Carboxylesterases mediated herb-drug interactions:a systematic review
Dan-Dan Wang,Yun-Qing Song,Ya-Di Zhu,Yi-Nan Wang,Hai-Feng Li,Guang-Bo Ge*,Ling Yang
1Institute of Interdisciplinary Integrative Medicine Research,Shanghai University of Traditional Chinese Medicine,Shanghai,China.2School of basic medical science,Shanghai University of Traditional Chinese Medicine,Shanghai,China.
Background
Drug metabolizing enzymes(DMEs)play a pivotal role in the metabolic clearance of drugs or other xenobiotic compounds by converting lipophilic molecules to more water-soluble metabolites,which can be readily excreted through the kidney or biliary clearance.Inhibition or induction of DMEs may affect the pharmacokinetic properties of therapeutic drugs and thus trigger clinical relevant drug/herb-drug interactions(DDIs or HDIs)[1-4].The regulatory agencies,such as the US Food and Drug Administration(FDA)and the European Medicines Agency(EMA),have issued guidelines for industry on evaluation the inhibition potentialsofdrugsunder development on the key human DMEs prior to approval[5,6].Drug metabolism is divided into phase I and phase II reactions.In phase I reactions,polar groups are introduced to the molecules through oxidation,reduction,and hydrolysis.In phase II reactions,phase I metabolites or the parental compounds themselves undergo conjugation reactions with hydrophilic moieties including glucuronic acid,sulfate,glutathione,or amino acids.Among all known DMEs involved in phase I reactions,cytochrome P450 enzymes(CYPs)play a crucial role in drug metabolism, followed by esterases, which contributed to the metabolism of~10%of the clinical drugs that contain ester or amide bonds.Over the past decade,CYPs mediated DDIs or HDIs have been well-summarized in several reviews,but the esterases mediated drug/herb-drug interactions have not been reviewed in depth[5].
Esterases belong to serine hydrolase enzyme family,which share a conserved catalytic mechanism that enlists a key serine nucleophile within a catalytic triad.As their name implies,esterases catalyse the hydrolysis of numerous compounds with ester/amide bonds into the corresponding alcohol and carboxylic acid,and thus play crucial roles in a wide range of physiological and pathological processes,such as xenobiotic metabolism,lipid homeostasis,cancer,diabetes and obesity[7,8].In mammals,carboxylesterases(CEs)are the most abundant esterases in the metabolic organ(such as liver,intestine and kidney),which play a pivotal role in hydrolysis of a variety of endogenous and xenobiotic esters and have been extensively studied over the past decade[9].In the human body,human carboxylesterase 1(hCE1)and human carboxylesterase 2(hCE2)are two key mediators responsible for the hydrolytic metabolism of various ester xenobiotics including ester drugs(such as oseltamivir,clopidogrel, irinotecan and capecitabine) and environmental toxicants(such as pyrethroids)[9,10].Human CE1 and human CE2 share 47%amino acid sequence identity,butthese two enzymes exhibit extremely different substrate distribution and specificity.Generally,hCE1 is abundant expressed in the human hepatocytes and adipocytes,with lesser amounts in the kidney,monocytes,lung,intestine,testis,heart,and macrophages.By contrast,hCE2 is expressed mainly in the small intestine and colon,and also detectable in kidney,liver,heart,brain and testis.Human CE1 and CE2 also exhibit distinct substrate specificities.Generally,hCE1 prefers to hydrolyze the ester substrates with a small alcoholic group and a large,bulky acyl group,such as enalapril, oseltamivir, imidapril, clopidogrel,meperidine,D-luciferin methyl ester,and the illegal drugs heroin and cocaine[9].By contrast,CE2 prefers to hydrolyse esters with a relatively large alcohol group and a smallacyl group,such asirinotecan,prasugrel,capecitabine,flutamide,and fluorescein diacetate[8].
Inhibition on hCEs may slow down the hydrolysis of hCEs substrate drugs in vivo,and thus modulate their pharmacological and toxicological effects.For instance,clopidogrel,one ofthe mostfrequently prescribed antiplatelet agent,the majority of which can be rapidly hydrolyzed to an inactive metabolite by hepatic hCE1,only a small proportion of which can be activated by CYPs to form 2-oxo-clopidogrel,followed by conversion to the active metabolite[11-14].Co-administration with hCE1 inhibitors may partially block the hydrolytic pathway of clopidogrel,while the formation rates of the active metabolite via CYP-mediated bioactivation will be increased, which may increase the exposure to clopidogrel active metabolite and enhance its antiplatelet effects.Furthermore,irinotecan,a hCE2 substrate drug,could triggersevere delayed diarrhea due to the overproduction of SN-38(the hydrolytic metabolite of irinotecan)in the small intestine,co-administration with potent hCE2 inhibitors may ameliorate CPT-11 associated life-threatening diarrhea in patients and thus improve the patient’s quality of life[15-18].With this goal in mind,many hCE2 inhibitors have been developed for alleviating irinotecan-induced toxicity or prolonging the half-lives of hCE2 substrate drugs.
The key roles of CEs in both human health and xenobioticmetabolism arousegreatinterestin the discovery of CEs inhibitors to modulate endogenous metabolism or to improve the outcomes of patients administrated ester drugs,as well as to avoid potential risks of DDIs or HDIs.Over the past decade,a panel of isoform-specificopticalprobesubstrates havebeen developed,which strongly facilitated high-throughput screening and characterization of CEs modulators and the investigations on hCEs associated DDIs or HDIs[19-22].With the help of these newly developed optical probe substrates,the inhibitory effects of herbal extracts and their constituents on hCEs have been well-investigated[9].Considering that herbal medicines are widely used in Asia countries for the treatment of various diseases in clinic,it is necessary to investigate the metabolic interactions of herbal constitutes with hCEs before combination use of herbal medicines and clinical drugs.With the intention of improving the reader’s knowledge of the HDIs associated with hCEs,the roles of hCEs in drug disposition,the inhibitory effects of herbal medicines,the inhibition potentials and action mechanism of herbal constitutes against hCEs have been well-summarized in this review.All information and knowledge presented in this review will be very helpful for the deep understanding the interactions between herbal constituents and hCEs,as well as for clinical clinicians to reasonable use herbalmedicines for alleviating hCEs-associated drug toxicity or avoiding the occurrence of clinically relevant hCEs-mediated HDIs.
