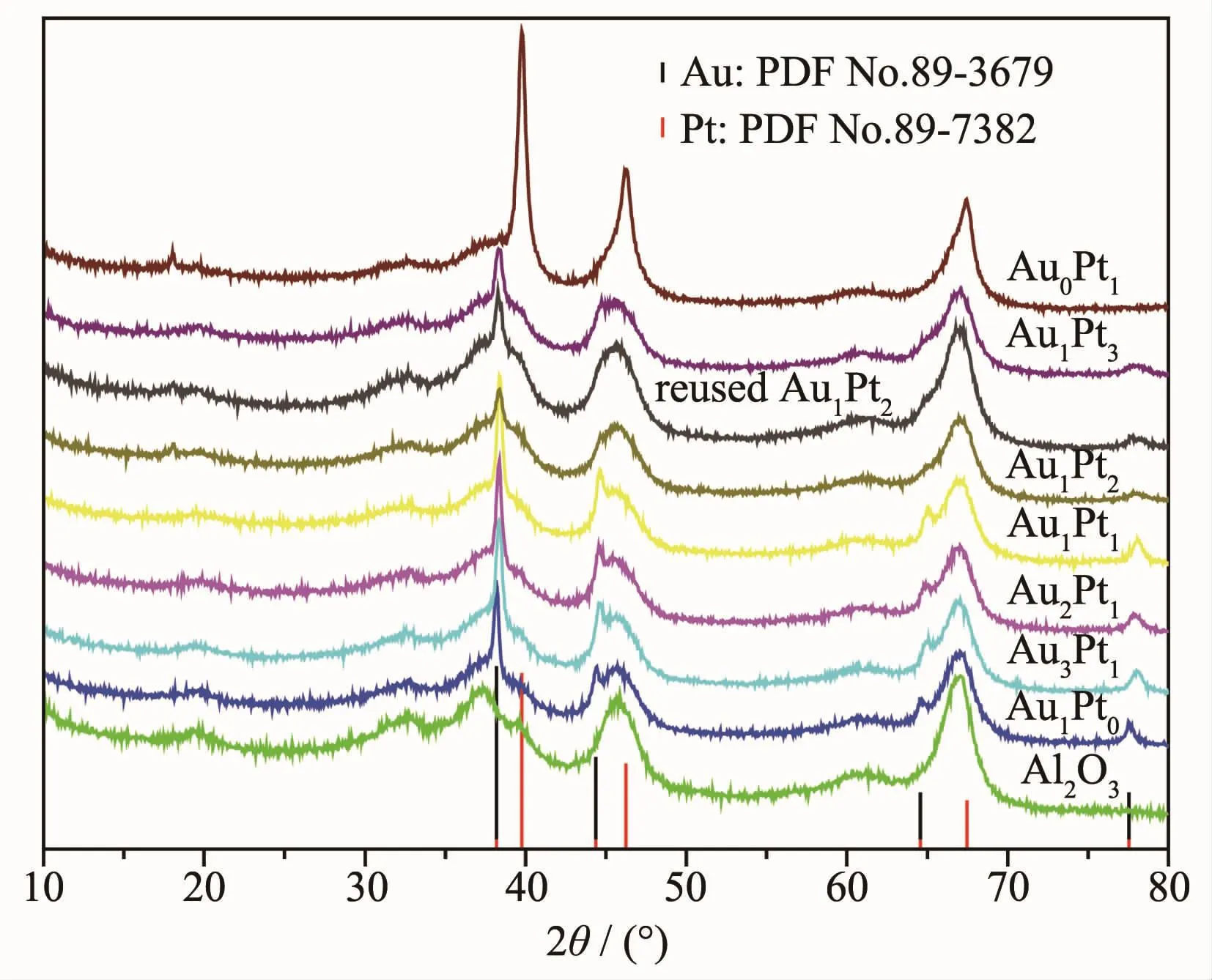
Fig.3 XRD patterns for Au@Pt NPs prepared with different molar ratios
2.3 Reducibility of catalyst
Table 1 shows the physical structure properties ofthe supported catalystand support γ-Al2O3.Compared with support γ-Al2O3,the specific surface area,pore size and pore volume of the supported catalyst were reduced,which may be due to the blockage of γ-Al2O3pores by some nanoparticles during the preparation process.However,the specific surface area and pore volume of Au1@Pt2/γ-Al2O3were relatively larger than other catalysts.The Au1@Pt2nanoparticles are mainly distributed on the surface of the support and have relatively less influence on the support structure.It is well known that the distribution of active components is one of the most important factors to affect the activity of the catalyst,especially for the gas-solid reactions.The more active components are located on the surface of the support,the more effective active sitesareprovided forthe reaction.What′s more,the time for the reactant to enter the inner pore and the product to diffuse out from the inner pores is shortened,thereby greatly improving the activity of the catalyst[23].
