Anticorrosion properties and thin film composite deposition of Zn-SiC-Cr3C2 coating on mild steel
A.A.Ayool ,O.S.I.Fyomi,A.P.I.Popool
a Chemical Engineering Department,Covenant University,P.M.B 1023,Ota,Ogun State,Nigeria
b Chemical,Metallurgical and Materials Engineering Department,Tshwane University of Technology,P.M.B.X680,Pretoria,South Africa
c Mechanical Engineering Department,Covenant University,P.M.B 1023,Ota,Ogun State,Nigeria
Keywords:Zn-SiC-Cr3C2 Electrodeposition Coatings Corrosion resistance Hardness
A B S T R A C T This work considered the in fluence of Cr3C2 particle loading on microstructure and mechanical properties of Zn-SiC-Cr3C2 nanocomposite produced via electrocodeposition are investigated.The surface nature of the nanocomposite coatings were characterized using scanning electron microscope(SEM)coupled with the energy dispersive spectrometer(EDS).Abrasive wear behaviour and hardness property of Zn-SiC-Cr3C2 nanocomposite produced were investigated using CERT UMT-2 multi-functional tribological tester and Dura Scan hardness tester.The corrosion property was evaluated through linear polarization approach.The result showed that the coatings exhibited good stability and Cr3C2 nanocomposite loading significantly improved the microstructural performance,hardness property,wear resistance as well as corrosion resistance of the coatings.
1.Introduction
Studies on nanocomposite coatings production through the electrolytic co-deposition with metal from plating baths has been carried out extensively[1-3].
Varied distinctive properties of nanocomposite materials include self-lubricity,ability to resist high temperature oxidation,high corrosion and wear resistance[2].In general,the choice of electrodeposition coating(as a good method of producing nanocomposite)is based on the low temperature of operation,good thickness ratio,excellent bonding characteristics,high rate of deposition and low operating cost.Investigations show that excellent composite coatings can be obtained from the codeposition of composite and ceramics materials(such as ZnO and Cr3C2particulates)with some metals or metallic alloys[4-7].And these coating materials affect the corrosion behaviour,physical and mechanical properties of the coated materials[8,9].Composite materials and alloys(e.g.Zn-SiC,Ni-Cr and Ni-SiC)are synthesized for surface treatment of certain materials due to their excellent interfacial properties and environmental friendliness[7-10].Improved corrosion resistance and hardness behaviour were experienced when pure zinc was coated with zinc coatings of SiC incorporated[10].Several studies on the use of Cr3C2particulate in surface engineering have been carried out by researchers in the past;however the use of the nanosized Cr3C2as composite reinforcement has not been adequately investigated.Hence,this study will consider the preparation of SiC-Cr3C2nanoparticles in Zn matrix,the electrodeposition of the coating materials through a simple bath,the in fluence of Cr3C2particulates loading on the corrosion behaviour,structural modification and mechanical properties of Zn Matrix/SiC-Cr3C2nanocomposite coating.
2.Methodology
2.1.Electrodeposition
The electrodeposition of Zn-SiC-Cr3C2nanocomposites was achieved using an electroplating cell having two zinc electrodes,as described by Refs.[6]and[10](Fig.1).The mild steel cathode is of the dimension 45×45×25 mm.And zinc sheet anode has 90×50×10 mm dimension.The elemental analysis of the mild steel sample used gives the composition shown in Table 1(with tolerance limit of±0.0001).Pure zinc,with 97.7%purity,was used as anode.Samples(mild steel)were given surface treatment,following established procedures[6,10].
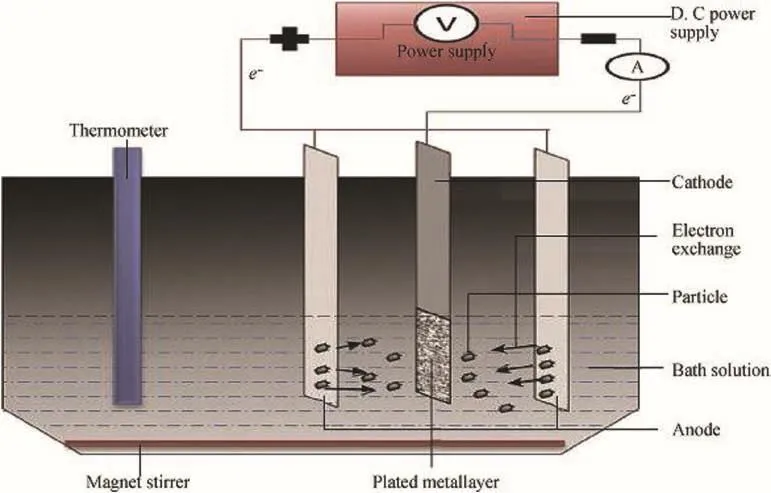
Fig.1.Experimental set up of the electrodeposition process([6,10]).