Human CEs substrate drugs
Human CEs are key enzymes from the serine hydrolase superfamily,which efficiently catalyze the hydrolysis of a variety of ester/amide-containing pharmaceutical products[23-25].It is widely recognized that the function of hCEs can influence drug metabolism and clinical outcomes.In this review,we outline the known substrate drugs of hCE1 and hCE2,and highlight the relevance of hCEs functions to contemporary pharmacotherapy[26,27].
As one ofthe mostimportantphase Idrug metabolizing enzymes, hCE1 involved in toxin detoxication and drug metabolism(Table 1).On one hand,hCE1 mediatesthe metabolic activation ofmany prodrugs(such as temocapril,oseltamivil,and sacubitril etc.)[27].On the other hand,hCE1 promotes the metabolic inactivation and clearance of some esterified drugs(such as clopidogrel,methylphenidate,and cocaine etc.).Recent study reported that a new class of promising anticancer compounds, phospho-nonsteroidal anti-inflammatory drugs(phospo-NSAIDs),arealso inactivated by hCE1 and hCE1 inhibitors will improve the efficacy of these phospho-NSAIDs both in vitro and in vivo.As for hCE2,it has been reported responsible for the activation of several anti-tumor prodrug,for instance CPT-11 and LY2334737(Table 1)[28].Actually,many factors including drugs,genetic factors,and disease status,have been reported can cause individuals and tissues differences in both expression and function of hCE1 and hCE2,and further influence the clinical outcomes of hCEs substrate drugs[29].
Genetic factor was one of the extensive studied factors affecting the clinical outcomes of CEs substrate drugs[44,45].Over the pastdecade,a vastnumber of single-nucleotidepolymorphisms(SNPs)have been reported in the NCBI SNP database.Notablely,the allele and haplotype frequencies of known SNPs showed significant differences among different ethnic groups.For instance,the D260fs and the G143E variants were two important functional SNPs in Caucasian populations,while these two CES1 genetic polymorphisms were not found in a Korean population.Up to now,many functional genetic variants of CES1 and CES2 have been reported,which may be associated with the individual difference in the responses to contemporary pharmacotherapy[10,46-49].Clopidogrel is a prodrug which has been widely used to inhibit platelet aggregation.Following oraladministration,more than 85% ofclopidogrel can be rapidly hydrolyzed to its carboxylic acid(an inactive metabolite)by hCE1.Zhu et al.Reported that the CES1 variants G143E and D260fs diminished thehCE1 activity,which impaired the metabolism of clopidogrel[46][10].Aspirin is an antiplatelet agent that frequently used for the prevention of cerebrovascular and cardiovascular events.Aspirin is also a CEs substrate drug which is mainly hydrolyzed by gastrointestinalCE2 to form itsactive hydrolytic metabolite.Tang et al.reported that the CES2 variant A139T decreased human CES2 activity and thus decreased aspirin hydrolysis [46].The association between SNPs in the human CES2 gene and CPT-11 hydrolysis has also been reported[48,50].Among Japanese volunteers,the CES2 variants rs72547531 and rs72547532 were associated with decreased human CE2 activity and reduced CPT-11 hydrolysis activity in vivo.[48]Moreover,disease statusalso can affectthe expression or function of CEs and drug response.Xu et al collected and analyzed 18 types of tumors,found 2 types(gallbladder tumor and lymphoma)did not express hCE2,5 types expressed weak hCE2,and 11 types expressed moderate to high hCE2 levels.Moreover,CE2 protein was highly variable among liver samples,with a 15-fold range in cytosol and a 3-fold range in microsome fractions.More importantly,liver microsomal hCE2 protein expression was significantly correlated with irinotecan activation to SN-38[51].LY2334737 is an oral prodrug of the clinically efficacious anticancer agent,gemcitabine. The hydrolysis of LY2334737 to gemcitabine is mediated by hCE2.Recent study exhibited the cellular hCE2 expression confers prodrug sensitivity[43].Since these two enzymes play crucial roles in the hydrolysisofa variety ofendogenousestersand ester-containing drugs,the strong inhibition on human CEs may slow down the hydrolysis of CEs substrates,which may affect their pharmacokinetic properties and thus trigger potential drug/herb-drug interactions.
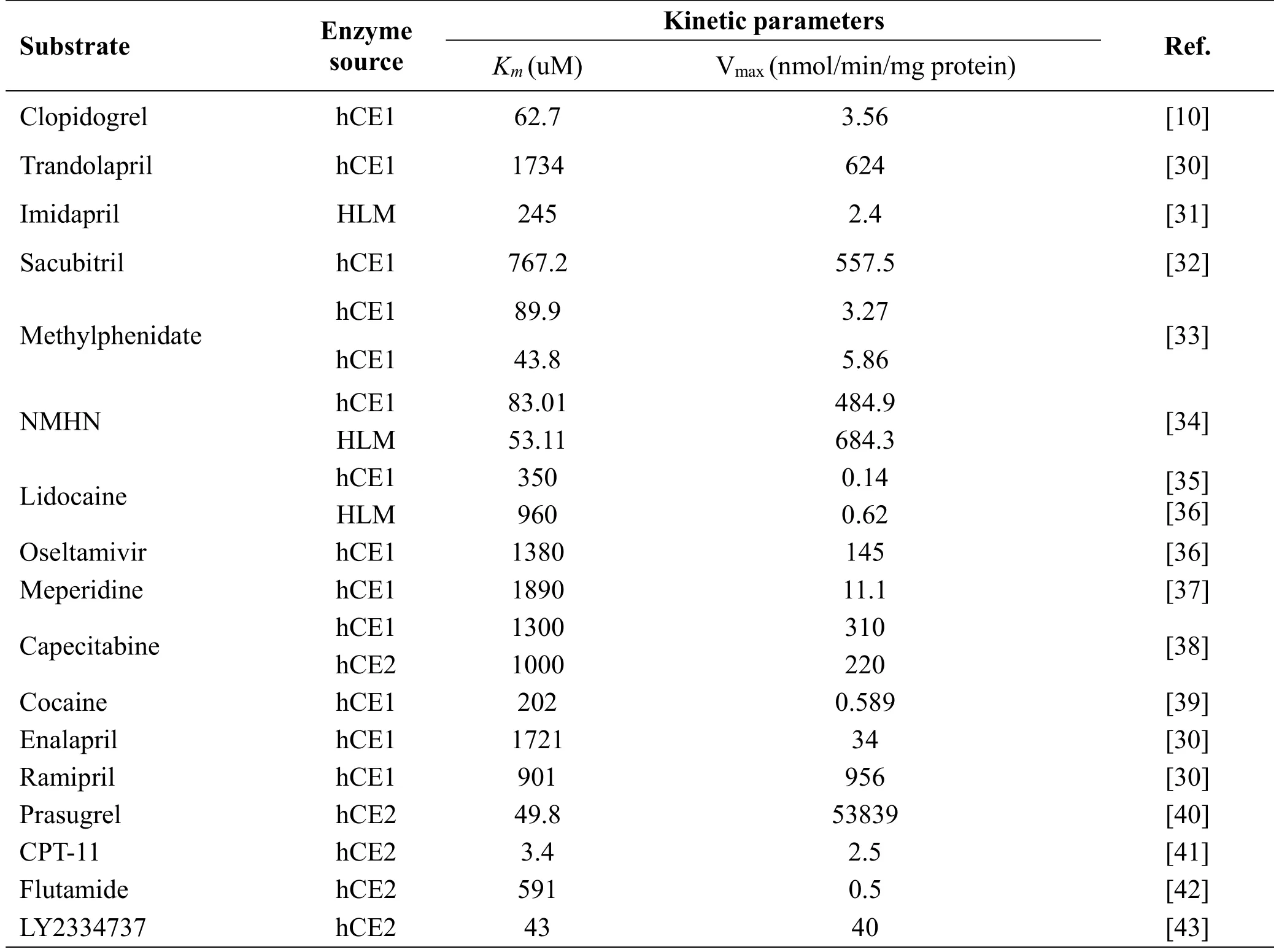
Table 1 The list of CEs substrate drugs
CEs mediated herb-drug interactions
As one important class of phase I drug metabolizing enzymes,hCEs play key role in toxin detoxication and drug metabolism.Since the catalytic activity of CEs has been reported to affect the efficacy and clinical outcomes of numerous esterified drugs,potent inhibition of the hCEs by herb ingredients may result in herb-drug interactions.Thus,the reported herb extracts or herbal constitutes that display potent inhibition towards CEs are summarized and discussed in the following section.
