肌动蛋白细胞骨架在小鼠第二生心区祖细胞部署发育中的作用
刘钟颖,黄霞,李紫怡,杨子豪,袁白银
肌动蛋白细胞骨架在小鼠第二生心区祖细胞部署发育中的作用
刘钟颖,黄霞,李紫怡,杨子豪,袁白银
武汉科技大学生命科学与健康学院,武汉 430065
脊椎动物心管起源于早期胚胎的生心中胚层细胞,随后从邻近的咽中胚层和内脏中胚层中逐渐添加第二生心区(second heart field, SHF)祖细胞而延长。SHF细胞向心管贡献受损,使心管不能最大限度地延长,导致一系列的心脏发育缺陷,包括最常见的右心室发育不良与流出道分隔旋转异常等先天性出生缺陷。SHF祖细胞构成非经典顶端-基底极性上皮,并具有顶部单纤毛、动态肌动蛋白(actin)富集基底端的丝状伪足等特点。本文总结了actin细胞骨架在小鼠SHF祖细胞部署发育过程中的研究进展,揭示actin细胞骨架在SHF祖细胞发育特别是SHF细胞向流出道部署过程中的重要性,以期为阐明和理解SHF祖细胞迁移、部署的细胞生物学特性提供一定的理论参考。
肌动蛋白;第二生心区;细胞部署;小鼠
先天性心脏病是目前人类最常见的出生缺陷,并且一直是引起婴幼儿死亡的首要原因,其中约30%的先天性心脏缺陷为流出道(outflow tract, OFT)发育缺陷[1,2]。OFT和右心室(right ventricle, RV)是由第二生心区(second heart field, SHF)祖细胞发育形成,SHF祖细胞发育缺陷导致OFT和RV发育异常。因此,阐明SHF的发育过程和深入探讨其调控机制对于破译OFT发育缺陷的起源、控制和降低先天性心脏病的发病率、寻找临床相关致病基因对其诊断、防治以及试管婴儿的筛选具有重要意义。
哺乳动物的心脏是由第一生心区(first heart field, FHF)和SHF两种主要的心脏祖细胞发育而来。位于前部侧板中胚层的FHF祖细胞贡献于早期分化的心肌细胞,形成原始心管,将来发育成左心室(left ventricle, LV)、房室通道、极小部分的右心室和心房心肌细胞[3~5]。位于咽中胚层(pharyngeal mesoderm, PM)和内脏中胚层(splanchnic mesoderm, SpM)的SHF祖细胞添加晚分化的心肌细胞于伸展的心管,贡献于OFT、RV及心房心肌细胞[4~6]。SHF祖细胞向心管贡献、部署的缺陷使OFT的长度不足以在心脏间隔形成期间正确的旋转,升主动脉和肺动脉干与左心室和右心室对齐,导致心室分隔和心室/动脉排列缺陷,引起双出口右室(double outlet right ventricle, DORV)、大动脉转位(transposition of the great arteries, TGA)、永存动脉干(persistent truncus arteriosus, PTA)等畸形[6,7]。
研究发现,SHF细胞构成非经典顶端-基底极性上皮,并具有顶部单纤毛、动态肌动蛋白(actin)富集基底端的丝状伪足等特点,这表明actin细胞骨架在SHF发育中具有重要作用[8,9]。本文对哺乳动物SHF的发现及特性、actin细胞骨架的特性、actin与细胞运动、actin与横纹肌肌纤维以及actin与哺乳动物SHF发育等方面进行了阐述,以期为科研人员更深入地解析actin细胞骨架与哺乳动物SHF发育之间的联系提供参考,为阐明和理解SHF细胞迁移、部署的细胞生物学行为及调控网络提供思路和方向,为寻找临床相关致病基因并对先天性心脏病的诊断、防治提供理论基础。
1 小鼠心脏第二生心区
1.1 第一生心区与第二生心区
心脏发育和形成是一个复杂的过程,涉及不同空间、不同细胞群体的整合,并被复杂的级联调控网络调控。心脏是由两种主要的心脏祖细胞发育而来,分别是FHF和SHF祖细胞[5]。研究表明,FHF和SHF祖细胞在心脏新月(cardiac crescent, CC)期之前与共同的心脏祖细胞分离;FHF首先与心脏共同的祖细胞分离出来,并贡献于心脏新月期和早期心管心肌细胞;而SHF是另外一个祖细胞群,它随后将新分化的心肌细胞贡献于生长的心管[4,6,10]。FHF来源的原始心管将来发育为左心室、小部分的右心室、房室管和大部分的心房[3,5]。SHF祖细胞通过心脏动脉极贡献于OFT和右心室,并通过静脉极贡献于心房心肌细胞[4~6]。
在小鼠()胚胎发育的E8.5~E10.5时期,位于心管后部咽中胚层和内脏中胚层的SHF祖细胞向原始心管添加分化的心肌细胞,使心管延伸并向右环化[11]。SHF祖细胞对心管的贡献使心管得以足够的伸展和生长,这是心脏形态发生所必 需的。SHF祖细胞向心管贡献、部署失败导致OFT缩短,使OFT的长度不足以在心脏间隔形成期间 正确的旋转以使升主动脉和肺动脉干与左心室和右心室对齐,导致心室分隔和心室/动脉排列缺 陷,如双出口右室、大动脉转位、永存动脉干及其他异常[6,12,13]。
1.2 第二生心区的发现及性质
20世纪70年代,de la Cruz及其同事利用体内氧化铁颗粒标记法揭示鸡()心脏远端OFT和RV实际是由后来添加的细胞形成,并且心脏的生长是通过添加位于早期心脏之外的细胞而实现[14]。另一项在小鼠中的实验也暗示,除了心脏新月以外,还有另外一种细胞群贡献于发育的心脏[15]。然而,这些早期心脏之外细胞的来源直到2001年才被确定。