The activation of the prepared mild steel samples was attained by inserting it into 10%0.5 M HCl solution for some period of time and then rinsed with deionized water.Chemicals used for bath preparation were technical grade.The constituents of the bath considered during coating are 75 g/L ZnSO2,15 g/L NaSO4,10 g/L SiC nanoparticles,5-15 g/L Cr3C2nanoparticles,0.5 g/L Sodium Chloride,5 g/L Thiourea(Table 2).100 nm SiC and 80 nm Cr3C2particle sizes were used in the course of this research.To achieve homogenous bath solution,the bath constituents were continuously stirred at 400 rpm and a constant 40°C heating temperature was maintained.
2.2.Analyses on the electrodeposited samples
Some of the analyses carried out on the Zn-SiC-Cr3C2samples are microstructural analysis,abrasive wear test,hardness test and corrosion test.The microstructures of the nanocomposite coatings obtained were evaluated using scanning electron microscope coupled with energy dispersive spectroscopy(JEOL FIELD EMMISSION-7600F SEM/EDS).Diamond pyramid indenter EMCO Test Dura-scan 10 micro-hardness tester was used to investigate the micro-hardness behaviour of the samples(before and after heattreatment).Previous works of[6-9]elaborate the standard procedures adopted during these analyses.
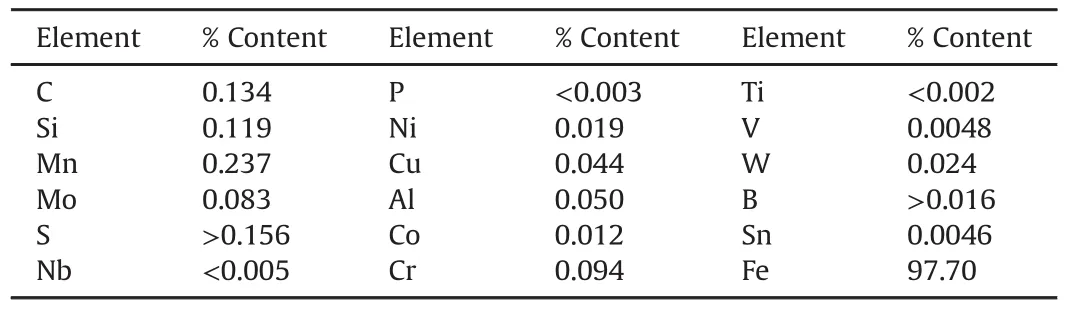
Table 1 Elemental components of the mild steel.