Herbal extracts with CEs inhibition activity
A number of studies have investigated the inhibitory effects of herb extracts on hCEs activity.The herbal extracts displaying inhibitory effects on hCEs are listed in Table 2.White Mulberry Root-bark(WMR)is an edible Chinese herbal used for the treatment of inflammation,nephritis,and asthma.The ethanolic extract from WMR displayed strong inhibitory effects against hCE2 and the IC50 value 30.32 μg/mL[52].The crude extract of Fructus Psoraleae(FP)also showed significant inhibitory effect towards hCE2-mediated FD hydrolysis,and the catalytic activity of hCE2 could be completely inhibited at a concentration of 12 μg/mL while the ethanol extract of FP displayed relatively weak inhibitory effects towards hCE1 at the same dose.The inhibitory effects on hCE2 by different extracts of Salvia miltiorrhiza(“Danshen”)prepared using hot water,acetone,or 56%ethanol.As summarized in Table 2,organic solvent extracts of“Danshen” roots exhibited the strongestinhibitory towards hCE2 with the IC50 value determined as low as 160 ng/ml[53],suggesting that potent hCE2 inhibitors are present within the acetone or ethanolic“Danshen root”extracts.It is worth note that the acetone extract of“Danshen root”,were capable to reduce the sensitivity of U373G cells expressing hCE2 to irinotecan,suggesting that the hCE2 inhibitors from “Danshen root”are cell permeable and may modulate SN-38 production in vivo.Another study found that St John’s wort,black cohosh and ginger root extract could potentially inhibit CEs mediated biotransformation of irinotecan.As shown in Table 2,the inhibition ability of these herbal extracts was ranked as black cohosh>Ginger>St John’s wort[54].Furthermore,Li et al has systematically collected and evaluated the inhibitory effects of 100 herbal extracts on hCE2 using FD as a probe substrate(Table 3),which provide important information for the further study on the herbal constitutes with hCEs inhibition activity[55].
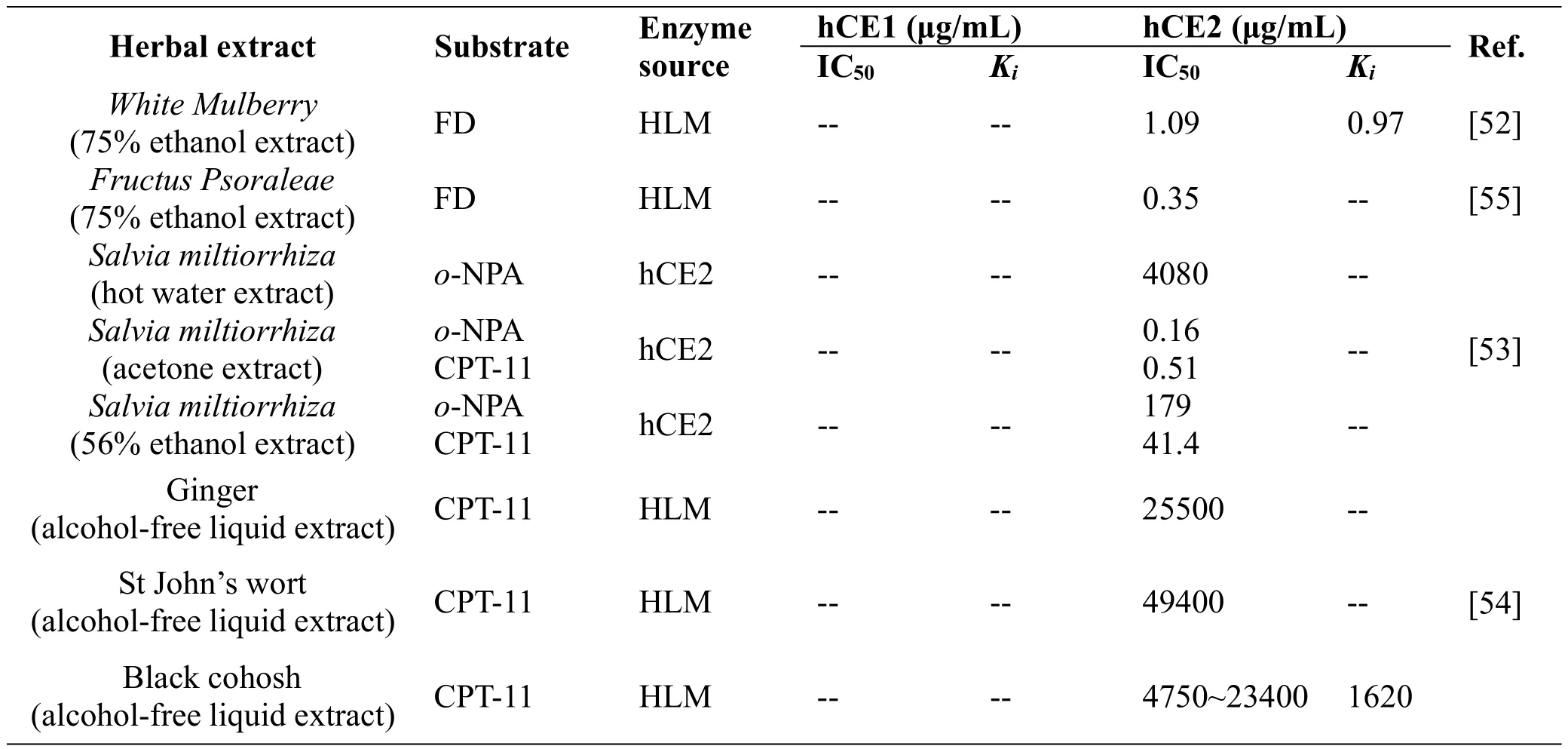
Table 2 The inhibitory effects of herbal extracts and inhibitory parameters on CEs