2001年发表的3项研究确定了在咽中胚层中的祖细胞群贡献于胚胎期延伸的OFT。Mjaatvedt等[16]确定了由de la Cruz等[14]发现的前部心脏形成区,该前部心脏形成区是由围绕在紧邻现有心管的主动脉囊周围的中胚层组成。此外,Waldo等[17]鉴定出在贡献于OFT期间,位于心包背壁(SpM)中表达和以及的祖细胞。同时,Kelly等[18]构建了受成纤维细胞生长因子10 ()基因调控元件驱动的转基因小鼠,发现在胚胎心脏的右心室和OFT以及相邻的PM和SpM中具有β-半乳糖苷酶活性;在转基因小鼠中使用DiI标记法确定RV和OFT中的心肌细胞是从咽弓核心和SpM中添加。这3项研究揭示了位于PM和SpM中的另外一个祖细胞群,它们在原始心管形成以后贡献于心脏。目前认为心脏SHF是由位于PM和SpM中的祖细胞群共同组成[4,6],研究SHF细胞成为心脏发育领域的焦点。
SHF又可分为两个亚细胞群:前部-SHF (A-SHF)和后部SHF (P-SHF),A-SHF细胞通过动脉极贡献于OFT和右心室,P-SHF在静脉极贡献于心房及心房的分隔[4,6,10]。到目前为止,尚未确定背侧心包壁中A-SHF祖细胞和P-SHF祖细胞之间的界限。利用染料-标记和谱系-追踪实验表明,P-SHF中存在能够同时贡献于OFT和心房心肌细胞的祖细胞群[19,20]。持续增殖和分化延迟是SHF祖细胞区别于FHF祖细胞的两个限定特性,这两个特性使SHF祖细胞能够被逐渐添加到伸展的心管中,并受动态咽信号环境和PM转录程序的调节[21]。FGF10是由Kelly团队鉴定得到的鼠科动物SHF细胞的第一个分子标记物,随后的研究发现SHF祖细胞具有表达、、、和等基因的特征[22~26]。
2 肌动蛋白细胞骨架
2.1 Actin细胞骨架及其解聚和加聚动态
Actin是真核生物中表达量最丰富的骨架蛋白之一[27]。动物细胞在不同环境中具有改变细胞形态的能力,这种能力主要是依赖actin细胞骨架系统[28]。细胞中actin具有两种存在形式,即游离的单体肌动 蛋白(globular actin, G-actin)和丝状肌动蛋白(filamentous actin, F-actin)。G-actin自身能够聚合形成F-actin。F-actin是肌纤维细肌丝系统的核心组分,并被组织成各种不同的结构以参与行使不同的生物学过程,如细胞周质、应力纤维、丝状伪足和板状伪足等[27,28]。
Actin细胞骨架是高度动态的骨架蛋白,在生理条件下F-actin能够持续在一端(正极端)聚合,在另一端(负极端)解聚。在细胞内,被组装成不同结构的F-actin不断进行动态且强烈地重组,使细胞适应环境。因此,actin细胞骨架的动态聚合和解聚在各种生理过程中发挥重要的调控作用[27],如细胞运动[29~32]、细胞分裂和胞质分裂[33,34]、细胞形状调节[35]及转录调控[36]等。在没有调节蛋白存在的生理离子条件下,actin自身能够经历自发的聚合、解聚和成核过程,但是其更新远比在生物体内缓慢的多。因此,为了适应体内F-actin快速更新的需求,需要大量的actin调节蛋白来调控actin成核、断裂、聚合和解聚等过程[37]。细胞内存在60多类G-actin和F-actin结合蛋白,协同调节actin的组装和拆卸[38,39]。例如,作为成核因子的Arp2/3复合体,具有促进分支状F-actin成核和交联F-actin的功能[40,41];WASP 家族(其中最有名的是WASP蛋白(Wiskott-Aldrich syndrome protein)和SCAR/WAVE (suppressor of cyclic AMP receptor/WASP-family verprolin-homologous protein))作为Arp2/3复合体的主要活化因子,能够结合并激活Arp2/3复合体,促进新的F-actin成核[42,43];Formins是另外一类actin成核因子,主要通过稳定actin二聚体,促进非分支F-actin成核和G-actin聚合[44,45];Profilin促进formins介导的actin聚合作用[41,44];ADF/cofilin是actin解聚的主要调控因子,通过促进F-actin切割和负极端G-actin解聚,增强actin的周转[46~48];WDR1/AIP1是ADF/cofilin的主要辅助因子,它能促进与ADF/cofilin结合的F-actin的解聚作用[49,50]。
2.2 Actin细胞骨架在细胞迁移、横纹肌中的调控作用
2.2.1 Actin细胞骨架与细胞迁移
细胞运动作为动物细胞的重要特征,是与器官发育、宿主防御功能以及疾病发展密切相关的基本生命过程[51]。细胞迁移在许多生理和病理过程中发挥重要作用,如在后生动物胚胎发育过程中,原肠胚呈现出广泛的细胞迁移[52],且在器官和腺体形成期间,存在细胞群的协调迁移[53];在正常生理条件下,成纤维细胞、血管内皮细胞等细胞迁移是伤口愈合所必需的[54,55];免疫细胞通过迁移穿过血管和淋巴管并寻找入侵物质[56,57]等。