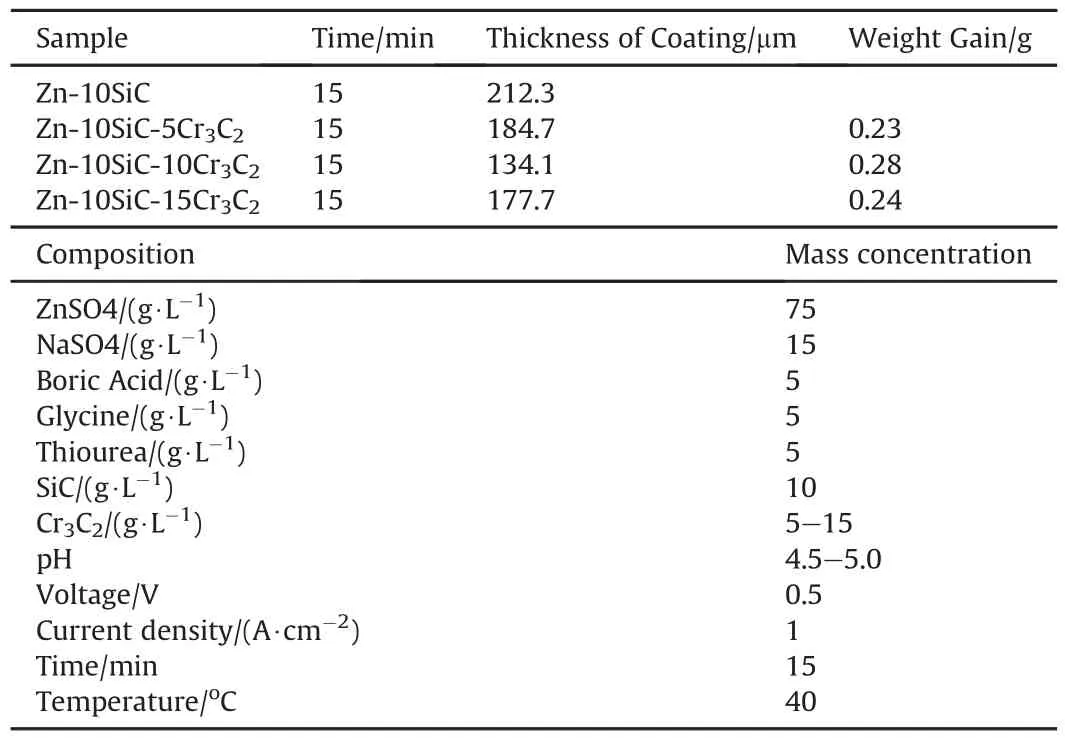
Table 2 Formulation of Zn-Matrix compositions and designed bath composition for Zn-SiCCr3C2 nano-composite.
The wear rate of the composite coatings was analysed using CERT UMT-2 multi-functional tribological tester(at 25°C).The reciprocating sliding tests involved the application of 5 N and 10 N load(separately)at 5 mm/s speed.A 4 mm Si3N ball was used for the examination of wear behaviour of the coated samples.After wear test,the likely structural deformation of the composite coatings was further studied through the use of high optic Nikon Optical microscope(OPM).
Corrosion resistance of the samples was investigated through the use of Autolab PGSTAT 101 Metrohm Potentiostat connected to an electrical cell consisting of three electrodes(silver electrode was used as the reference electrode)and 3.5%NaCl solution at 25°C.The potentiodynamic potential scan was fixed from-2.5 V to+0.5 V with scan rate of 0.012 V/s.
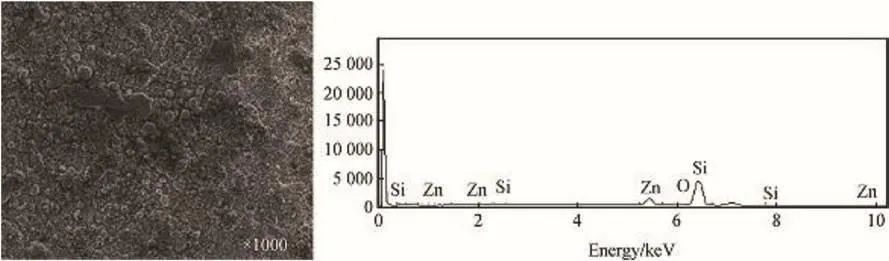
Fig.2.Surface morphology of Zn-10SiC coated mild steel using SEM/EDS.
3.Results and discussion
3.1.SEM/EDS analysis of the deposited composites
SEM/EDS analysis in Fig.2 shows the surface structure of Zn-10SiC coated mild steel.The figure showed that there is no presence of Cr3C2in Zn-10SiC matrix.Considering Fig.3,EDS diagram clearly showed the presence of Chromium peaks for Zn-10SiC-15Cr3C2nanocomposites matrix.SEM analysis of the microstructures of the two samples revealed uniform grains distribution for both.That is the coating was very good in both cases.However,the micro-crystallites grains were finer and better linked in Fig.3,compared to the pattern observed in Fig.2.It is therefore reasonable to say that the change in the microstructure was due to the loading of the Cr3C2nanoparticles leading to improved orientation thereby strengthen the composite in the zinc metal matrix[9-11].The improved lustrous surface of Zn-10SnO2-15Cr3C2made the coating composite to adhere firmly to the surface of the mild steel[10-12].