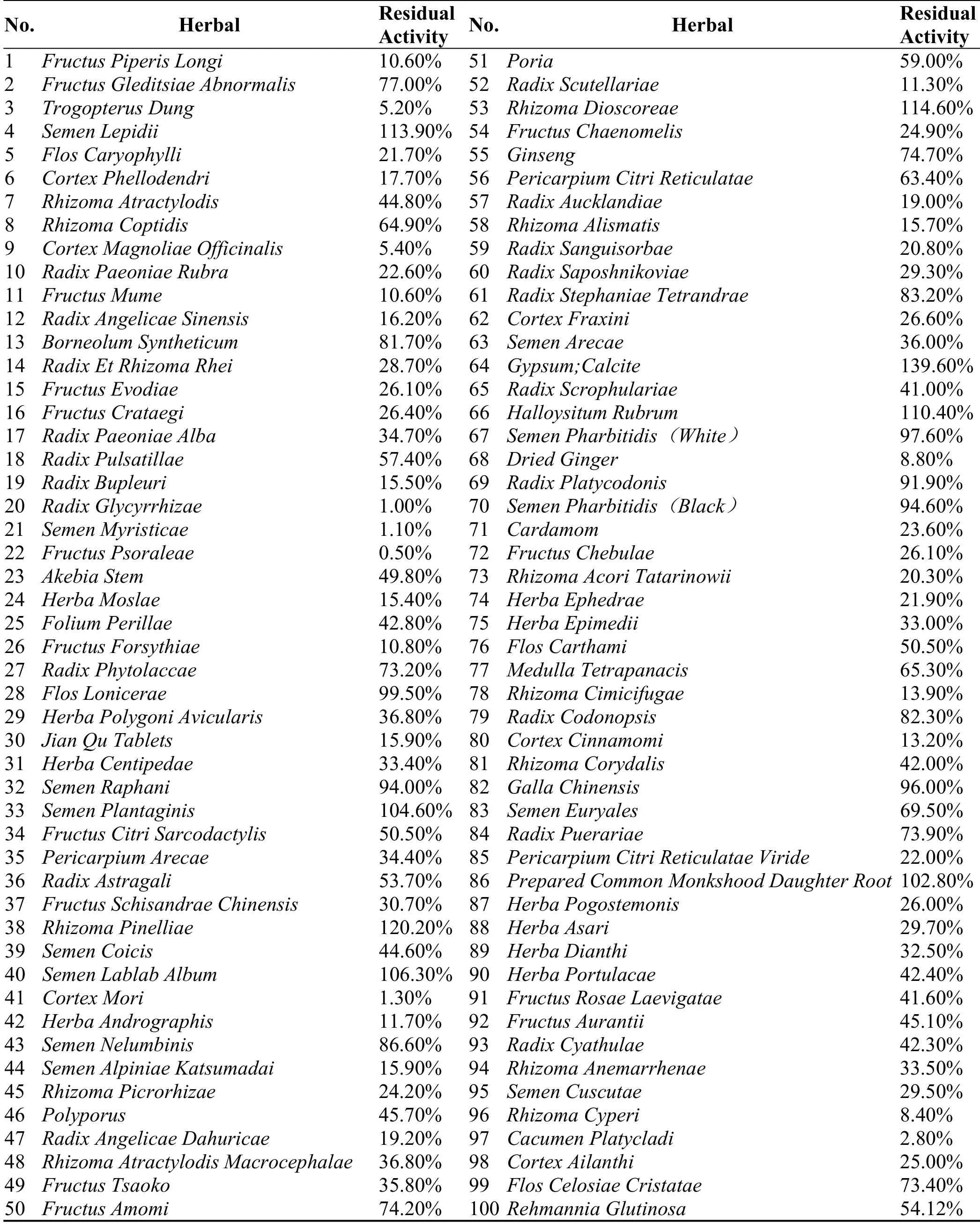
Table 3 Preliminary inhibition screening of herbal extracts(75%ethanol extracts)towards hCE2
Inhibition of herbal constitutes on human CEs
FlavonoidsFlavonoids are polyphenolic compounds that widely distributed in vegetables,fruits,and beverages,such as tea and wine,which fulfilling pharmacological properties.Recent studies have demonstrated that some natural flavonoids, including 5,6-dihydroxyflavone,hispidulin, eupatilin, isorhamnetin and apigenin 7-O-methyl ether,are strong inhibitors against hCE2[56],while nevadensin,an abundant natural constitute from Lysionotus pauciflorus Maxim.,is a relative specific inhibitor of hCE1[57].Sun et al have found the major ingredients of FP, including neobavaisoflavone,corylifolinin, coryfolin, psoralidin, corylin, and bavachinin showed strong inhibition towards the activity of hCE1 in a dose-dependent manner[58].Li et al have reported that the major constitutes in Fructus Psoraleae,including neobavaisoflavone, isobavachalcone,bavachinin,corylifol A,and bakuchiol can potently inhibit hCE2-mediated FD hydrolysis in HLM[55].Both Lineweaver–Burk and Dixon plots demonstrated that these five natural flavonoids against hCE2 in HLM functioned as non-competitive inhibitor against hCE2-mediated FD hydrolysis in HLM,with the Kivalues evaluated as 3.89 μM,1.64 μM,1.12 μM,0.62 μM,and 2.12 μM,respectively.Liu et al have identified and characterized the major flavonoids in White Mulberry Root-bark are naturally occurring hCE2 inhibitors,using chemical fingerprinting analysis combined with hCE2 inhibition assays[52].On the basis of LC retention times,UV and MS spectral data,three major constitutes in White Mulberry Root-bark are efficiently identified as SD(sanggenone D),KG(kuwanon G)and SC(sanggenone C).The IC50values of SD,KG and SC against CE2 in HLM were evaluated as 1.09 μM,1.14 μM,and 1.02 μM,respectively[52].These findings are very helpful for the medicinal chemists to design and develop more potent and highly selective flavonoid-type hCE2 inhibitors[64].