尽管在不同细胞、组织及研究系统中,细胞迁移会有不同的类型和机制,但它们可被有效的概念化为一个循环过程:首先细胞发生极化,随后在其前部形成突起,建立、稳定细胞-基质粘附作为细胞迁移的牵引位点,最后在其后部细胞脱离和缩回。细胞迁移过程是actin细胞骨架发生重大重塑的极端情况,而actin细胞骨架重塑是调控细胞迁移过程的关键和必需事件[29,58,59]。在细胞向前运动过程中,actin在细胞前端聚合以形成突起,同时这种聚合与后端板状伪足的解聚相匹配,为细胞随后的actin聚合反应提供所需要的再循环G-actin[28,37];另外,肌球蛋白马达拉动F-actin在迁移细胞尾部产生的收缩活性,提供细胞向前移动的动力[29,60]。F-actin作为肌球蛋白Ⅱ马达的支架,是肌动球蛋白收缩活动的先决条件[61]。目前,已有大量研究确定actin细胞骨架的动态特性是细胞运动所必需,使用actin解聚或稳定F-actin的药物处理运动细胞,将会终止细胞运动[62,63]。
2.2.2 横纹肌中的actin细胞骨架
脊椎动物的骨骼肌和心肌是典型的横纹肌,秀丽隐杆线虫()的体壁肌是斜横纹肌[64]。在横纹肌纤维中,基于actin的细肌丝、基于肌球蛋白的粗肌丝和其他相关蛋白被组织成收缩装置的最小重复单元—肌小节(sarcomere),以产生肌肉组织的收缩力[65,66]。肌小节是位于两个相邻Z盘之间的区域,粗肌丝位于肌小节的中部,并具有双极方向的肌球蛋白头部;被原肌球蛋白-肌钙蛋白复合物包裹的细肌丝在每个肌小节单元的末端以相反的方向取向;actin细肌丝的正极端被a-actinin蛋白锚定并交联在Z带上,并被加帽蛋白CapZ所加帽,而被加帽蛋白Tropomodulin加帽的细肌丝负极端则未被锚定在特定的结构上[67,68]。
虽然在迁移的成纤维细胞和肿瘤细胞中,F-actin形成的调控机制已经被广泛研究,但在发育的肌细胞中,肌小节细肌丝的形成及调控过程目前仍不清楚。针对肌纤维的组装,Sanger等提出了前肌原纤维模型[69,70]。在肌肉发育过程中,肌原纤维组装是actin细胞骨架发生的主要形态改变,此外G-actin和F-actin的比率也发生剧烈的变化[71]。在成熟的横纹肌肌小节中,整个actin细肌丝经历了不同速率的周转[72~78]。有趣的是,虽然成熟肌小节细肌丝经历actin解聚和加聚动态,但细肌丝却具有相似的长度[79,80]。横纹肌肌纤维的形成、成熟肌小节细肌丝的结构及动态等特点预示在肌小节组装和功能维持过程中,必然存在一套精确的调控系统来调节actin细肌丝的组装和分解(表1)。大量的研究已经表明,细肌丝正极端和负极端的加帽蛋白CapZ[81,82]和Tropomodulin[76,83~86]能够调节肌小节细肌丝的组织和actin解聚和加聚动态;其他actin相关蛋白如Nubulin[87~90]、Tropomyosin[91,92]、ADF/cofilin[78,93,94]、WDR1[95,96]、Leiomodin[86,97~99]和FHOD3[100]等也参与了肌小节细肌丝的组织和actin动态的调控。作为actin解聚因子ADF/cofilin的辅助因子,WDR1能够促进与ADF/cofilin结合的F-actin的解聚。本课题组的研究表明,分别在胚胎期、幼年期和成年期心肌细胞中敲除,均会导致F-actin堆积、肌纤维的组装和功能维持受损等表型[101,102]。
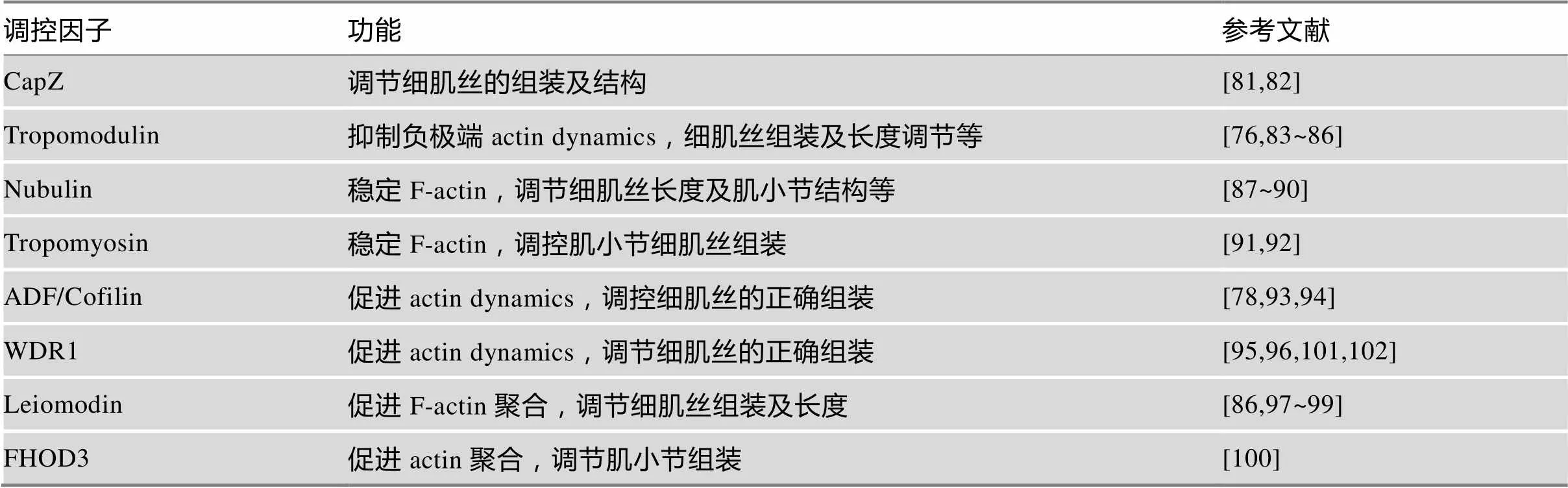
表1 横纹肌中重要的肌动蛋白动态调控因子
在遗传和/或环境因素等影响的病理条件下,肌小节细肌丝会发生改变。先天性肌病是一种遗传性的肌肉病变,其特征是骨骼肌无力,并存在含有actin或其他肌纤维蛋白的棒状物或聚集体[103]。在杆状体肌病(nemaline myopathy)、肌动蛋白肌病和细胞核内杆状体肌病这3种主要的先天性肌病中,均涉及编码ACTIN及相关蛋白的基因突变[68,103],包括cofilin (CFL2)[104,105]、a-actin (ACTA1)[106~110]、a-和b-原肌球蛋白(TPM3和TPM2)[111,112]、肌钙蛋白T (TNNT1)[113~115]和nubulin (NEB)[116~118]等。
3 Actin细胞骨架在第二生心区祖细胞部署发育中的调控作用
近年来,一些调控SHF祖细胞部署发育的转录因子和信号通路相继被报道,这些转录因子及信号通路缺失或改变导致OFT缩短、心脏环化受损等SHF发育的缺陷,并伴随SHF细胞内actin细胞骨架的破坏。这些结果揭示了actin细胞骨架在SHF细胞发育特别是SHF祖细胞向OFT部署过程的重要性(表2)。
3.1 Wnt5a-Dvl-PCP通路与SHF祖细胞actin细胞骨架
2005年,Kirby研究团队发现鸡()中与远端OFT相邻的SpM SHF细胞具有假复层柱状上皮细胞层的特点[121]。直到2012年,这种紧密结合、上皮样细胞层的SHF细胞形态才在小鼠SpM 中被发现,而靠近尾部SpM处的SHF细胞具有板状伪足状突起的细胞形态。因此有研究者提出一种假说:位于尾部SpM中松散堆积的间充质样SHF祖细胞经历间充质细胞到上皮细胞的转化过程,以形成SpM中上皮样SHF细胞层,通过细胞插入(cell intercalation)的方式促进SHF祖细胞对OFT的贡献;此外,在PCP活性被抑制的突变体中,尾部SpM处SHF祖细胞中actin聚合和板状伪足的活性被破坏;该研究推测Wnt5a-PCP信号可能通过调节尾部SpM处SHF祖细胞内actin聚合作用促进SHF祖细胞的迁移部署,影响OFT延伸和心脏环化过程[8]。