3.2.Hardness property of the samples
The hardness test shown in Fig.4 was carried out to determine the hardness performance of Zn-SiC sample and all the Zn-SiCCr3C2nanocomposite coatings.As the concentration of Cr3C2loading increase,the hardness of the samples also increased.That is,Zn-10SiC sample(with no Cr3C2content)had the least hardness behaviour and the sample matrix Zn-10SiC-15Cr3C2(with highest Cr3C2content)showed the highest hardness property.
The hardness value obtained for Zn-SiC sample was 82.0μHV,while the hardness values of 264.5 and 280.5μHV were obtained for Zn-10SiC-15Cr3C2sample before and after heat treatment respectively.

Fig.4.Hardness property of Zn-10SiC-15Cr3C2 coatings before and after heat treatment.
Also,the figure shows that the hardness property of the samples increased when subjected to heat treatment.Increase in hardness due to heat treatment,as observed,could be attributed mainly to the increased strain energy in the periphery of the particles in the matrix thereby making the sample to be more compact[12-14].Also,one can inferred that the coatings were very good,this is because the micro-hardness values increase tremendously in their values after thermal treatment[12-14].These results indicate excellent dispersion of grains of Cr3C2on the surface of Zn-SiC.Thus,the microstructural enhancement as a result of agglomerate of fine particulate of Cr3C2enhances the hardness of the coating[14].
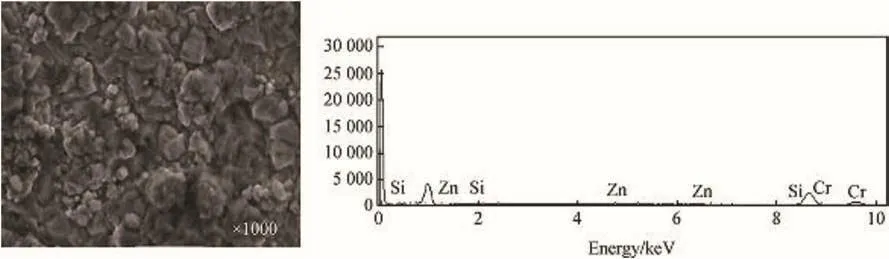
Fig.3.Surface morphology of Zn-10SiC-15Cr3C2 coating using SEM/EDS.

Fig.5.Optical micrographs of:(a)Zn-10SiC-5Cr3C2,(b)Zn-10SiC-10Cr3C2,(c)Zn-10SiC-15Cr3C2,after heat treatment.
To further evaluate the microstructure of the Zn-SiC-Cr3C2samples after heat treatment,optical microscope was used for the analysis(Fig.5).The examination clearly showed that as the concentration of Cr3C2loading increases in the formulations,the morphological arrangement of the coated materials becomes more finely and uniformly,and this implies that the addition of Cr3C2results in improvement in the grain refining.Also,the heat treatment aids the stabilisation of the material matrix and the firmness of the deposited sample[15-25].
3.3.Abrasive wear analysis

Fig.6.Wear rate of the deposited samples.
Fig.6 shows the wear rate of all matrix composite and Zn-10SiC mild steel substrate,when subjected to the application of 5 N and 10 N loads separately.All the coated samples demonstrated significant improvement in wear resistance,compared to Zn-10SiC mild steel sample(with no Cr3C2loading).Also,the results show that as the concentration of Cr3C2loading increases,the rate at which the composite coating experienced wear decreases.For 5 N load application system,the wear rates were 0.05 g/min,0.021 g/min,0.009 g/min and 0.006 g/min for Zn-10SiC,Zn-10SiC-5Cr3C2,Zn-10SiC-10Cr3C2and Zn-10SiC-15Cr3C2matrix respectively.
That is,increase in coating concentration produces better antiwear activities by forming a more stable compound between Cr3C2and the steel[23-26].
Comparatively,Fig.5 also showed that the application of 10 N load on the coated Zn-10SiC mild steel produced higher rate of wear loss of the coated materials,when compared to the results obtained with the application of 5 N.For instance,Zn-10SiC-10Cr3C2had a wear loss of 0.009 g/min when 5 N load was exerted on the coated mild steel while the wear loss value was 0.014 g/min when a load of 10 N was exerted.As the force exerted increased,the intermolecular bonds got distorted.This resulted into slight cracks which eventually graduated into gradual wearing away of the coating materials on the mild steel[14].