TriterpenoidsTriterpenoids are a diverse group of natural products with wide distribution,high chemical diversity and important pharmacological properties.Zou et al collected a series of natural triterpenoids and tested their inhibitory effects against CEs using D-Luciferin methyl ester (DME), and 6,8-dichloro-9,9-dimethyl-7-oxo-7,9-dihydroacridin-2-yl benzoate(DDAB)as specific optical substrate for hCE1,and hCE2,respectively.Following screening of these natural triterpenoids,oleanolic acid(OA),and ursolic acid(UA)were found with strong inhibitory effects on hCE1 while showed weak inhibitory effects on hCE2[59].Twelve new and ten known protostane triterpenoids were isolated from the rhizome of Alismaorientale,while four of them(Alismanol B,25-O-Ethylalisol A,Alismanol D,Alismanol F)showed moderate inhibitory activities and were selective toward hCE2 enzymes,with IC50values of 8.68,4.72,4.58,and 2.02 μM,respectively [65].Moreover,the inhibition kinetics of Alismanol F toward hCE2-mediated 4-benzoyl-N-butyl-1,8-naphthalimide(MPN)hydrolysis were established,and the Kivalue was determined as low as 1.76 μM using a mixed inhibition model.
Fatty acidsFatty acids are present in many herbal extracts.A recent work reported the inhibition of hCEs activity using THP1 monocytes/macrophages and hCEs by fatty acids.Crow et al.found that most naturally occurring fatty acids strongly inhibited the hydrolytic activities of hCE1,with the IC50values within the micromolar range,and unsaturated fatty acids displayed better inhibitory effects on hCE1 than saturated ones,but they did not display strong inhibition towards hCE2(Table 4).Among these fatty acids tested,5Z,8Z,11Z,14Z-Eicosatetraenoic acid(arachidonic acid,C20:4 ω6)showed the strongest inhibitory effects toward hCE1,with IC50value 2µM[60].
OthersBesides the above mentioned compounds,other compounds with carboxylesterase inhibition capacity have also been reported.Wang et.al obtained phenolic glycosidesand monoterpenoidsfrom the rootsof Euphorbia ebracteolata,allofthem showed the inhibitory effect against hCE2 by MPN-based fluorescence bioassay in vitro,with the strongest inhibitor scopoletin-7-O-β-d-(6’-galloyl)-glucopyranoside (IC507.17 μM)[61].Shikonin,a natural naphthoquinone compound derived from the herb Lithospermumerythrorhizon,is widely used for its various pharmacological activities.A recent study exhibits that shikonin significantly inhibits the activity of CE2 when FD and NCEN are used as substrates[62].A chemical investigation of the roots of Euphorbia ebracteolata identified eighteen diterpenoids and glycosides and most of them showed moderate inhibitory effects against hCE2[63].Recent studies showed that some tanshinones are potent hCEs inhibitors toward both hCE1 and hCE2 in vitro,such as tanshinone IIA and tanshinone I.Meanwhile,their ability to effect intracellular inhibition ofhCE2wasassayedusing4-methylumbelifferone acetate(4-MUA)as a substrate.By using cells expressing hCE2,tanshinone IIA and tanshinone I were proved could reduce the sensitivity of cells to CPT-11,due to reducing the production of SN-38 [53].A recentwork demonstrated that tanshinone IIA, tanshinone I,dihydrotanshinone and cryptotanshinone were all irreversible inhibition of hCEs,and can inactivate human CEs both in vitro and in cell culture systems and can modulate the metabolism of the esterified drug oseltamivir[64].
Conclusion and future perspectives
Over the past decade,the key roles of hCEs in hydrolysis of a variety of endogenous and xenobiotic esters have been well-investigated.Considering that the crucial roles of hCEs in both endogenous and xenobiotic metabolism,it is necessary to evaluate the regulatory effects of clinical drugs and herbal medicines on hCEs,and to predict the potential beneficial or undesirable effects of hCEs-associated herb-endobiotic interactions or herb-drug interactions(HDIs).Over the past ten years,the biochemists have made significant breakthrough on the developmentofpracticaland specific opticalsubstrates for sensing hCE1 or hCE2 in complicated biological systems[66-69],which strongly facilitate high-throughput screening and characterization of hCE1 modulators(such as inhibitors,inactivators,simulators and inducers) and further investigations on hCEs-associated HDIs.With these probe substrates in hands,the inhibition or induction assays of herbal extracts orherbalconstitutes on hCEs in tissue preparations or living systems can be conducted in a more convenient and efficient way.Up to now,a variety of herbal extracts and herbal constitutes have been found with hCEs inhibition activity.However,most of previous investigations on hCEs inhibition were conducted in liver microsomes,and the capability of all reported herbal constitutes targeting intracellular hCEs and their potency against hCEs in living systems have not been well investigated.Thus,it is urgent necessary to construct more practical methods for screening and characterization the inhibitory effects of herbal constitutes targeting intracellular hCEs in living systems or in vivo[70].For those herbal extracts with strong hCEs inhibition activity,it is necessary to further identify the major natural inhibitors from herbs. In these cases, chemical fingerprinting analysis should be used in combination with fluorescence-based inhibition assays,such strategy has been successfully used to identity and characterize the naturally occurring inhibitors of hCE2 in several herbal medicines[55].Furthermore,to betterpredictthe clinically relevant hCEs-associated HDIs,it is very necessary to conduct in vitro-in vivo extrapolation(IVIVE)using reliable data about both human beings and hCEs inhibitors,including the physiological parameters of the particular patients,the pharmacokinetic data and inhibition constants of major hCEs inhibitors in human tissues.Taken together,current available data call for more in-depth studies on hCEs-associated herb-endobiotic interactions or herb-drug interactions(HDIs),such as the biological functions of hCEs in endogenous metabolism,the relevance of hCEs to human diseases,the response of hCEs inhibitors on mammalian CEs from various species,as well as the interactions between hCEs and their ligands.All these studies will be very helpful for further investigations on hCEs-associated HDIs and the possible consequences.
