Effects of the Syzygium aromaticum L. extract on antioxidation and inhibition of matrix metalloproteinase in human dermal fibroblast
Da Eun Kim, Yeon Sil Hwang, Bo Yoon Chang, Dae Sung Kim, Hyoung Kwon Cho, Sung Yeon Kim✉
1Institute of Pharmaceutical Research and Development, College of Pharmacy, Wonkwang University, 460 Ikandae-ro, Iksan 570-749, Jeollabuk-do,Republic of Korea
2Hanpoong Pharm. CO., Ltd, 333-24 1st Palbok-dong, Deokjingu, Jeonju 561-841, Jeonbuk, Republic of Korea
Keywords:Syzygium aromaticum L Antioxidant Anti-aging Elastase Matrix metalloproteinase-1
ABSTRACT Objective: To investigate cosmetic potential of Syzygium aromaticum L. (S. aromaticum L.) and to determine its antioxidant and anti-wrinkling effects. Methods: Using highperformance liquid chromatography, eugenol component was quantitated. The antioxidant activity of S. aromaticum L. was analyzed by 2,2-diphenyl-1-picrylhydrazyl radical scavenging and superoxide dismutase like activities. To determine cell viability, elastase and matrix metalloproteinase-1 (MMP-1) activity, human dermal fibroblasts (HS68) were treated with S. aromaticum L. The inhibitory effect of S. aromaticum L. on tumor necrosis factor alpha induced MMPs expression in HS68 was analyzed by realtime-PCR. Results: The eugenol content was confirmed in S. aromaticum L. S. aromaticum L. was observed to have high 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity and superoxide dismutase like activity. S.aromaticum L. had no cytotoxicity against the HS68 and dose-dependently increased elastase inhibition. Moreover, S. aromaticum L. significantly decreased MMP-1 content and inhibited gene levels of MMP-1, MMP-2, MMP-3 and MMP-9. Conclusions: The findings suggest that S. aromaticum L. has great potential as a cosmeceutical ingredient with antioxidant and antiwrinkling effects.
1. Introduction
The skin is the outermost part of our body and plays a defensive role against various external environments including ultraviolet(UV) rays. Skin aging is manifested by wrinkles, decreased elasticity, and pigmentation. There are two major causes of skin aging that can cause wrinkles[1]. Intrinsic aging naturally occurs as people age, which means hormone secretion decreases, cell activity decreases, and the skin wrinkles due to the decrease of biosynthesis of constitutive proteins caused by mutation and cell metabolism imbalance. The occurrence of exogenous aging means the occurrence of skin wrinkles caused by environmental factors such as UV rays, gravity, and polluted air[2,3]. The occurrence of wrinkles is caused by the deformation of the dermal tissue of the skin. The dermis is the connective tissue underlying in the epidermis and occupies most of the skin volume. It is not densely packed like the epidermal keratinocyte, and has many extracellular spaces. It is composed of extracellular matrix and fibroblast, which is a macromolecular network[4]. Fibroblast is responsible for the expression of collagen and elastin, which are fibrous proteins,and helps maintaining the flexibility, elasticity and tension of the skin. When the action of fibroblasts that produce extracellular matrix is degraded by UV, collagen biosynthesis is decreased and degenerated elastin is increased[5]. Furthermore, the expression of matrix metalloproteinases (MMPs), a metalloprotease that degrades extracellular matrix, has been shown to result in deformation of cells and extracellular matrix, causing damage to the length and distribution of collagen fibers. The epidermis-dermal boundary is destroyed, harmful components are introduced and dermal decomposition is accelerated[6].
The skin always comes into contact with oxygen and is constantly exposed to UV resulting in skin protein denaturation and cell damage caused by reactive oxidant species (ROS). As the intracellular ROS increases, it promotes intracellular oxidative damage and degrades the enzymatic function of the antioxidant enzyme, thereby impairing the skin defense system against the aging of the skin and the external harmful environment[7,8].
