On the formation of Basu's Type III(peeled orange)gunshot residues
Felice Nunziata ,Matteo Donghi
a Professional Member of the Chartered Society of Forensic Science,Naples,Italy
b Arma dei Carabinieri,Reparto Investigazioni Scientifiche,Parma,Italy
Keywords:Peeled orange particles GSR CDR Charged particles Electrostatic attraction Rayleigh droplet stability
A B S T R A C T In a famous paper published in 1982,a very special class of gunshot residue particles(GSR)was named by Samarendra Basu “peeled orange”,due to their particular structure,consisting of a barium/antimony core covered by an outer lead lea flet.In this class of GSR particles the surface may show nodular structures of lead.Basu proposed an explanation in terms of a nucleus of antimony and barium that captures lead vapours produced after the explosion of a cartridge into a firearm:as solidification points of antimony and barium are close one another,both higher than solidification point of lead,he stated that lead occurs as a layer around the core in peeled orange GSR particles.In this paper we study the thermodynamic of the barium/antimony alloy and we hypothesize a formation process in terms of colloidal metal growth,charged particles and electrostatic attraction.We propose an updated model of formation for peeled orange GSR particles that explains the existence of outer lead lea flet and nodules in terms of electrostatic attraction of lead nanoparticles and instability of lead droplets.
1.Introduction
In criminalistics,the possibility of scientifically verifying the connection between a suspect and a discharged firearm has always been,for investigators,one of the most sought after target[1].
The study of gunshot residues(GSR)formation processes[2]is very important for recognition and classification of particles found on the collected samples.The case of peeled orange particles is the most particular among all types of GSR.
According to a definition firstly proposed in 1982 by Samarendra Basu[3],Peeled orange or Category III or Type III GSR particles,are a minority group(5%-6%of the total),their core usually contains a uniform distribution of antimony and barium and lead occurs around the core as a ring-like layer.These particles,on their surface,may show nodular lead structures.This latter case,a barium/antimony core covered with lead nodules,is of particular interest:we could define these particular particles as “strawberry”like GSR,rather than “peeled orange”ones.In these particles,often recurrent especially in cartridge cases,lead nodules raise directly from the BaSb core,as in the two attached SEM photos.In this case no external lead layer-that should be few microns thick according to Basu-is present,as can be seen in the backscattered electron images(see Figs.1 and 2),where white nodules(Pb)emerge on a gray background(BaSb).It is common experience to find such particles:the following images and spectra are related to a judicial case of our day.
In this paper we study the thermodynamic of barium/antimony alloy and we hypothesize the formation process of these kind of GSR in terms of metal colloidal growth,charged particles and electrostatic attraction.
2.Theory/calculation
Observing typical Type III GSR particles,we noted that super ficial nodules are very regular and don't appear like splattering particles in relative collisional motion with the core:they don't show the typical impact shape with non continue filaments.Splashed liquid droplet,solidifying after an impact onto a rigid substrate,are expected to show patterns of symmetry breakdown both in radial and azimuthal direction.Moreover,because of the high-velocity of impact between liquid droplets and rigid curved surface,the impact effect should also consist in a relevant loss of mass of the molten particles.On the contrary,in Type III GSR particles the interface between nodules and surface is regular and nodules seem to be inserted into the surface.

Fig.1.Typical“strawberry”like GSR particle.Spectrum shows nodules consisting of lead alone.
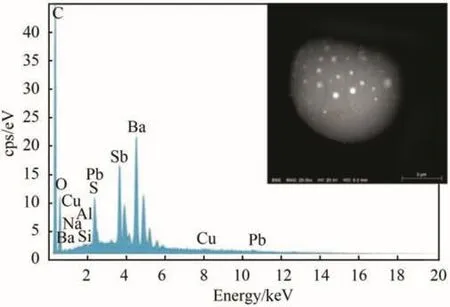
Fig.2.Typical“strawberry”like GSR particle.Spectrum shows the core consisting of barium,antimony(and sulphur,the latter deriving from antimony sulphide used as fuel in the primer mixtures).
In a collisional model,an extra pressure inside each colliding droplet is expected.Such pressure is generated,during the impact,by conversion of the momentum of the impacting droplet into the momentum of flow along the impact surface:when inner pressure exceeds surface tension,splashing occurs.Liquid along the contact area is compressed and the extra pressure P propagates into the liquid as a shock wave at the speed of sound(water hammer effect).When the front of the shock wave pressure reaches the free surface of the liquid droplet,splashing manifests.According to Allievi's equation,extra pressure P is expressed as
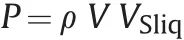
whereρis the liquid density,V is the droplet impact velocity,and VSliqis the speed of sound in the liquid.The reason for droplet spherical shape after impact might be explained by conservative surface tension of the solidifying liquid.We believe that circumstance is not sufficient to explain regular morphology of the spheroids presenton core's surface:in fact,being collisions random phenomena,nodules should be irregular,with presence of filaments.