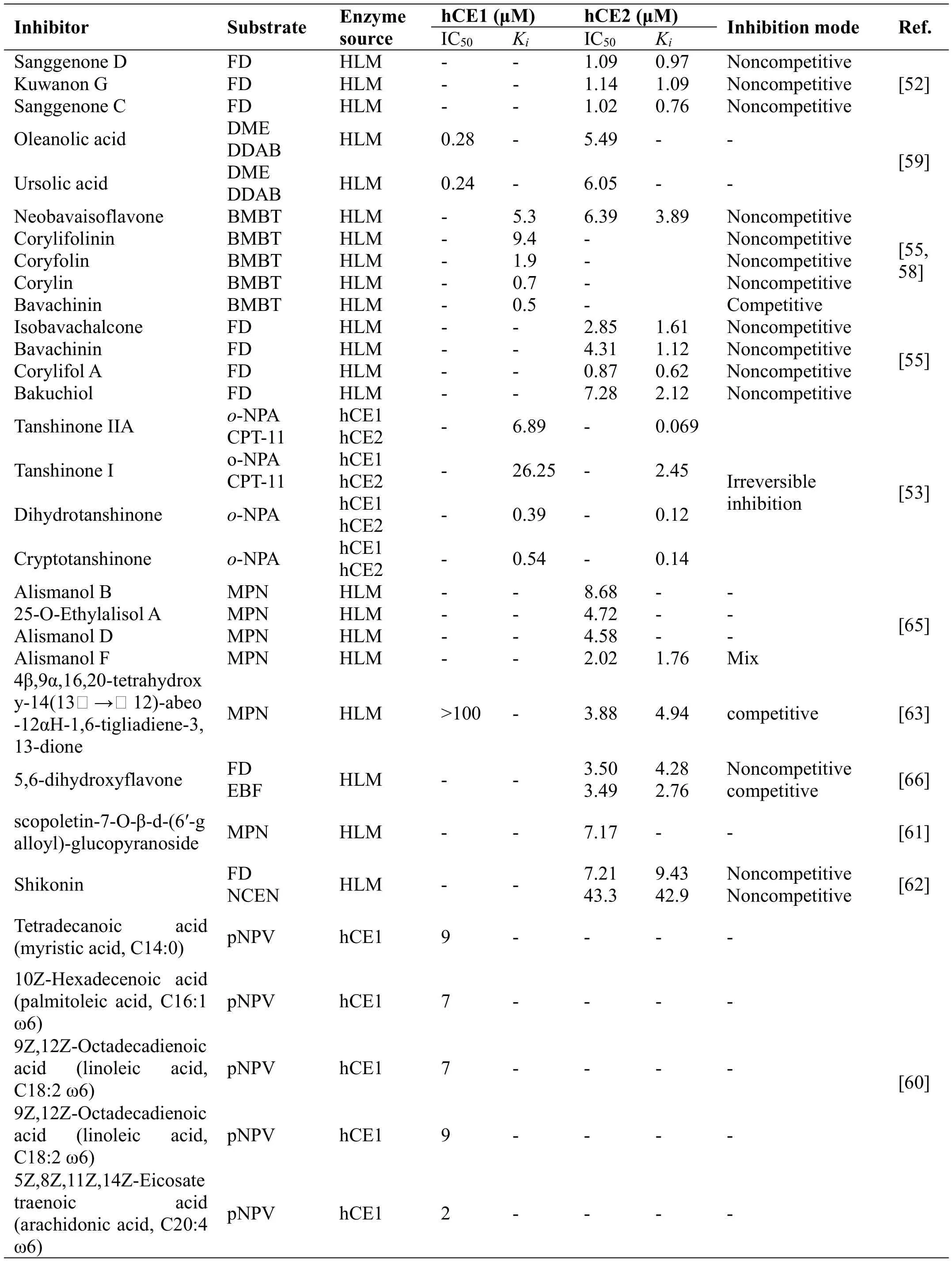
Table 4 The inhibitory effects of herbal constitutes on hCEs
1. Fang ZZ,Zhang YY,Wang XL,et al.Bioactivation of herbal constituents:simple alerts in the complex system.Expert Opin.Drug Metab.Toxicol.2011;7:989-1007.
2. Hanlon JT,Sloane RJ,Pieper CF,et al.Adverse Drug Reactions(ADRs)are Associated with both Drug-Drug and Drug-Disease Interactions in Frail Elderly Outpatients.,J Am Geriatr Soc2010;58:166-166.
3. Hu ZP,Yang XX,Ho PCL,et al.Herb-drug interactions-A literature review,Drugs 2005;65:1239-1282.
4. Izzo AA.Herb-drug interactions:an overview of the clinicalevidence,Fund Clin Pharmacol2005;19:1-16.
5. Schreck I,Yasuda S,Beck S,et al.Assessment of Cyp450 Enzyme Induction in Fresh Human Hepatocytes:Comparing Fda and Ema Ddi Guidelines,Drug Metab Rev 2015;47:127-128.
6. Barberan O,Ijjaali I,Dubus E,,et al.Prediction of inhibition-based drug-drug interactions using aurscope ADME/DDI(R)knowledge base from in vitro andinvivodata.CasestudyonFDA recommended in vivo probe substrates,Drug Metab Rev 2006;38:79-80.
7. Fu SN,Yang L,LiP,etal.Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity,Nature 2011;473:528-531.
8. Dominguez E,Galmozzi A,Chang JW,et al.Integrated phenotypic and activity-based profiling links Ces3 to obesity and diabetes,Nat Chem Biol 2014;10:113-121.
9. WangDD,ZouLW,JinQ,etal.Human carboxylesterases:a comprehensive review.Acta Pharmaceutica Sinica B 2018,8(5):699-712.
10.Zhu HJ,Wang XW,GawronskiBE,etal.Carboxylesterase 1 as a Determinant of Clopidogrel Metabolism and Activation, Journal of Pharmacology and Experimental Therapeutics 2013;344:665-672.
11.Neuvonen M,Tarkiainen EK,Tornio A,et al.Effects of Genetic Variants on Carboxylesterase 1 Gene Expression,and Clopidogrel Pharmacokinetics and Antiplatelet Effects,Basic Clin Pharmacol 2018;122:341-345.