The flower bud of Syzygium aromaticum L. (S. aromaticum L.)has a strong unusual smell. Its flavor is spicy and it is not toxic.S. aromaticum L. was considered as a cure for diarrhea, most liver, stomach and bowel ailments, and as a stimulant for the nerves for centuries. Traditionally S. aromaticum L. has been used to treat flatulence, nausea and vomiting[9]. In foods and drinks,S. aromaticum L. is used as a flavoring. In manufacturing, S.aromaticum L. is used in toothpaste, soaps, cosmetics and perfumes.Studies on S. aromaticum L. have been conducted on antioxidant,antimicrobial, antiviral, anticoagulant, immediate hypersensitivity suppression, antiallergic, vasodilation, and anesthetic effects[10-14].In order to improve skin wrinkles and prevent aging, it is important to find antioxidants that remove active oxygen, materials that increase collagen synthesis, or inhibit MMPs, enzymes that degrade extracellular matrix. We hypothesized that S. aromaticum L. could be beneficial to skin wrinkles based on antioxidant properties. However,there are only a few studies on the improvement of wrinkles using S. aromaticum L.[15]. Therefore, in this paper, we planned to confirm the wrinkle-improving activity and underlying mechanisms of S.aromaticum L.
2. Materials and methods
2.1. Materials
Reagents used were of analytical grade purchased from suppliers such as Sigma-Aldrich (St. Louis, MO, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),dimethyl sulfoxide, 2,2-diphenyl-1-picrylhydrazyl (DPPH),N-succinyl-(L-Ala)-3-p-nitroanilide and elastase. Dulbecco modified eagle medium, penicillin/streptomycin, phosphate-buffered saline and fetal bovine serum were obtained from Life Technologies Corporation.
Tumor necrosis factor alpha (TNF-α) was obtained from R&D Systems Inc. (MN, USA). Easy-BlueTMTotal RNA extraction kit was purchased from Intron Biotechnology, Inc. (iNtRON Biotechnology,Seongnam, Korea). Superoxide dismutase (SOD) Assay Kit-WST was purchased from Dojindo Laboratories (Kumamoto, Japan).MMP-1 Biotrack activity Assay Kit was purchased from Amersham Bioscience (Amersham, UK) and a procollagen type-Ⅰ C peptide(PIP) EIA kit was purchased from Takara Bio Inc. (Takara bio,Tokyo, Japan). TaqManRNA-to-CtTM1-Step Kits were purchased from Applied Biosystems (USA).
2.2. Extract of S. aromaticum L.
S. aromaticum L. were purchased through Heungil Pharmaceuticals(Korea) and extracted by the Hanpoong Pharm and Foods Company(Korea). S. aromaticum L. (1 kg) were boiled with 1 L of 50%ethanol for 3 h. The extract was filtered with a 1 μm filter, and the residue was subjected to secondary extraction under the same conditions. The solvent was independently evaporated under reduced pressure at 60 ℃, and the extract was completely dried to obtain a S.aromaticum L. extract (SAE). The percentage yield of the dry SAE was found to be 18.5%.
2.3. High-performance liquid chromatography analysis(HPLC)
The eugenol content of SAE was determined by HPLC. HPLC analyses were performed on a Waters 2695 HPLC system (Milford,USA) equipped with 250 mm × 4.6 mm Capcellpak UG-120 ODS column (Shiseido, Japan). The mobile phase consisted of (A)methanol/water/acetic acid (10:88:2, v/v/v) and (B) methanol/water/acetic acid (98:2:2, v/v/v). The flow rate was set at 1 mL/min, peaks were acquired at 260 nm[16]. The presence of eugenol was confirmed by comparing the peak of the standard eugenol (Sigma-Aldrich,USA) and the residence time.
2.4. DPPH free radical and superoxide radical scavenging activity
The DPPH radical scavenging assay was conducted according to the method described by Manivasagan et al.[17].
A total of 0.1 mL SAE (0, 10, 50, 100 and 500 μg/mL) samples were added to 0.1 mM DPPH dissolved in methanol (0.1 mL)solution and mixed thoroughly. After incubation for 30 min in the dark at room temperature, the absorbance was measured at 520 nm.Vitamin C was used as a positive control.