A possible explanation could be suggested by the experimental evidence that triboelectric and combustion processes,after discharging a firearm,impart electrostatic charge on projectiles,smoke,unburned propellant and other debris[4-7].
In agreement with Basu,we can consider the core of barium and antimony already solidified when lead is still in vapour state.From Lichtestein et al.[8],we note that the eutectic point of the barium/antimony alloy is 555°C,higher than the solidification point of pure lead,i.e.327°C.We believe that eutectic point value for the alloy is here more important than pure element solidification points themselves(antimony 630.5°C and barium 725°C)as previously proposed by Basu.In the solidification process of the core,it's very difficult that one element may solidify before the other.Heat transfer from an elemental aggregate to another is such relevant that the forming two-element(barium/antimony)nucleus has to be considered an eutectic alloy.Hence,according to Basu's prevision,the core should aggregate and solidify before lead coating.
Because lead is distributed like an outer lea flet around the core,then it must to be attracted when it is still in vapour state.The suggested attraction mode is the electrostatic one.
In a first stage,nanoscale lead particles are instable and tend to agglomerate because,at short interparticle distances,they are attracted each other by van der Waals electrostatic or magnetic forces.The general principle of growth mechanism is due to coalescence:interaction potential between two identical spherical particles depends on electrostatic stabilization,according to the Guy-Chapman model.This model consists in an inner Stern compact layer and an outer diffusive layer,corresponding to a decrease in counter-ion and co-ion concentration with respect to the distance from the particle surface.During its formation,the double layer around the nanoparticle consists in a spherical distribution of electric charges,mutually opposite in sign(see Fig.3).
In a second stage,these lead particles in formation are attracted by nucleus surface:electrostatic interaction between lead particles and core surface,using a linear superposition approximation[9],is
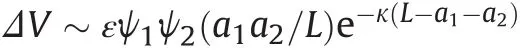
where ε is the dielectric constant of the medium,Ψ1and Ψ2are the surface potentials of the interacting particles,a1and a2are the radii of the interacting particles,L=a1+a2+h is the distance between particle centers and 1/κis the characteristic Debye-Hückel length.
A further stage consists in a colloidal assembly,corresponding to the rearrangement of particles originating from attractive capillary forces.
It has been suggested[10]that free energy of capillary interaction can be expressed by
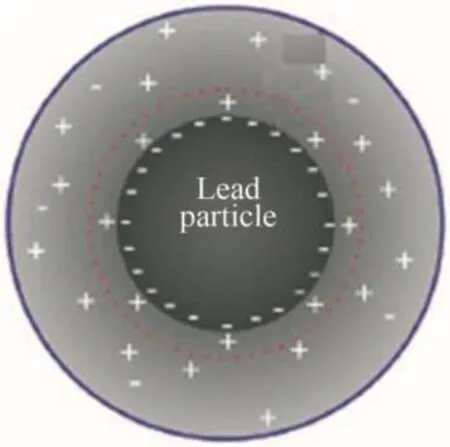
Fig.3.Spherical distribution of electric charges,mutually opposite in sign,in lead nanoparticle in formation.
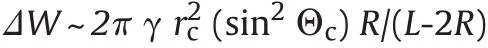
where(see Fig.4)γis surface tension,rc=[h(2R-h)]1/2is the radius of the contact line at the surface of the particle,Θc=arcsin(rc/R)-α is the mean slope angle of the meniscus(of the semiliquid front on nucleus's surface constituted by previous impacts)at the contact line,L is the distance between the particles,andαis the contact angle.
It's plausible that the combination between electrostatic and capillary forces promotes the covering of barium/antimony nucleus in the form of an outer lea flet.At this point,the outer lea flet increase its thickness.Moreover,part of lead vapours can aggregate into droplets.
The proposed hypotheses explains both “peeled orange”and“strawberry”GSR particles:in the first case lead aggregates as a crust on the Ba/Sb core,in the second only lead nodules are present on Ba/Sb core.
Considering that particles in formation are electrostatically charged,hereinafter we focalize our attention on the stability criteria of a conducting droplet.We assume a conducting liquid mass that differs from a sphere;the spheroid equation is
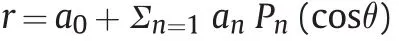
where r is the distance from the origin to the surface of the spheroid,an≪a0for all n,Pnis the Legendre Polynomial of order n,andθis the angle between x axis and radial direction.