12.Shao H,Lu J,Xu YT,et al.Metabolic Interaction Potential between Clopidogrel and Sulfonylurea Antidiabetic Agents: Effects on Clopidogrel Bioactivation,Pharmacology 2016;97:18-24.
13.Zou JJ,Ding L,Tan J,et al.Pharmacokinetics of clopidogrel in healthy Chinese volunteers,Pharmazie 2012;67:792-794.
14.Zhu YQ,Zhou J.Identification of the Significant Involvement and Mechanistic Role of CYP3A4/5 in Clopidogrel Bioactivation,Acs Med Chem Lett 2012;3:844-849.
15.Lokiec F,Canal P,MathieuBoue A,et al.CPT-11 metabolism in blood,bile and urine in cancer patients,Eur J Cancer 1995;31A:947-947.
16.Yano H,Kayukawa S,Iida S,et al.Overexpression of carboxylesterase-2 results in enhanced efficacy of topoisomerase I inhibitor,irinotecan(CPT-11),for multiple myeloma,Cancer Sci 2008;99:2309-2314.
17.Wierdl M,Tsurkan L,Hyatt JL,et al.An improved human carboxylesterase for enzyme/prodrug therapy with CPT-11,Cancer Gene Ther 2008;15:183-192.
18.Tobin PJ,Seale P,Lee S,et al.The in vitro metabolism of irinotecan (CPT-11) by carboxylesterase and beta-glucuronidase in human colorectal tumours., J Clin Oncol 2005;23:283s-283s.
19.Wang DD,Jin Q,Zou LW,et al.A bioluminescent sensor for highly selective and sensitive detection of human carboxylesterase 1 in complex biological samples,Chem Commun 2016;52:3183-3186.
20.Feng L,Liu ZM,Xu L,et al.A highly selective long-wavelength fluorescent probe for the detection of human carboxylesterase 2 and its biomedical applications, Chem Commun 2014;50:14519-14522.
21.Feng L,Liu ZM,Hou J,et al.A highly selective fluorescent ESIPT probe for the detection of Human carboxylesterase 2 and its biological applications,Biosens Bioelectron 2015;65:9-15.
22.Jin Q,Feng L,Wang DD,et al.A Two-Photon Ratiometric Fluorescent Probe for Imaging Carboxylesterase 2 in Living Cells and Tissues,Acs Appl Mater Inter 2015;7:28474-28481.
23.Potter PM,Wolverton JS,Morton CL,et al.Cellular localization domains of a rabbit and a human carboxylesterase:Influence on irinotecan(CPT-11)metabolism by the rabbit enzyme,Cancer Res 1998;58:3627-3632.
24.Sanghani SP,Sanghani PC,Schiel MA,et al.Human Carboxylesterases:An Update on CES1,CES2 and CES3,Protein Peptide Lett 2009;16:1207-1214.
25.Satoh T,Hosokawa M.Structure,function and regulation of carboxylesterases,Chem-Biol Interact 2006;162:195-211.
26.Ross MK,Crow JA.Human Carboxylesterases and their role in xenobiotic and endobiotic metabolism,J Biochem Mol Toxic 2007;21:187-196.
27.Hosokawa M.Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs,Molecules 2008;13:412-431.
28.ImaiT,Ohura K.The Role of Intestinal Carboxylesterase in the Oral Absorption of Prodrugs,Curr Drug Metab 2010;11:793-805.
29.Xu YJ,Zhang CL,He WX,et al.Regulations of Xenobiotics and Endobiotics on Carboxylesterases:A Comprehensive Review,Eur J Drug Metab Ph 2016;41:321-330.
30.Thomsen R,Rasmussen HB,Linnet K.In Vitro Drug Metabolism by Human Carboxylesterase 1 with Focus onAngiotensin-Converting Enzyme Inhibitors,Drug Metab Rev 2014;45:192-193.
31.Takahashi S,Katoh M,Saitoh T,et al.Allosteric Kinetics of Human Carboxylesterase 1:Species Differences and Interindividual Variability,J Pharm Sci-Us 2008;97:5434-5445.
32.Shi J,Wang XW,Nguyen J,et al.Sacubitril is selectively activated by carboxylesterase 1(CES1)in the liver and the activation is affected by CES1 genetic variation,Faseb Journal 2016;30.
33.Sun ZJ, Murry DJ, Sanghani SP, et al.Methylphenidate is stereoselectively hydrolyzed by human carboxylesterase CES1A1,J Pharmacol Exp Ther 2004;310:469-476.
34.Lv X,Wang DD,Feng L,et al.A highly selective marker reaction for measuring the activity of human carboxylesterase 1 in complex biological samples,RSC Adv 2016;6:4302-4309.
35.Higuchi R,Fukami T,Nakajima M,et al.Prilocaineand Lidocaine-Induced Methemoglobinemia Is Caused by Human Carboxylesterase-,CYP2E1-,and CYP3A4-Mediated Metabolic Activation,Drug Metabolism and Disposition 2013;41:1220-1230.
36.Parker RB,Hu ZY,Meibohm B,et al.Effects of Alcohol on Human Carboxylesterase Drug Metabolism,Clin Pharmacokinet 2015;54:627-638.
37.Zhang J,Burnell JC,Dumaual N,et al.Binding and hydrolysis of meperidine by human liver carboxylesterase hCE-1,Journal of Pharmacology and Experimental Therapeutics 1999;290:314-318.
38.Quinney SK,SanghaniSP,Davis WI,etal.Hydrolysis of capecitabine to 5'-deoxy-5-fluorocytidine by human carboxylesterases and inhibition by loperamide,The Journal of pharmacology and experimental therapeutics 2005;313:1011-1016.
39.Hatfield MJ,Tsurkan L,Hyatt JL,et al.Biochemical and molecular analysis of carboxylesterase-mediated hydrolysis of cocaine and heroin,Brit J Pharmacol 2010;160:1916-1928.
40.Williams ET,Jones KO,Ponsler GD,et al.The biotransformation of prasugrel,a new thienopyridine prodrug,by the human carboxylesterases 1 and 2,Drug Metab Dispos 2008;36:1227-1232.
41.Fukami T,Takahashi S,Nakagawa N,et al.In Vitro Evaluation of Inhibitory Effects of Antidiabetic and Antihyperlipidemic Drugs on Human Carboxylesterase Activities,Drug Metabolism and Disposition 2010;38:2173-2178.
42.Watanabe A,Fukami T,Nakajima M,et al.Human Arylacetamide Deacetylase Is a Principal Enzyme in Flutamide Hydrolysis,Drug Metabolism and Disposition 2009;37:1513-1520.