SOD activity of SAE was evaluated on 96-well plates using a SOD Assay Kit-WST (Dojindo Molecular Technologies, Japan) following the procedure provided by the supplier.
Briefly, 20 μL of a sample solution and 200 μL of water-soluble tetrazolium salt working solution were mixed in the well. The reaction was initiated by the addition of 20 μL of enzyme working solution and the plate was incubated at 37 ℃ for 20 min[18]. The absorbance was measured at 450 nm using a microplate reader(BioTek, VT, USA). Trolox was used as a positive control.
2.5. Measurement of elastase activity assay
The inhibition of elastase activity was evaluated by the hydrolysis of N-succinyl-(L-Ala)3-p-nitroanilide under the specified conditions(at 25 ℃ and pH 8.0)[19].
The reaction mixture contained 0.2 M Tris-HCl buffer (pH 8.0),SAE samples (50, 100 and 500 μg/mL) or 500 μg/mL ursolic acid, 1 unit/mL elastase, and 1 mM N-succinyl-(L-Ala)3-p-nitroanilide. The reaction mixture was incubated for 20 min at 25 ℃, and absorbance was measured at 405 nm.
2.6. Cell culture and cell viability
HS68 human fibroblast cell lines were purchased from ATCC(Manassas, VA, USA). Dulbecco modified eagle medium used for cell culture was mixed with 10% fetal bovine serum and 1% penicillin/streptomycin. Cell incubation was performed in a 5% CO2incubator at 37 ℃. The cell viability was assessed using an MTT assay. HS68 cells were seeded in a 96-well plate at a density of 3 × 104cells/well and treated with various concentrations of SAE (0, 10, 25, 50,100 and 200 μg/mL) for 24 h. MTT media at 1 mg/mL were added into each well and incubated for 3 h at 37 ℃. The MTT formazan precipitate was then dissolved in 200 μL of dimethyl sulfoxide, and the absorbance was measured at 540 nm[20].
2.7. Quantification of MMP-1 activity
To examine inhibitory effects of SAE on MMP-1 activity, HS68 human fibroblast cells were cultured with SAE or adenosine for 24 h and then the supernatants were collected to quantify the level of MMP-1. SAE were treated at 25, 50, and 100 μg/mL concentrations and simultaneously treated with TNF-α (10 ng/mL) to induce MMP-1 expression.
MMP-1 activity was measured with an MMP-1 Human Biotrak ELISA System (Amersham Biosciences Corp, NJ). Activity assays were performed according to the manufacturer’s instructions and the absorbance was measured at 450 nm (BioTek, VT, USA)[21].
2.8. Measurement of MMPs mRNA expression
The mRNA was isolated with the easy-Blue Total RNA extraction kit. The concentration of RNA was determined using the ND-1000 Spectrophotometer (NanoDrop Technologies, DE). Total RNA was used in the existence of the TaqMan®RNA-to-CTTM1-Step Kit(Applied Biosystems) according to the manufacturer’s instructions.Analysis of relative gene expression was based on the△Ct method[22].
2.9. Statistical analysis
Data were expressed as mean ± SD. Significant differences were compared using one-way ANOVA followed by the Tukey multiple range test. Statistical significance was defined as P < 0.05. All statistical analyses were performed using GraphPad Software.
3. Results
3.1. Eugenol composition of SAE
Eugenol content of SAE quantitated by HPLC was found to be 37 mg/g. The chromatogram of eugenol was shown in Figure 1.