It is known to all that the reducibility of catalysts can play an important role in the redox reactions.For metaloxide catalysts,H2-TPR measurementcan reflect the reduction of high-valent metal ions to lowvalent metal ions or metal-atom,as well as the potential to remove or absorb oxygen[24].The H2-TPR profiles of Au@Pt catalysts are shown in Fig.4.For Au/Al2O3,there were two reduction peaks.The first weak peak around 324℃is attributed to the reduction of AuxOy[25],which coincides with the results of XRD with the presence of oxides.And the second peak obtained at 598℃can correspond to the reduction of gold ions(Au+)in the subsurface of support[26].For Pt/Al2O3,the first peak at 93℃can be assigned to the reduction of PtOx,which contains the contribution of the surface oxygen adjacent to Pt species due to the spillover effect induced by the metal-support interaction.The second peak at 502℃is ascribed to the reduction of the surface oxygen far away from Pt particles and the reduction of subsurface oxygen[27].The third peak around 680℃observed in all the samples may be ascribed to the reduction of surface A13+on γ-Al2O3[28].
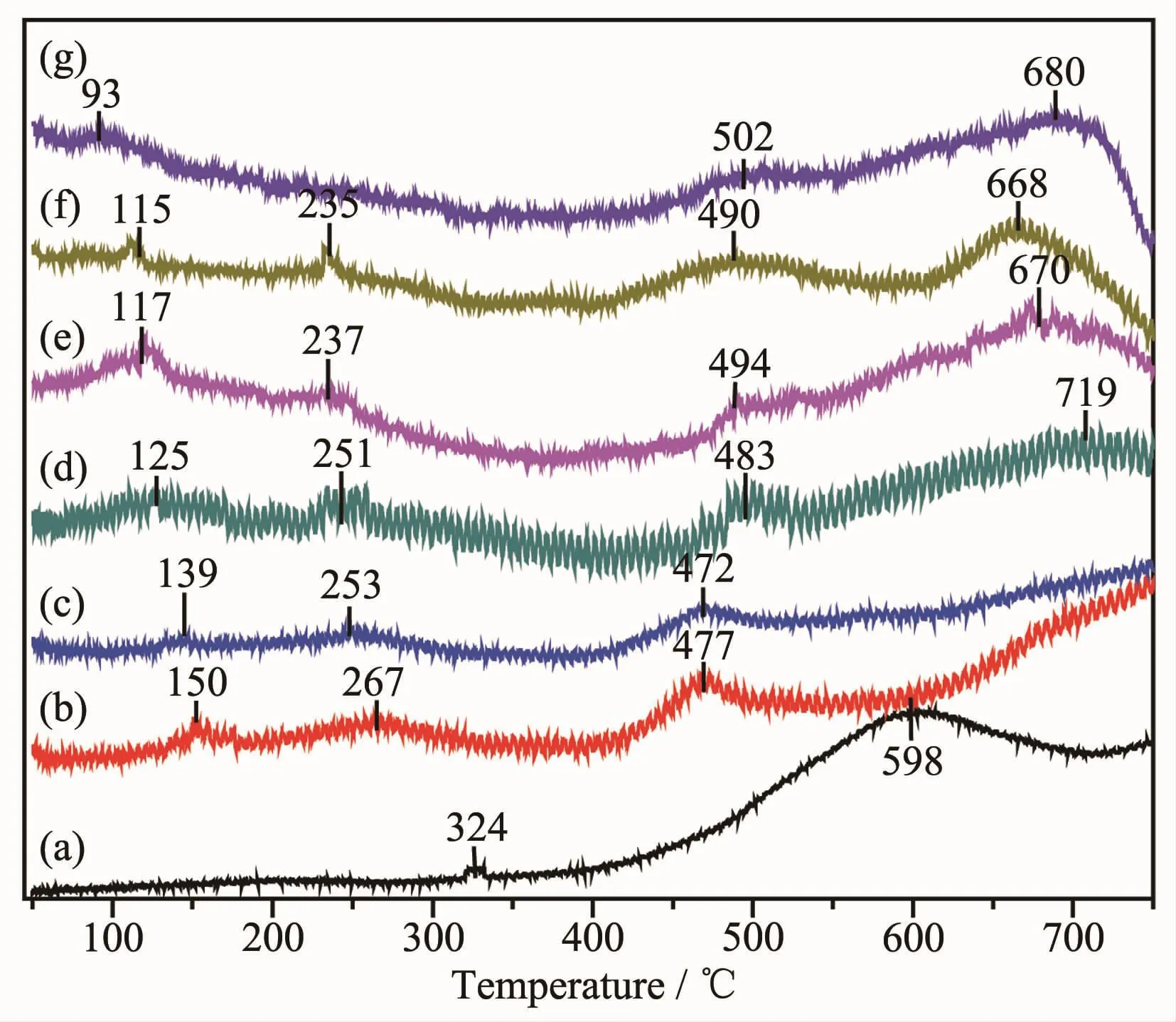
Fig.4 H2-TPR profiles of Au@Pt catalysts
For bimetallic Au@Pt catalyst,the reduction peak between 115 and 150℃is attributed to the reduction of PtOx,and the reduction peak between 235 and 275℃belongs to the reduction of AuxOy.In the high temperature (477~490 ℃),the reduction peak was basically similar to metal Pt.Because the amount of metal oxides in the catalyst account for a small part,which is in line with the results of XPS,various reduction peaks were weak.In addition,the reduction peaks in the low-temperature region gradually shifted to lower temperatures as the Pt content increased,showing Au and Pt mutually exerts positive influence on their respective reductions.In particular,both Au1@Pt2and Au1@Pt3reduction peaks were slightly lower than other bimetallic catalysts,indicating the surface oxygen species has a faster migration rate and oxygen vacancies are easier to generate.The position of the reduction peak of the bimetallic Au@Pt catalyst depends on the degree of interaction between Au and Pt.These results demonstrate that there is a strong interaction between Au,Pt and support[29],which improves catalytic activity.