43.Pratt SE,Durland-Busbice S,Shepard RL,et al.Human Carboxylesterase-2 Hydrolyzes the Prodrug of Gemcitabine(LY2334737)and Confers Prodrug Sensitivity to Cancer Cells,Clin Cancer Res 2013;19:1159-1168.
44.Sai K,Saito Y,Tatewaki N,et al.Association of carboxylesterase 1A genotypes with irinotecan pharmacokinetics in Japanese cancer patients,British journal of clinical pharmacology 2010;70:222-233.
45.Yoshimura M,Kimura T,Ishii M,et al.Functional polymorphisms in carboxylesterase1A2(CES1A2)gene involves specific protein 1(Sp1)binding sites,Biochemical and biophysical research communications 2008;369:939-942.
46.Tang M,Mukundan M,Yang J,et al.Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol,Journal of Pharmacology and Experimental Therapeutics 2006;319:1467-1476.
47.Shi J,Wang XW,Eyler RF,et al.Association of Oseltamivir Activation with Gender and Carboxylesterase 1 Genetic Polymorphisms,Basic Clin Pharmacol 2016;119:555-561.
48.KuboT,Kim SR,SaiK,etal.Functional characterization of three naturally occurring single nucleotide polymorphismsin the CES2 gene encoding carboxylesterase 2 (HCE-2), Drug Metabolism and Disposition 2005;33:1482-1487.
49.Sai K,Saito Y,Tatewaki N,et al.Association of carboxylesterase 1A genotypes with irinotecan pharmacokinetics in Japanese cancer patients,British journal of clinical pharmacology 2010;70:222-233.
50.Nemoda Z,Angyal N,Tarnok Z, et al.Carboxylesterase 1 gene polymorphism and methylphenidate response in ADHD,Neuropharmacology 2009;57:731-733.
51.XuG,ZhangWH,MaMK,etal.Human carboxylesterase 2 is commonly expressed in tumor tissue and is correlated with activation of irinotecan,Clinical Cancer Research 2002;8:2605-2611.
52.Liu YJ,Li SY,Hou J,et al.Identification and characterization of naturally occurring inhibitors against human carboxylesterase 2 in White Mulberry Root-bark,Fitoterapia 2016;115:57-63.
53.Hatfield MJ,Tsurkan LG,HyattJL,etal.ModulationofEsterifiedDrugMetabolism by Tanshinones from Salvia miltiorrhiza("Danshen"),Journal of Natural Products 2013;76:36-44.
54.Gorman GS,Coward L,Darby A,et al.Effects of herbal supplements on the bioactivation of chemotherapeutic agents,J Pharm Pharmacol 2013;65:1014-1025.
55.Li YG,Hou J,Li SY,et al.Fructus Psoraleae contains natural compounds with potent inhibitory effects towards human carboxylesterase 2,Fitoterapia 2015;101:99-106.
56.Weng ZM,Ge GB,Dou TY,et al.Characterization and structure-activity relationship studies of flavonoids as inhibitors against human carboxylesterase 2,Bioorganic Chemistry 2018;77:320-329.
57.Wang YQ,Weng ZM,Dou TY,et al.Nevadensin is a naturally occurring selective inhibitor of human carboxylesterase 1,Int J Biol Macromol 2018;120:1944-1954.
58.Sun DX,Ge GB,Dong PP,et al.Inhibition behavior of fructus psoraleae's ingredients towards human carboxylesterase 1 (hCES1),Xenobiotica2016;46:503-510.
59.Zhuang S,Wang H,Ding K,et al.Interactions of benzotriazole UV stabilizers with human serum albumin:Atomic insights revealed by biosensors,spectroscopies and molecular dynamics simulations,Chemosphere 2016;144:1050-1059.
60.Crow JA,Herring KL,Xie S,et al.Inhibition of carboxylesterase activity of THP1 monocytes/macrophages and recombinant human carboxylesterase 1 by oxysterols and fatty acids,Bba-Mol Cell Biol L 2010;1801:31-41.
61.Wang AH,Huo XK,Feng L,et al.Phenolic glycosides and monoterpenoids from the roots of Euphorbia ebracteolata and their bioactivities,Fitoterapia 2017;121:175-182.
62.Yoon KJ,Qi J,Remack JS,et al.Development of an etoposide prodrug for dual prodrug-enzyme antitumor therapy,Molecular Cancer Therapeutics 2006;5:1577-1584.
63.Wang AH,Tian XG,Cui YL,et al.Diterpenoids from the roots of Euphorbia ebracteolata and their inhibitory effects on human carboxylesterase 2,Phytochemistry 2018;146:82-90.
64.Hatfield MJ,Binder RJ,Gannon R,et al.Irreversible Inhibition of Human Carboxylesterases by Tanshinone Anhydrides Isolated from Salvia miltiorrhiza("Danshen"),J Nat Prod 2018.
65.MaiZP,ZhouK,GeGB,etal.Protostane Triterpenoids from the Rhizome of Alisma orientale Exhibit Inhibitory Effects on Human Carboxylesterase 2,Journal of Natural Products 2015;78:2372-2380.
66.Wang DD,Zou LW,Jin Q,et al.Recent progress in the discovery of natural inhibitors against human carboxylesterases.Fitoterapia,2017,117:84-95.
67.Zou LW,Jin Q,Wang DD,et al.Carboxylesterase inhibitors:an update,Curr Med Chem,2018,25:1627-1649.
68.Ma HY,Yang JD,Hou J,,et al.Comparative metabolism of DDAO benzoate in liver microsomes from various species.Toxicol in Vitro,2017,44:280-286.
69.Jin Q,Feng L,Wang DD,et al.A highly selective near-infrared fluorescent probe for carboxylesterase 2 and its bioimaging applications in living cells and animals.Biosens Bioelectron,2016,83:193-199.
70.Lei W,Wang DD,Dou TY,et al.Assessment of the inhibitory effects of pyrethroids against human carboxylesterases.Toxicol Appl Pharmacol,2017,321:48-56.
 TMR Modern Herbal Medicine2019年1期
TMR Modern Herbal Medicine2019年1期
- TMR Modern Herbal Medicine的其它文章
- Traditional Chinese Medicine in the New Era
——New Year Message 2019 - Clinical metabolic analysis combined with traceability of biosynthetic pathway: a new approach to quality marker of Chinese materia medica
- Effects of Dingjifumai Decoction on Electrocardiogram and sodium potassium pump of rats with ventricular arrhythmia
- An introduction of the experience in treating Ventricular premature beat with stasis heat