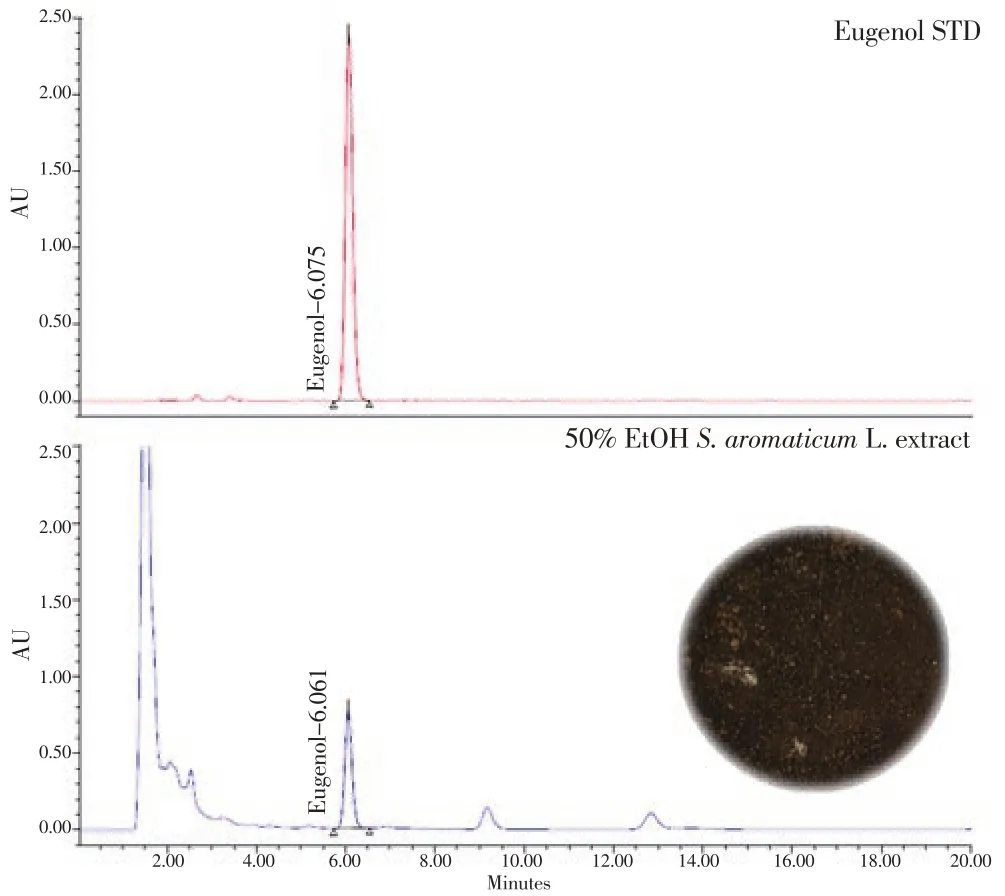
Figure 1. HPLC of eugenol in SAE at 260 nm.
3.2. Antioxidant activities
To determine the antioxidant effect of SAE, DPPH and superoxide radical scavenging activities of SAE were measured. The DPPH radical scavenging activities at 10 and 50 μg/mL were 51.0% and 95.3%, respecitvely (IC50= 16.1 μg/mL). DPPH scavenging activity of 50 μM vitamin C (a positive control) was 65.8% (Figure 2a). The superoxide radical scavenging activities of SAE at 10, 50 and 100 μg/mL were 35.8%, 77.6% and 90.3% respectively (IC50= 23.6 μg/mL),while that of 500 μM trolox (a positive control) was 58.4% (Figure 2b). Antioxidant activity of SAE was displayed in a dose-dependent manner.

Figure 2. Anti-oxidant activities of SAE determined by (a) DPPH radical scavenging activity and (b) superoxide radical scavenging activity.
3.3. Measurement of elastase inhibitory activity
The elastase inhibitory activity of SAE was shown in Figure 3.The elastase activities of SAE at 100 and 500 μg/mL were 9.5% and 10.8% each, while that of 500 μg/mL ursolic acid (a positive control)was 39.1% (Figure 3). The ability of SAE to inhibit elastase activity dose dependently has been observed, and it has shown significant effects.
3.4. Cell viability of HS68 cells after treatment of SAE
The cells were treated with various concentrations (0, 10, 25, 50,100 and 200 μg/mL) of SAE for 24 h. SAE did not induce any significant cytotoxicity up to 200 μg/mL concentration. Therefore,SAE did not show any effects on cell proliferation (Figure 4a).