In terms of Legendre Polynomial,we write
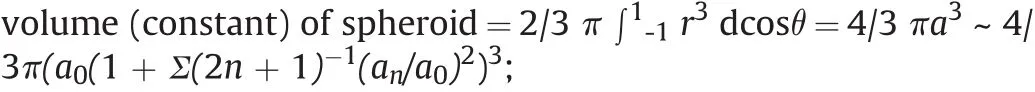
-surface area of spheroid= ∫02π ∫0π (r2sinθ/cosν)dθ dΦ=4πa2+2πΣ(n-1)(n-2)(2n+1)-1,whereθandΦare the spherical coordinate variables andνis the angle between the normal to surface and a radial line from origin through the surface point;
-potential energy of capillarity (PEC)=2πγΣ(n-1) (n-2)(2n+1)-1,whereγis surface tension(relative to the sphere of equilibrium);
-electrostatic potential energy(EPE)=½ QΞ=-Σ(n-1)(Q2/2a2)(2n+1)-1(relative to the sphere of equilibrium),whereΞis electrostatic potential on the surface;
-kinetic energy of the motion of the liquid(KIN)=2πa3ρΣn--1(2n+1)-1,whereare generalized coordinates for spheroid.
The Lagrangian function is L=KIN-PEC-EPE.
Lagrange equation of motion for anis

inasmuch in our model the term K=0,so it reduces to
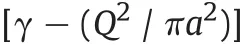
namely the comparison between coulombian and tensive surface contribution.
Note that electrostatic potential energy is opposed to surface tension;stability of the droplet is determined by two competing factors:surface tension,that tends to stabilize a droplet in a spherical shape,and electrostatic repulsion between charges,that tends to increase the surface where charges are distributed.
Finally,if we consider
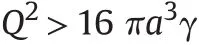
or in pure Rayleigh notation

where κ=1/(4πε0)=8987·10-9Nm2C-2.In this case the droplet is unstable for all values of n below a certain limit and it may break into stable droplets of smaller radii or it may eject a jet of very small droplets which carry away enough charge and mass so that the remaining droplet is stable again.
3.Results and discussion
If we consider a generic lead droplet with radius a=1μm,γ=0,545 N/m the surface tension of molten lead[11],ε0=8,85 10-12C2/Nm2,then Rayleigh limit of droplet fissility is Q~10-14C.However if consider a=0.1μm then Rayleigh limit of droplet fissility is Q~10-6C.
But in cited literature on tribocharged shot debris,charge orders of magnitude are Q~10-8C to 10-12C,so we have to hypothesize that tribocharging contributes to instability of liquid lead droplets.
This means that lead particles are formed by nucleation,then they aggregate and grow until they become unstable,also due to the tribocharge.Hence a lot of lead droplets,with radius in the order of microns,are subject to the effects of instability inducted by triboelectric effects.
4.Conclusion
Basu interpreted the origin of peeled orange GSR particles,consisting of a barium/antimony core covered by an outer lead lea flet,in terms of a nucleus that captures lead vapour produced by the explosion of a cartridge into a firearm.In fact,as solidification points of antimony(630.5°C)and barium(725°C)are very close one another,and both higher than the solidification point of lead(327°C),he stated that lead occurs as a layer around the barium/antimony core.We believe that,in the Ba/Sb alloy,it is very difficult that one element may solidify before the other.Heat transfer from an elemental aggregate to another is such relevant that the forming two-element nucleus has to be considered an eutectic alloy.We propose an updated model for the formation of peeled orange GSR particles,which explains the presence of the outer lead lea flet and nodules in terms of electrostatic attraction of lead nanoparticles and instability of lead droplets.Finally,we propose a mechanism of formation for the specific,often recurrent especially inside cartridge cases,sub-category of Type III GSR particles we indicate as“strawberry”like GSR,hypothesizing that the presence of Pb nodules on the surface of a Ba/Sb core is due to electrostatic attraction of lead nanoparticles and instability of lead droplets.

Fig.4.Geometric scheme of the particle surface used for calculation.
- Defence Technology的其它文章
- Fluorine-containing oxidizers for metal fuels in energetic formulations
- Interaction of TATB with Cu and Cu+1.A DFT study
- Control of exterior ballistic properties of spin-stabilized bullet by optimizing internal mass distribution
- Crystal lattice free volume and thermal decomposition of nitramines
- Tribological and vibrational characteristics of AISI 316L tested at elevated temperature and 600 Torr vacuum
- Underwater explosion effects of 60 mm H.E.mortar bomb on a cylindrical concrete structure-PIT