2014年,王建波研究团队的结果表明:位于小鼠SpM中的SHF祖细胞被组织成上皮样细胞层,在细胞层中的单个细胞显示出多边形形态,并且在细胞顶端皮质周围富集F-actin 的细胞被紧密挤在一起;SHF祖细胞在SpM某处经历间充质到上皮样细胞的转变,被组织形成上皮样细胞层,并以紧密结合、上皮样细胞层的形式而非单细胞的方式向OFT迁移[7]。该研究与Sinha等[8]在缺失突变体小鼠中的研究结果一致,即抑制Wnt5a导致SpM处SHF祖细胞表现出减少且紊乱的actin聚合、细胞的形态和细胞排列方向被破坏等表型,进一步揭示了SpM处SHF祖细胞中actin细胞骨架、细胞形态及排列方向在SHF祖细胞向OFT部署中的重要性。
2016年,王建波研究团队报道了利用突变体小鼠研究SpM中SHF祖细胞向OFT部署的另一项研究成果,进一步揭示Wnt5a-PCP信号通路在SpM SHF祖细胞向OFT部署过程中的时空调控作用[119]。在缺失的突变体小鼠中,通过Islet1- Cre驱动SHF细胞过表达,能够挽救SpM处SHF细胞中actin聚合、细胞极性的缺陷。在Islet1- Cre驱动SHF细胞过表达的突变体小鼠中,与远端OFT相邻的SpM处上皮样SHF细胞的细胞粘附连接被破坏,SHF细胞滞留在该处,形成突起而不向OFT部署。虽然该研究并未通过实验证明过表达影响SpM处SHF细胞粘附连接的具体机制,然而研究表明上皮细胞极性和细胞连接相互影响,两者的建立和维持需要actin细胞骨架的协同调控作用[35,122]。该研究进一步揭示actin细胞骨架及细胞排列和极性在SHF祖细胞部署中的必要作用。
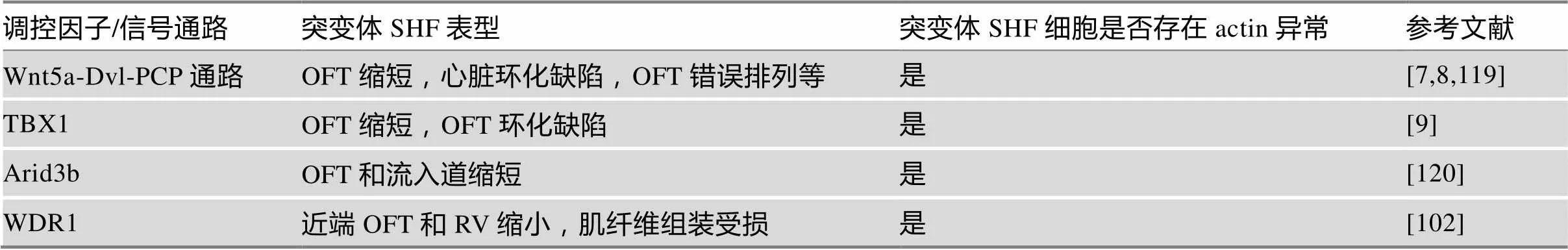
表2 影响SHF祖细胞actin细胞骨架的调控因子及信号通路

图1 SHF祖细胞特异性敲除Wdr1小鼠的表型
E9.5 (A~F)、E10.5 (G~L)对照组(CTL)和SHF祖细胞敲除组(Wdr1/F; Mef2c-AHF-Cre)胚胎的显微和组织学分析。与对照组胚胎相比,敲除组胚胎具有RV缩小、近端OFT和RV心肌细胞排列紊乱(黑箭头)的表型。
3.2 TBX1与SHF祖细胞actin细胞骨架
2014年,Francou等[9]研究证明:位于SpM及远端OFT处的SHF细胞组成非经典顶端-基底极性的上皮,并具有顶部单纤毛、动态actin富集基底端的丝状伪足等特点;该研究还发现,除了调节SHF祖细胞的增殖和分化外,还通过参与调节SHF细胞上皮细胞极性、SHF祖细胞基底端actin动态和丝状伪足活性,影响SHF祖细胞向OFT的部署。该研究推测,在心管伸展过程中,SHF祖细胞基底端动态actin重塑驱动的丝状伪足运动可能在维持SpM处SHF祖细胞状态所需的信号通路中发挥作用。
3.3 Arid3b与SHF祖细胞actin细胞骨架
2014年,Uribe等[120]研究发现,通过基因捕获(gene trap)方法构建的突变体胚胎具有OFT和流入道(inflow tract, IFT)长度缩短、房室垫发育受损等一系列心脏发育缺陷的表型;在E9.5的突变体胚胎中观察到Islet1阳性的SHF祖细胞在OFT区域积累,且SpM处SHF祖细胞中actin细胞骨架和细胞结构均发生改变;Dil标记、追踪实验证明在突变体胚胎中,SHF细胞向心管的贡献受损。该研究推测Arid3b可能通过actin细胞骨架调节SHF祖细胞向心管的运动,表明actin细胞骨架在SHF祖细胞迁移部署中的重要作用。
3.4 WDR1与SHF祖细胞actin细胞骨架
本课题组最近的研究揭示actin解聚调控因子WDR1在哺乳动物SHF发育中的必要作用[102]。通过Mef2c-SHF-Cre构建SHF细胞特异性敲除小鼠,该突变体小鼠从E11.5开始死亡,且在E10.5表现出近端OFT和RV缩小的表型;敲除并不影响SHF细胞的数量以及SHF祖细胞由SpM向远端OFT的部署过程,但近端OFT和右心室细胞的空间排列和心肌细胞肌纤维的组装过程被严重破坏(图1)。我们推测:WDR1介导的actin解聚和加聚动态可能通过调节上皮样SHF祖细胞向心肌细胞分化过程中心肌细胞空间排列的重塑过程,进一步调控OFT和RV大小。关于Mef2c-SHF-Cre敲除并不影响SHF祖细胞由SpM向远端OFT的部署过程,推测可能有两方面原因:首先,基因不完全删除导致WDR1的少量存留;其次,前人的研究表明SpM和远端OFT处的SHF细胞是伸展、极化的上皮细胞,且在这种上皮样细胞层中可能存在一种推动力以驱动SHF细胞向远端OFT部署[7,119],暗示SHF祖细胞向远端OFT部署过程中可能对actin解聚和加聚动态的需求较少。
4 结语与展望
SHF祖细胞整合、部署的精确、具体细胞机制尚不完全清楚,如是否通过主动细胞迁移、细胞凝聚及细胞张力、细胞插入还是有方向的细胞分裂等机制,仍需要进行深入的研究来阐明。然而,进一步研究SHF祖细胞部署的细胞生物学过程及生物力学等特性,需要开发高分辨率动态成像技术。SHF细胞具有非典型上皮样的顶端-基底细胞极性、细胞连接、排列和动态actin在基底端细胞膜富集等特点,这些研究揭示actin细胞骨架在SHF祖细胞部署、发育中发挥关键的调控作用。因此,利用actin细胞骨架作为切入点,通过分子细胞生物学手段构建SHF祖细胞标记、追踪体系,结合活细胞成像和高穿透性电子显微成像系统,将会为深入阐明、理解SHF祖细胞迁移、部署的细胞生物学特征以及生物力学提供新的研究思路和方向。
[1] Bruneau BG. The developmental genetics of congenital heart disease.,2008,451(7181): 943–948.