3.5. Quantification of MMP-1
As a result, it was confirmed that the vehicle group and the TNF-α treated group produced 2.0 ng/mL and 60.4 ng/mL of MMP-1,respectively, and the amount of MMP-1 induced by TNF-α increased.MMP-1 inhibition rates of SAE were 55.3%, 76.3% and 85.0% at 25,50 and 100 μg/mL, respectively (IC50=24.9 μg/mL), when compared with the TNF-α treatment group (Figure 4b).

Figure 3. Inhibitory effects of SAE on elastase.

Figure 4. Effect of SAE on (a) cell viability and (b) MMP-1 content in HS68 human fibroblast cells.
3.6. Effects of SAE on MMPs mRNA levels
To determine whether SAE modulates skin aging-related factors at the molecular level, MMP-1, MMP-2, MMP-3 and MMP-9 mRNA levels were examined. The expressions of MMP-1, MMP-2,MMP-3, and MMP-9 increased after being treated with 10 ng/mL of TNF-α. The inhibition rates of MMP-1 expression by SAE were 59.7%, 79.6% and 80.7% at 25, 50 and 100 μg/mL respectively,when compared with the TNF-α treatment group. Inhibition rates of MMP-2 expression at 25, 50 and 100 μg/mL were 24.5%, 34.4% and 52.0%, respectively. Moreover, MMP-3 expression inhibition rates were 36.6%, 64.5% and 74.7% while MMP-9 expression inhibition rates were 57.4%, 86.6% and 93.7% at 25, 50 and 100 μg/mL,respectively (Figure 5).

Figure 5. Effects of SAE on mRNA expressions of (a) MMP-1, (b) MMP-2,(c) MMP-3 and (d) MMP-9.
4. Discussion
The skin consists of three layers, from the outside to the epidermis,dermis and subcutaneous fat layer. The dermis is located between the epidermis and the fat layer, and consists of matrix and connective tissue composed of fiber protein such as collagen and elastin[23,24].Collagen gives strength and tension to the skin, protects the skin,occupies more than 90% of the dermis, and 80% of the extracellular matrix. Collagen is composed of 80%-85% type-Ⅰ collagen and 15%-20% type-Ⅲ collagen and fibronectin. Elastin accounts for about 3%-4% of the extracellular matrix and maintains the elasticity of the skin[25,26].
Skin aging reduces the synthesis of collagen and elastin in the dermal fibroblast, collagen degradation by MMPs increases, the elasticity reduces, and wrinkles are generated. In addition, reduced collagen in skin that is continuously exposed to ROS and UVB is also an important cause of wrinkle formation[27]. To maintain the normal state of the human body, restraint of homeostasis and excessive stress is important. Antioxidant systems which protect the tissue cells from oxidative damage of lipid, protein and nucleic acid play a critical role.
These antioxidant systems are classified into two types, antioxidant enzymes and non-enzymatic antioxidants. Especially, catalase and SOD are typical examples of enzymatic defense mechanisms[28,29].DPPH radical scavenging ability and SOD-like activity, which can predict the inhibition of the initial reaction of lipid peroxidation,were measured in order to examine the antioxidant activity of SAE.The antioxidative effect of SAE showed a concentration-dependent increase which was higher than positive controls in experimental conditions. Several constituents of S. aromaticum L. have been identified, mainly eugenol, eugenol acetate, vanillin, eugenin,rhamnetin, kaempferol, α-humulene, β-caryophyllene, biflorin and caryophyllene[30,31]. Also some flavonoids including kaempferol,rhamnetin and β-caryophyllene were reported to have the antiinflammatory and antibacterial activity[32,33]. Antioxidant activity of S. aromaticum L. might be due to the higher concentration of phenolic compounds such as eugenol, eugenol acetate, β-caryophyllene and thymol[34,35]. A strong antioxidant effect of SAE is expected to protect the skin cells and maximize the wrinkle improvement effect.
Decreased elastin causes wrinkles and loss of elasticity as well as promotes skin aging. Elastase decomposes the elastin of the stratum corneum, reducing the elasticity of the skin and causing the skin to age. Elastase is a protease corresponding to MMP-12 in MMPs and acts specifically on the degradation of elastin. Substances that inhibit elastase activity have the effect of improving skin wrinkles[36]. The elastase inhibitory activity of SAE was lower than that of the positive control, but it still showed significant inhibitory activity.