The Pt4f and Au4f orbital XPS spectra of Au@Pt catalysts are shown in Fig.5.Because the Al2p peak overlapped with the Pt4f peak in a range of 68~80 eV,it is necessary to separate the Al2p peak from the spectra.For Pt/Al2O3,when the Al2p was used at 73.9 eV,the Pt4f7/2spectra can be deconvoluted into two peaks at 70.1 and 71.0 eV,and the Pt4f5/2spectra can be also divided into two peaks at 73.4 and 74.3 eV,corresponding to Pt0and Pt2+[30],respectively.In bimetallic system,the Al2p needed to be separated from Pt4f.The Pt4f and Au4f fine spectra of bimetallic Au@Pt can be fitted into two sets of peaks,corresponding to the 4f7/2and 4f5/2orbitals of Pt0and Pt2+,Au0and Auδ+,respectively.The binding energy of Au shifted slightly to lower value (Fig.5B),and the binding energy of Pt increased (Fig.5A)compared to pure Au and Pt with the increase of Pt content,which demonstrates that electronic structure has significantly changed in bimetallic Au@Pt catalysts,and the interand intra-atomic charges transfers between Au and Pt[31].In addition,the difference in electronegativity of Au and Pt(2.54 and 2.28)may imply potential electronwithdrawing effect from Au to neighboring Pt[32],which suggests that the oxidation state of Pt shell is affected by the Au core.Table 2 shows the binding energy and relative content of Au4f and Pt4f for bimetallic Au@Pt catalysts,and it can be seen that the Au0and Pt0contents occupy the majority of the catalyst,indicating that Au0and Pt0are the main active species on the catalyst surface.According to the XPS data of Au1@Pt2after the reaction,the Au0/Auδ+and Pt0/Pt2+proportion were slightly lower than that before the reaction,indicating that the number of main active centers with Au0and Pt0decrease asthe reaction proceeds.However,the range of variation is small,which indicates that the catalyst has good stability and conforms to the characterization results of XRD and TEM.So the modified electronic structure and active species would improve their catalytic properties.