[2] Vincent SD, Mayeuf-Louchart A, Watanabe Y, Brzezinski JA IV, Miyagawa-Tomita S, Kelly RG, Buckingham M. Prdm1 functions in the mesoderm of the second heart field, where it interacts genetically with tbx1, during outflow tract morphogenesis in the mouse embryo.,2014,23(19): 5087–5101.
[3] Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development.,2010,107(12): 1428– 1444.
[4] Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells.,2005,6(11): 826–835.
[5] Vincent SD, Buckingham ME. How to make a heart: The origin and regulation of cardiac progenitor cells.,2010,90: 1–41.
[6] Dyer LA, Kirby ML. The role of secondary heart field in cardiac development.,2009,336(2): 137– 144.
[7] Sinha T, Li D, Théveniau-Ruissy M, Hutson MR, Kelly RG, Wang J. Loss of wnt5a disrupts second heart field cell deployment and may contribute to oft malformations in digeorge syndrome.,2015,24(6): 1704–1716.
[8] Sinha T, Wang B, Evans S, Wynshaw-Boris A, Wang J. Disheveled mediated planar cell polarity signaling is required in the second heart field lineage for outflow tract morphogenesis.,2012,370(1): 135–144.
[9] Francou A, Saint-Michel E, Mesbah K, Kelly RG. Tbx1 regulates epithelial polarity and dynamic basal filopodia in the second heart field.,2014,141(22): 4320–4331.
[10] Kelly RG. The second heart field.,2012,100: 33–65.
[11] van den Berg G, Abu-Issa R, de Boer BA, Hutson MR, de Boer PA, Soufan AT, Ruijter JM, Kirby ML, van den Hoff MJ, Moorman AF. A caudal proliferating growth center contributes to both poles of the forming heart tube.,2009,104(2): 179–188.
[12] Mesbah K, Harrelson Z, Théveniau-Ruissy M, Papaioannou VE, Kelly RG. Tbx3 is required for outflow tract development.,2008,103(7): 743–750.
[13] Cortes C, Francou A, De Bono C, Kelly RG. Epithelial properties of the second heart field.,2018,122(1): 142–154.
[14] de la Cruz MV, Sánchez Gómez C, Arteaga MM, Argüello C. Experimental study of the development of the truncus and the conus in the chick embryo.,1977,123(Pt 3): 661–686.
[15] Viragh S, Challice CE. Origin and differentiation of cardiac muscle cells in the mouse.,1973,42(1): 1–24.
[16] Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field.,2001,238(1): 97–109.
[17] Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field.,2001,128(16): 3179–3188.
[18] Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from fgf10-expressing cells in pharyngeal mesoderm.,2001,1(3): 435–440.
[19] Rana MS, Théveniau-Ruissy M, De Bono C, Mesbah K, Francou A, Rammah M, Domínguez JN, Roux M, Laforest B, Anderson RH, Mohun T, Zaffran S, Christoffels VM, Kelly RG. Tbx1 coordinates addition of posterior second heart field progenitor cells to the arterial and venous poles of the heart.,2014,115(9): 790–799.
[20] Domínguez JN, Meilhac SM, Bland YS, Buckingham ME, Brown NA. Asymmetric fate of the posterior part of the second heart field results in unexpected left/right contributions to both poles of the heart.,2012,111(10): 1323–1335.
[21] Rochais F, Mesbah K, Kelly RG. Signaling pathways controlling second heart field development.,2009,104(8): 933–942.
[22] Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart.,2003,5(6): 877–889.
[23] Chen L, Fulcoli FG, Tang S, Baldini A. Tbx1 regulates proliferation and differentiation of multipotent heart progenitors.,2009,105(9): 842–851.
[24] Guo C, Sun Y, Zhou B, Adam RM, Li X, Pu WT, Morrow BE, Moon A, Li X. A tbx1-six1/eya1-fgf8 genetic pathway controls mammalian cardiovascular and craniofacial morphogenesis.,2011,121(4): 1585–1595.
[25] Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development.,2006,133(12): 2435–2445.
[26] Robertson EJ, Charatsi I, Joyner CJ, Koonce CH, Morgan M, Islam A, Paterson C, Lejsek E, Arnold SJ, Kallies A, Nutt SL, Bikoff EK. Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice.,2007,134(24): 4335–4345.
[27] Chhabra ES, Higgs HN. The many faces of actin: Matching assembly factors with cellular structures.,2007,9(10): 1110–1121.
[28] Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility.,2014,94(1): 235–263.
[29] Svitkina T. The actin cytoskeleton and actin-based motility.,2018,10(1).
[30] Callan-Jones AC, Voituriez R. Actin flows in cell migration: from locomotion and polarity to trajectories.,2016,38: 12–17.
[31] Inagaki N, Katsuno H. Actin waves: origin of cell polarization and migration?,2017,27(7): 515–526.
[32] Allard J, Mogilner A. Traveling waves in actin dynamics and cell motility.,2013,25(1): 107–115.
[33] Sun SC, Kim NH. Molecular mechanisms of asymmetric division in oocytes.,2013,19(4): 883–897.
[34] Yu XJ, Yi Z, Gao Z, Qin D, Zhai Y, Chen X, Ou-Yang Y, Wang ZB, Zheng P, Zhu MS, Wang H, Sun QY, Dean J, Li L. The subcortical maternal complex controls symmetricdivision of mouse zygotes by regulating F-actin dynamics.,2014,5: 4887.
[35] Engl W, Arasi B, Yap LL, Thiery JP, Viasnoff V. Actin dynamics modulate mechanosensitive immobilization of e-cadherin at adherens junctions.,2014,16(6): 587–594.