Collagen is a major substrate protein produced in the fibroblasts of the skin and serves to protect the body from external physiological and chemical stimulation. As aging progresses, collagen production decreases and degradation increases, thus inducing depression of the dermal layer of the skin to produce wrinkles[37]. Procollagen contains a peptide sequence called propeptide at the amino terminus and carboxyl terminus. It is known to help the folding of procollagen molecules in the endoplasmic reticulum and to cleave and separate from the collagen molecules when the collagen polymerization takes place. Therefore, by measuring the amount of the separated propeptide, the degree of collagen biosynthesis in the cell can be determined[38]. Procollagen biosynthesis of SAE did not promote collagen synthesis (data not shown).
The type Ⅰ collagen occupies 80% of the total collagen. In normal skin, the synthesis of type Ⅰ collagen and its degrading enzyme,MMP-1, are balanced to maintain the elasticity of the skin. MMP-1 activity reduces collagen degradation, maintains elasticity of skin tissue and prevents wrinkle formation[39].
The amount of MMP-1 was confirmed by the treatment of SAE in fibroblast. As a result, the inhibitory effect of 55.3% and the significant difference were confirmed at 25 μg/mL used in the experiment. MMPs are endopeptidases that require zinc ions to participate in the degradation of all the intracellular matrix’s components. These MMPs are classified into 5 types according to their substrate specificity. MMP-1 is collagenase 1, which is involved in various body processes such as cell or tissue growth.MMP-3 is stromelysin 1, which degrades type Ⅳ collagen of basement membrane and activates pro-MMP-1, a zymogen. MMP-9 is gelatinase B, which hydrolyses products degraded by collagenase to a smaller extent[40-42]. This study found that mRNA expressions of MMP-1, MMP-2, MMP-3, and MMP-9 by SAE treatment were inhibited in a concentration-dependent manner.
This study also confirmed the antioxidant and wrinkle-improving effects of S. aromaticum L. Antioxidant activity, cell viability,elastase inhibitory activity, MMP-1 level and mRNA expressions of MMPs (MMP-1, MMP-2, MMP-3, MMP-9) were investigated in SAE. The antioxidant activity of SAE was confirmed by measuring DPPH and SOD-like activities. As a result, it increased in a concentration-dependent manner and showed a high antioxidative effect compared with the positive controls. SAE did not show any cytotoxicity of the cells at all concentrations tested.
SAE significantly decreased the amount of MMP-1 induced by TNF-αtreatment, confirming the MMP-1 inhibitory effect. Elastase inhibition activity of SAE also showed significant results. Besides,MMPs mRNA expressions were inhibited by SAE treatment.
These results suggest that the effect of improving wrinkles of SAE is due to the strong antioxidant effect and the inhibition of elastase and MMPs. Therefore, SAE can be considered to be highly useful as a wrinkle improving material.
Conflict of interest statement
The authors declare that there are no conflicts of interests regarding the publication of this article.
Funding
This paper was supported by Wonkwang University in 2018.
 Asian Pacific Journal of Tropical Biomedicine2019年2期
Asian Pacific Journal of Tropical Biomedicine2019年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Melissa officinalis and rosmarinic acid in management of memory functions and Alzheimer disease
- In vitro evaluation of anti-acetylcholinesterase and free radical scavenging potential of leaf extracts of some selected medicinal plants
- Fruiting increases total content of flavonoids and antiproliferative effects of Cereus jamacaru D.C. cladodes in sarcoma 180 cells in vitro
- In vivo hypoglycemic investigation, antihyperglycemic and antihyperlipidemic potentials of Pereskia bleo Kunth. in normal and streptozotocin-induced diabetic rats
- Effects of Brassica oleracea extract on impaired glucose and lipid homeostasis in highfat diet-induced obese mice
- Significance of IL-1Ra and IL-6 gene variants in Turkish patients with Crimean-Congo hemorrhagic fever