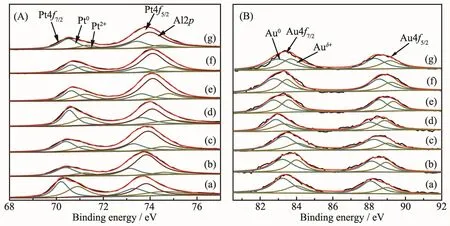
Fig.5 Pt4f(A)and Au4f(B)XPS spectra of prepared catalysts
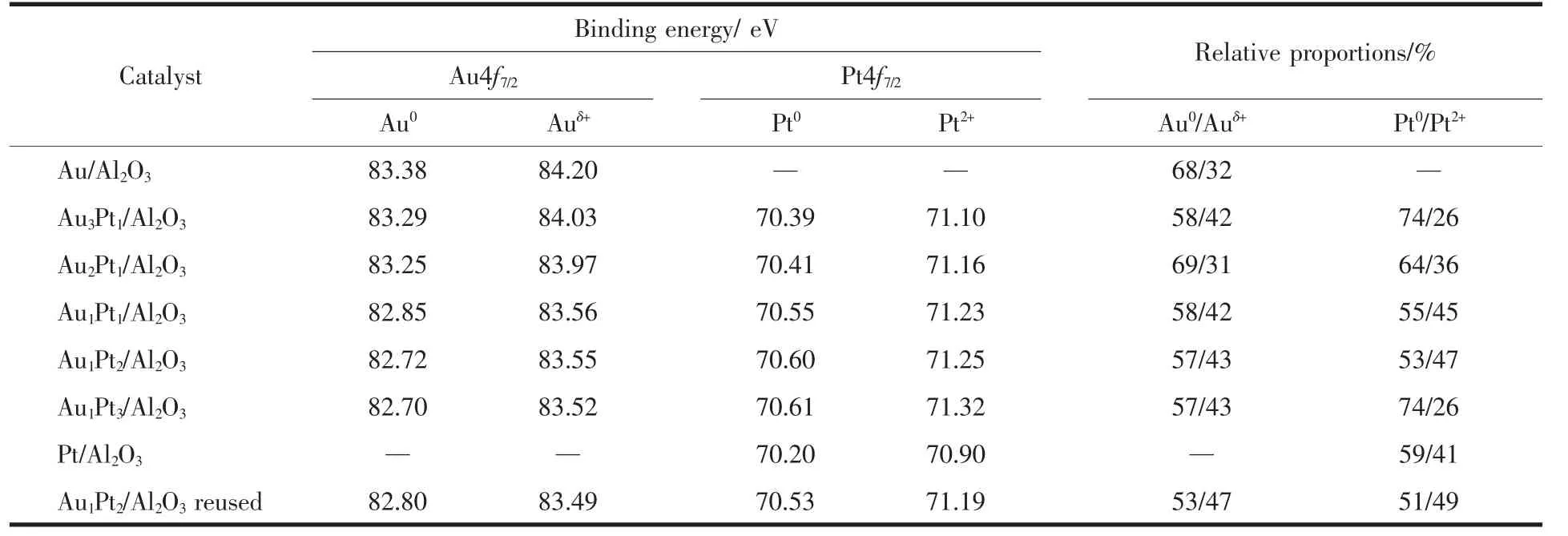
Table 2 Binding energies and proportion of Au4f and Pt4f of prepared catalyst
2.4 Catalytic activity of Au@Pt catalysts for toluene oxidation
Fig.6 shows catalytic oxidation of toluene by Au@Pt catalysts prepared with different molar ratios of Au and Pt.In the reaction,the products were only CO2and water,and CO and other organic small molecules were not detected.The catalytic activity for 98%oxidation of toluene were given in the order of Au1Pt2≥Au1Pt3>Au1Pt1>Au2Pt1>Au3Pt1>Pt>Au.And the T98corresponding to the above-mentioned sequential catalyst is 195,195,210,215,230,230 and 270℃,respectively.However,the Au1Pt2catalyst exhibited excellent catalytic activity,which might be due to that it has smaller particle size and better dispersibility.And it also illustrates that the coexistence of Au and Pt in the bimetallic Au1Pt2catalyst has higher activity for toluene oxidation compared with monometallic Au or Pt.