[36] Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions.,2010,11(5): 353–365.
[37] Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments.,2003,112(4): 453–465.
[38] Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells.,2000,29: 545–576.
[39] Paavilainen VO, Bertling E, Falck S, Lappalainen P. Regulation of cytoskeletal dynamics by actin-monomer- binding proteins.,2004,14(7): 386– 394.
[40] Pollard TD, Beltzner CC. Structure and function of the arp2/3 complex.,2002,12(6): 768–774.
[41] Insall RH, Machesky LM. Actin dynamics at the leading edge: From simple machinery to complex networks.,2009,17(3): 310–322.
[42] Miki H, Takenawa T. Regulation of actin dynamics by wasp family proteins.,2003,134(3): 309– 313.
[43] Stradal TE, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by wasp and wave family proteins.,2004,14(6): 303–311.
[44] Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. An actin nucleation mechanism mediated by bni1 and profilin.,2002,4(8): 626–631.
[45] Kovar DR, Pollard TD. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces.,2004,101(41): 14725–14730.
[46] Bamburg JR. Proteins of the adf/cofilin family: essential regulators of actin dynamics.,1999,15: 185–230.
[47] Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of adf/cofilin.,2006,24(1): 13–23.
[48] Bernstein BW, Bamburg JR. Adf/cofilin: a functional node in cell biology.,2010,20(4): 187–195.
[49] Ono S. Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1: new blades for twisted filaments.,2003,42(46): 13363–13370.
[50] Nadkarni AV, Brieher WM. Aip1 destabilizes cofilin-saturated actin filaments by severing and accelerating monomer dissociation from ends.,2014,24(23): 2749–2757.
[51] Rottner K, Stradal TE. Actin dynamics and turnover in cell motility.,2011,23(5): 569– 578.
[52] Keller R. Cell migration during gastrulation.,2005,17(5): 533–541.
[53] Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties.,2008,322(5907): 1502–1505.
[54] Shaw TJ, Martin P. Wound repair: a showcase for cell plasticity and migration.,2016,42: 29–37.
[55] Shaw TJ, Martin P. Wound repair at a glance.,2009,122(Pt 18): 3209–3213.
[56] Nourshargh S, Alon R. Leukocyte migration into inflamed tissues.,2014,41(5): 694–707.
[57] Weninger W, Biro M, Jain R. Leukocyte migration in the interstitial space of non-lymphoid organs.,2014,14(4): 232–246.
[58] Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process.,1996,84(3): 359–369.
[59] Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion.,1996,84(3): 371–379.
[60] Zaidel-Bar R, Zhenhuan G, Luxenburg C. The contractome—a systems view of actomyosin contractility in non-muscle cells.,2015,128(12): 2209– 2217.
[61] Pandya P, Orgaz JL, Sanz-Moreno V. Actomyosin contractility and collective migration: may the force be with you.,2017,48: 87–96.
[62] Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility.,2001,292(5521): 1502– 1506.
[63] Carlier MF, Pernier J, Montaville P, Shekhar S, Kühn S, Cytoskeleton Dynamics and Motility Group. Control of polarized assembly of actin filaments in cell motility.,2015,72(16): 3051–3067.
[64] Ono K, Ono S. Actin-adf/cofilin rod formation in caenorhabditis elegans muscle requires a putative f-actin binding site of adf/cofilin at the c-terminus.,2009,66(7): 398–408.
[65] Squire JM. Architecture and function in the muscle sarcomere.,1997,7(2): 247–257.
[66] Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function.,2002,18: 637–706.
[67] Sparrow JC, Schöck F. The initial steps of myofibril assembly: integrins pave the way.,2009,10(4): 293–298.
[68] Ono S. Dynamic regulation of sarcomeric actin filamentsin striated muscle.,2010,67(11): 677–692.
[69] Sanger JW, Kang S, Siebrands CC, Freeman N, Du A, Wang J, Stout AL, Sanger JM. How to build a myofibril.,2005,26(6–8): 343–354.
[70] Sanger JW, Wang J, Fan Y, White J, Sanger JM. Assembly and dynamics of myofibrils.,2010,2010: 858606.
[71] Shimizu N, Obinata T. Actin concentration and monomer-polymer ratio in developing chicken skeletal muscle.,1986,99(3): 751–759.
[72] Dome JS, Mittal B, Pochapin MB, Sanger JM, Sanger JW. Incorporation of fluorescently labeled actin and tropomyosin into muscle cells.,1988,23(1–2): 37–52.
[73] Imanaka-Yoshida K, Sanger JM, Sanger JW. Contractile protein dynamics of myofibrils in paired adult rat cardiomyocytes.,1993,26(4): 301–312.
[74] Shimada Y, Suzuki H, Konno A. Dynamics of actin in cardiac myofibrils and fibroblast stress fibers.,1997,22(1): 59–64.
[75] Suzuki H, Komiyama M, Konno A, Shimada Y. Exchangeability of actin in cardiac myocytes and fibroblasts as determined by fluorescence photobleaching recovery.,1998,30(2): 274–280.
[76] Littlefield R, Almenar-Queralt A, Fowler VM. Actin dynamics at pointed ends regulates thin filament length in striated muscle.,2001,3(6): 544–551.
[77] Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of z-band based proteins in developing skeletal muscle cells.,2005,61(1): 34–48.
[78] Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres.,2009,122(Pt 12): 2119–2126.
[79] Littlefield R, Fowler VM. Defining actin filament length in striated muscle: rulers and caps or dynamic stability?,1998,14: 487–525.
[80] Littlefield RS, Fowler VM. Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler.,2008,19(6): 511–519.
[81] Schafer DA, Hug C, Cooper JA. Inhibition of capz during myofibrillogenesis alters assembly of actin filaments.,1995,128(1–2): 61–70.
[82] Hart MC, Cooper JA. Vertebrate isoforms of actin capping protein beta have distinct functions.,1999,147(6): 1287–1298.
[83] Gregorio CC, Weber A, Bondad M, Pennise CR, Fowler VM. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes.,1995,377(6544): 83–86.
[84] Sussman MA, Baqué S, Uhm CS, Daniels MP, Price RL, Simpson D, Terracio L, Kedes L. Altered expression of tropomodulin in cardiomyocytes disrupts the sarcomeric structure of myofibrils.,1998,82(1): 94–105.
[85] Gokhin DS, Ochala J, Domenighetti AA, Fowler VM. Tropomodulin 1 directly controls thin filament length in both wild-type and tropomodulin 4-deficient skeletal muscle.,2015,142(24): 4351–4362.