3.4.Corrosion test
Table 3 and Fig.7 show corrosion resistance behaviours of the matrix Zn-SiC-Cr3C2composite coating.The results of these corrosion properties favours increasing loading of Cr3C2as corrosion inhibitor.For instance,the potential,current density,corrosion rate and polarization resistance changes from-1.4381 V,0.0104 A/cm2,1.10000 mm/year and 272.50Ωcm2respectively for Zn-SiC sample to-1.0075 V,0.000105 A/cm2,0.01851 mm/year and 562.96Ωcm2respectively for Zn-10SiC-15Cr3C2sample.Hence,Cr3C2nanoparticles in the coating enhance the corrosion resistance of the steel.That is,Zn-10SiC-15Cr3C2coated sample showed the most preferred potentiodynamic polarization data(increased potential,reduced current density,reduced corrosion rate and increased polarization resistance)compared to any other coated materials or uncoated Zn-10SiC.The gradual improvement in the corrosion resistance of the coated material could be attributed to the increased concentration of the Cr3C2coating which inhibits the corrosion process.And this showed that the coated samples had improved corrosion resistance when compared to Zn-SiC sample.Hence,the increasing order of corrosion resistance of the coated samples are Zn-10SiC-5Cr3C2,follow by Zn-10SiC-10Cr3C2then Zn-10SiC-15Cr3C2.
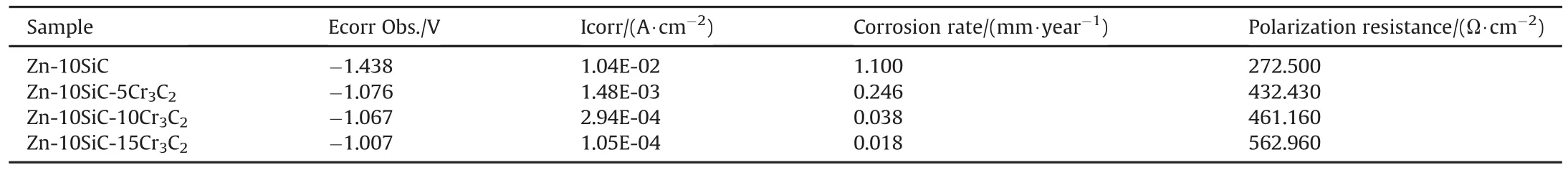
Table 3 Linear potentiodynamic polarization data for matrix Zn-SiC-Cr3C2 composite.
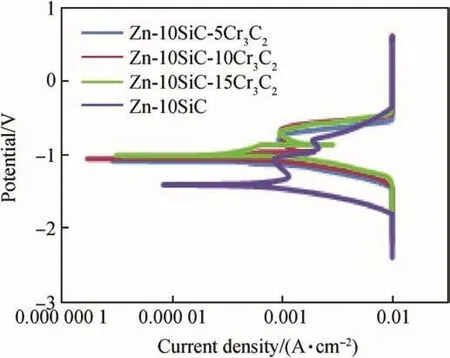
Fig.7.Polarization curves of Zn-10SiC and Zn-10SiC-Cr3C2 coated samples.
4.Conclusion
1.Cr3C2nanoparticle was used in the production of Zn-10SiCCr3C2composite coating from sulphate bath.
2.SEM/EDS analysis established the incorporation of Cr3C2in the coating
3.The hardness property of the substrate was enhanced by the incorporation of the SiC/Cr3C2nano composite particles in the zinc matrix.
4.Increase in the concentration of Cr3C2composite loading resulted into increase in the micro-hardness property,wear resistance and corrosion resistance of the samples.
Acknowledgement
National Research Foundation,Surface Engineering Research Centre(SERC),and Tshwane University of Technology,Pretoria,South Africa were acknowledge for their support.The authors would also like to thank Covenant University Centre for Research Innovation and Discovery(CUCRID)Ota,Nigeria for the provision of financial support towards the publication of this work.
- Defence Technology的其它文章
- Fluorine-containing oxidizers for metal fuels in energetic formulations
- On the formation of Basu's Type III(peeled orange)gunshot residues
- Interaction of TATB with Cu and Cu+1.A DFT study
- Control of exterior ballistic properties of spin-stabilized bullet by optimizing internal mass distribution
- Crystal lattice free volume and thermal decomposition of nitramines
- Tribological and vibrational characteristics of AISI 316L tested at elevated temperature and 600 Torr vacuum