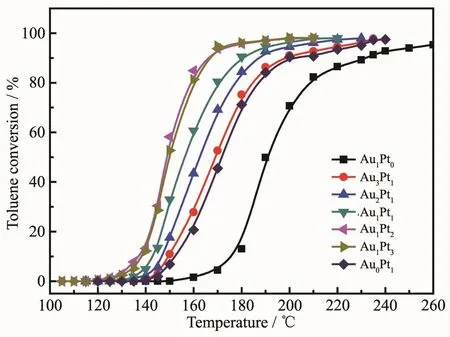
Fig.6 Catalytic oxidation of toluene with by Au@Pt catalysts prepared with different molar ratios of Au and Pt
2.5 Catalyst stability test
To examine the stability of Au,Pt and Au1@Pt2catalysts and maintain the reaction temperature at their respective T98under GHSV at 18 L·g-1·h-1with 1×10-3(V/V)toluene,the catalysts were taken at regular intervals and analyzed on-line.Fig.7 shows the relationship between toluene conversion,selectivity of carbon dioxide and reaction time.It could be seen that the removal rate of toluene is over 98%and the selectivity of CO2is 100%within 0~50 h,which indicates the catalysts have high stability and selectivity.
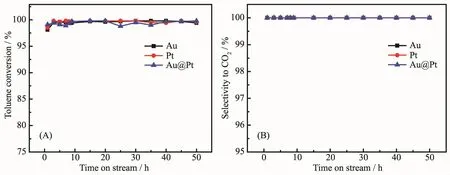
Fig.7 Stability test of toluene oxidation(A)and selectivity to CO2(B)with time-on-stream over Au/Al2O3,Pt/Al2O3,Au1@Pt2/Al2O3catalysts
In order to examine the effect of water vapor over the Au1@Pt2/Al2O3catalytic activity,we performed toluene oxidation in the presence of water vapor(volume fraction:5.0%)over the Au1@Pt2/Al2O3and the result is shown in Fig.8.The results showed that the addition of water vapor decreased the toluene conversion by 9%.When the water vapor was cut off,the toluene conversion rate gradually increased.This phenomenon indicates that there is strong competitive adsorption on the surface of the catalyst in the three components of water vapor,toluene and oxygen[33].
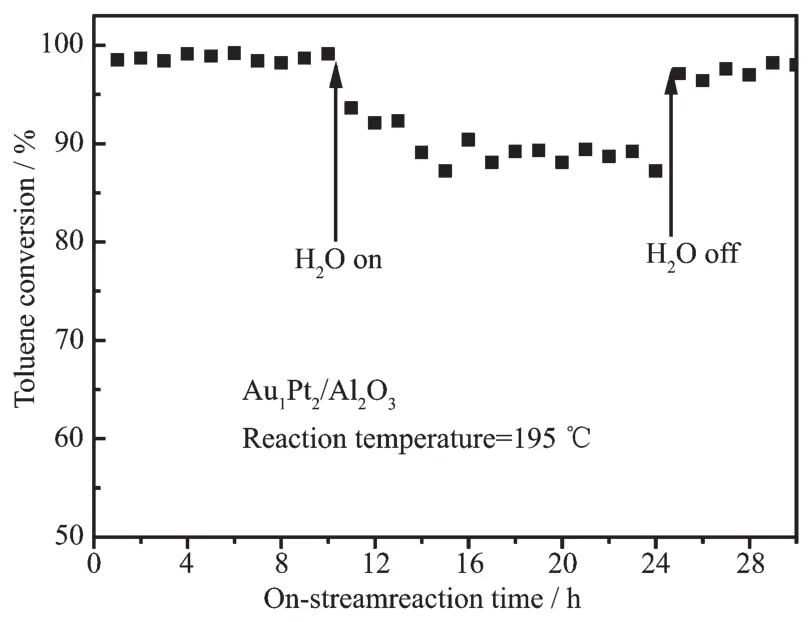
Fig.8 Effect of water vapor on toluene conversion overAu1Pt2/Al2O3catalyst
3 Conclusions
In summary,the bimetallic Au@Pt core-shell nanoparticles prepared by two-step reduction method have been loaded on the surface of Al2O3in a highly dispersed state.The Au@Ptcore-shellstructure prepared by this method is not only simple but also controllable,which showed higher activity than monometallic Pt or Au catalyst for the oxidation of toluene.The catalytic activity for toluene was given in the order of Au1Pt2≥Au1Pt3>Au1Pt1>Au2Pt1>Au3Pt1>Pt>Au.Among the catalysts,the Au1@Pt2/Al2O3exhibited higher catalytic activity (T98=195℃)and better stability.These results suggest that the enhancement effect of Au may arise from the electron transfer from Au to Pt,thereby increasing the reactive oxygen species on the surface of Pt.The core-shell structure may find potentialapplications in the catalytic combustion of VOCs.
Supporting information is available at http://www.wjhxxb.cn