[86] Nworu CU, Kraft R, Schnurr DC, Gregorio CC, Krieg PA. Leiomodin 3 and tropomodulin 4 have overlapping functions during skeletal myofibrillogenesis.,2015,128(2): 239–250.
[87] McElhinny AS, Schwach C, Valichnac M, Mount-Patrick S, Gregorio CC. Nebulin regulates the assembly and lengths of the thin filaments in striated muscle.,2005,170(6): 947–957.
[88] Bang ML, Li X, Littlefield R, Bremner S, Thor A, Knowlton KU, Lieber RL, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle.,2006,173(6): 905–916.
[89] Witt CC, Burkart C, Labeit D, McNabb M, Wu Y, Granzier H, Labeit S. Nebulin regulates thin filament length, contractility, and z-disk structure.,2006,25(16): 3843–3855.
[90] Pappas CT, Krieg PA, Gregorio CC. Nebulin regulates actin filament lengths by a stabilization mechanism.,2010,189(5): 859–870.
[91] Ono S, Ono K. Tropomyosin inhibits adf/cofilin- dependent actin filament dynamics.,2002,156(6): 1065–1076.
[92] Yu R, Ono S. Dual roles of tropomyosin as an F-actin stabilizer and a regulator of muscle contraction in caenorhabditis elegans body wall muscle.,2006,63(11): 659–672.
[93] Kremneva E, Makkonen MH, Skwarek-Maruszewska A, Gateva G, Michelot A, Dominguez R, Lappalainen P. Cofilin-2 controls actin filament length in muscle sarcomeres.,2014,31(2): 215–226.
[94] Chatzifrangkeskou M, Yadin D, Marais T, Chardonnet S, Cohen-Tannoudji M, Mougenot N, Schmitt A, Crasto S, Di Pasquale E, Macquart C, Tanguy Y, Jebeniani I, Pucéat M, Morales Rodriguez B, Goldmann WH, Dal Ferro M, Biferi MG, Knaus P, Bonne G, Worman HJ, Muchir A. Cofilin-1 phosphorylation catalyzed by erk1/2 alters cardiac actin dynamics in dilated cardiomyopathy caused by lamin a/c gene mutation.,2018, 27(17): 3060–3078.
[95] Ono S. The caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments.,2001,152(6): 1313–1319.
[96] Mohri K, Ono K, Yu R, Yamashiro S, Ono S. Enhancement of actin-depolymerizing factor/cofilin- dependent actin disassembly by actin-interacting protein 1 is required for organized actin filament assembly in the caenorhabditis elegans body wall muscle.,2006,17(5): 2190–2199.
[97] Yuen M, Sandaradura SA, Dowling JJ, Kostyukova AS, Moroz N, Quinlan KG, Lehtokari VL, Ravenscroft G, Todd EJ, Ceyhan-Birsoy O, Gokhin DS, Maluenda J, Lek M, Nolent F, Pappas CT, Novak SM, D'Amico A, Malfatti E, Thomas BP, Gabriel SB, Gupta N, Daly MJ, Ilkovski B, Houweling PJ, Davidson AE, Swanson LC, Brownstein CA, Gupta VA, Medne L, Shannon P, Martin N, Bick DP, Flisberg A, Holmberg E, Van den Bergh P, Lapunzina P, Waddell LB, Sloboda DD, Bertini E, Chitayat D, Telfer WR, Laquerrière A, Gregorio CC, Ottenheijm CA, Bönnemann CG, Pelin K, Beggs AH, Hayashi YK, Romero NB, Laing NG, Nishino I, Wallgren-Pettersson C, Melki J, Fowler VM, MacArthur DG, North KN, Clarke NF. Leiomodin-3 dysfunction results in thin filament disorganization and nemaline myopathy.,2014,124(11): 4693–4708.
[98] Chereau D, Boczkowska M, Skwarek-Maruszewska A, Fujiwara I, Hayes DB, Rebowski G, Lappalainen P, Pollard TD, Dominguez R. Leiomodin is an actin filament nucleator in muscle cells.,2008,320(5873): 239–243.
[99] Tsukada T, Pappas CT, Moroz N, Antin PB, Kostyukova AS, Gregorio CC. Leiomodin-2 is an antagonist of tropomodulin-1 at the pointed end of the thin filaments in cardiac muscle.,2010,123(Pt 18): 3136–3145.
[100] Taniguchi K, Takeya R, Suetsugu S, Kan-O M, Narusawa M, Shiose A, Tominaga R, Sumimoto H. Mammalian formin fhod3 regulates actin assembly and sarcomere organization in striated muscles.,2009,284(43): 29873–29881.
[101] Yuan B, Wan P, Chu D, Nie J, Cao Y, Luo W, Lu S, Chen J, Yang Z. A cardiomyocyte-specific wdr1 knockout demonstrates essential functional roles for actin disassembly during myocardial growth and maintenance in mice.,2014,184(7): 1967–1980.
[102] Hu J, Shi Y, Xia M, Liu Z, Zhang R, Luo H, Zhang T, Yang Z, Yuan B. Wdr1-regulated actin dynamics is required for outflow tract and right ventricle development.,2018,438(2): 124–137.
[103] Clarkson E, Costa CF, Machesky LM. Congenital myopathies: diseases of the actin cytoskeleton.,2004,204(4): 407–417.
[104] Agrawal PB, Greenleaf RS, Tomczak KK, Lehtokari VL, Wallgren-Pettersson C, Wallefeld W, Laing NG, Darras BT, Maciver SK, Dormitzer PR, Beggs AH. Nemaline myopathy with minicores caused by mutation of the cfl2 gene encoding the skeletal muscle actin-binding protein, cofilin-2.,2007,80(1): 162–167.
[105] Ockeloen CW, Gilhuis HJ, Pfundt R, Kamsteeg EJ, Agrawal PB, Beggs AH, Dara Hama-Amin A, Diekstra A, Knoers NV, Lammens M, van Alfen N. Congenital myopathy caused by a novel missense mutation in the cfl2 gene.,2012,22(7): 632–639.
[106] Ilkovski B, Cooper ST, Nowak K, Ryan MM, Yang N, Schnell C, Durling HJ, Roddick LG, Wilkinson I, Kornberg AJ, Collins KJ, Wallace G, Gunning P, Hardeman EC, Laing NG, North KN. Nemaline myopathy caused by mutations in the muscle alpha-skeletal-actin gene.,2001,68(6): 1333–1343.
[107] Feng JJ, Marston S. Genotype-phenotype correlations in acta1 mutations that cause congenital myopathies.,2009,19(1): 6–16.
[108] Laing NG, Dye DE, Wallgren-Pettersson C, Richard G, Monnier N, Lillis S, Winder TL, Lochmüller H, Graziano C, Mitrani-Rosenbaum S, Twomey D, Sparrow JC, Beggs AH, Nowak KJ. Mutations and polymorphisms of the skeletal muscle alpha-actin gene (acta1).,2009,30(9): 1267–1277.
[109] Friedman B, Simpson K, Tesi-Rocha C, Zhou D, Palmer CA, Suchy SF. Novel large deletion in the acta1 gene in a child with autosomal recessive nemaline myopathy.,2014,24(4): 331–334.
[110] Jain RK, Jayawant S, Squier W, Muntoni F, Sewry CA, Manzur A, Quinlivan R, Lillis S, Jungbluth H, Sparrow JC, Ravenscroft G, Nowak KJ, Memo M, Marston SB, Laing NG. Nemaline myopathy with stiffness and hypertonia associated with an acta1 mutation.,2012,78(14): 1100–1103.
[111] Mroczek M, Kabzińska D, Chrzanowska KH, Pronicki M, Kochanski A. A novel tpm2 gene splice-site mutation causes severe congenital myopathy with arthrogryposis and dysmorphic features.,2017,58(2): 199–203.
[112] Marttila M, Lehtokari VL, Marston S, Nyman TA, Barnerias C, Beggs AH, Bertini E, Ceyhan-Birsoy O, Cintas P, Gerard M, Gilbert-Dussardier B, Hogue JS, Longman C, Eymard B, Frydman M, Kang PB, Klinge L, Kolski H, Lochmüller H, Magy L, Manel V, Mayer M, Mercuri E, North KN, Peudenier-Robert S, Pihko H, Probst FJ, Reisin R, Stewart W, Taratuto AL, de Visser M, Wilichowski E, Winer J, Nowak K, Laing NG, Winder TL, Monnier N, Clarke NF, Pelin K, Grönholm M, Wallgren-Pettersson C. Mutation update and genotype-phenotype correlations of novel and previously described mutations in tpm2 and tpm3 causing congenital myopathies.,2014,35(7): 779–790.
[113] Fox MD, Carson VJ, Feng HZ, Lawlor MW, Gray JT, Brigatti KW, Jin JP, Strauss KA. Tnnt1 nemaline myopathy: Natural history and therapeutic frontier.,2018, 27(18): 3272–3282.
[114] Konersman CG, Freyermuth F, Winder TL, Lawlor MW, Lagier-Tourenne C, Patel SB. Novel autosomal dominant tnnt1 mutation causing nemaline myopathy.,2017,5(6): 678–691.
[115] Abdulhaq UN, Daana M, Dor T, Fellig Y, Eylon S, Schuelke M, Shaag A, Elpeleg O, Edvardson S. Nemaline body myopathy caused by a novel mutation in troponin t1 (tnnt1).,2016,53(4): 564–569.
[116] Piga D, Magri F, Ronchi D, Corti S, Cassandrini D, Mercuri E, Tasca G, Bertini E, Fattori F, Toscano A, Messina S, Moroni I, Mora M, Moggio M, Colombo I, Giugliano T, Pane M, Fiorillo C, D'Amico A, Bruno C, Nigro V, Bresolin N, Comi GP. New mutations in neb gene discovered by targeted next-generation sequencing in nemaline myopathy italian patients.,2016,59(3): 351–359.
[117] Lehtokari VL, Pelin K, Sandbacka M, Ranta S, Donner K, Muntoni F, Sewry C, Angelini C, Bushby K, van den Bergh P, Iannaccone S, Laing NG, Wallgren-Pettersson C. Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy.,2006,27(9): 946–956.
[118] Tsunoda K, Yamashita T, Motokura E, Takahashi Y, Sato K, Takemoto M, Hishikawa N, Ohta Y, Nishikawa A, Nishino I, Abe K. A patient with slowly progressive adult-onset nemaline myopathy and novel compound heterozygous mutations in the nebulin gene.,2017,373: 254–257.
[119] Li D, Sinha T, Ajima R, Seo HS, Yamaguchi TP, Wang J. Spatial regulation of cell cohesion by wnt5a during second heart field progenitor deployment.,2016,412(1): 18–31.
[120] Uribe V, Badía-Careaga C, Casanova JC, Domínguez JN, de la Pompa JL, Sanz-Ezquerro JJ. Arid3b is essential for second heart field cell deployment and heart patterning.,2014,141(21): 4168–4181.
[121] Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart.,2005,281(1): 78–90.
[122] Campos Y, Qiu X, Gomero E, Wakefield R, Horner L, Brutkowski W, Han YG, Solecki D, Frase S, BongiovanniA, d'Azzo A. Alix-mediated assembly of the actomyosin- tight junction polarity complex preserves epithelial polarity and epithelial barrier.,2016,7: 11876.
The role of actin cytoskeleton in regulating the deployment process of mouse cardiac second heart field progenitor cells
Zhongying Liu, Xia Huang, Ziyi Li, Zihao Yang, Baiyin Yuan
,,,
The vertebrate heart tube originates from cardiogenic mesodermal cells in the early embryo, and subsequently elongates by progressive addition of second heart field (SHF) progenitor cells from adjacent pharyngeal mesoderm and splanchnic mesoderm. Insufficient addition of SHF cells to the heart tube causes the failure of maximal elongation of the heart tube, which results in a series of developmental defects including the most common congenital birth defects, such as right ventricular dysplasia and outflow tract septation, and alignment anomalies. SHF cells form an atypical, apicobasally polarized epithelium which is characterized by apical monocilia, and dynamic actin-rich basal filopodia. In this review, we summarize recent research progresses of actin cytoskeleton in the deployment process of mouse SHF progenitor cells, and reveal the significance of actin cytoskeleton in SHF development, especially in the deployment of SHF cells to the outflow tract, to provide theoretical reference for elucidating and understanding the biological characteristics of SHF deployment.
actin; second heart field; cell deployment; mouse
2018-10-29;
2019-01-18
国家自然科学基金项目(编号:31701266)资助[Supported by the National Natural Science Foundation of China (No. 31701266)]
刘钟颖,硕士研究生,专业方向:遗传及细胞生物学。E-mail: 757967423@qq.com
袁白银,博士,讲师,研究方向:遗传及发育生物学。E-mail: yuanby@wust.edu.cn
10.16288/j.yczz.18-293
2019/1/29 16:14:30
URI: http://kns.cnki.net/kcms/detail/11.1913.R.20190129.1614.002.htm
(责任编委: 杨中州)

