Clinical metabolic analysis combined with traceability of biosynthetic pathway: a new approach to quality marker of Chinese materia medica
Zhi-Rui Yang, Ai-Ting Wang, Juan Liu, Xin-Yu Yang, Xiao-Fang Wang, Lei Zhang, Na Guo, Jia-Chen Zi,Cheng Peng, Shu-Xun Yan, Dan Yan23*
1Beijing Shijitan Hospital, Capital Medical University, Beijing, China. 2Beijing Key Laboratory of Bio-characteristic Profiling for Evaluation of Rational Drug Use, Beijing, China. 3International Cooperation & Joint Laboratory of Bio-characteristic Profiling for Evaluation of Rational Drug Use, Beijing, China. 4Experimental Research Center, China Academy of Chinese Medical Sciences, Beijing, China. 5JiNan University, Guangzhou, China. 6Chengdu University of Traditional Chinese Medicine, Chengdu, China. 7The first affiliated hospital, Henan University of China Medicine,Zhengzhou, China.
Background
Quality control of Chinese materia medica (CMM) is an important basis for ensuring the safety and efficacy of CMM, and it is also a key and difficult issue in Chinese medicine standardization research for a long time. Quality marker (Q-marker) of CMM has become a hot issue in traditional Chinese medicine with the prominent role in quality control of CMM [1]. Its research idea and technique have become key scientific issues demanded to be expanded and improved. At present, the separation and bioactivity test of active constituents are the main research strategy for discovering Q-marker of CMM.However, this approach might be largely blind, uncertain,time-consuming and laborious. In order to enrich and improve the theory, technical methods, and research paths of Q-marker of CMM, it is necessary to promote the transformations and developments of CMM quality evaluation model with Q-marker as the core. The strategy based on the reverse analysis of drug metabolism in clinic and traceability of botanic biosynthetic pathways to discovery Q-marker of CMM will be a useful supplement to expand the current Chinese medicine Q-marker research ideas and approaches.
Hence, from the conception, principle and original intention of Q-marker of CMM, we propose a novel research idea for discovery and validation of Q-marker,selecting Jinqi Jiangtang Tablets (JQJTT) with significant clinical efficacy as a model drug [2, 3]. Based on the fact that quality standard of CMM should be correlated with clinical efficacy, it would be effective to start with the identification of bioactive constituents in clinical samples(e.g. blood, urine), followed by traceability through botanic biosynthetic pathways to discover the bioactive and inherent key secondary metabolites, which might be the potential Q-marker. With final validation by classic hypoglycemic evaluation model, the confirmed Q-marker can be transmitted and traced, allowing its availability in quality control of the entire production process of JQJTT.This strategy emphasizes “REVERSE”-from clinical drug metabolism profile to key botanic intermediate in biosynthetic pathways- which ensures the discovered Q-marker is clinically and botanically-related, directly connecting the natural property and clinical significance of CMM. This new conception of Q-marker of CMM with “Discovery of clinically active constituents as guidance, Reverse analysis of metabolic transformations as link, and Traceability of biosynthesis pathways as key”,provides a useful strategy for theoretical innovation,technological breakthrough, and research idea expansion of quality control of CMM.
JQJTT, a classical ancient formula, first appeared in Qianjin Fang, which published in 652 A.D. (Tang Dynasty of China), is recorded in the Chinese Pharmacopoeia 2015 edition (volume 1), which consists of Coptis c hinensis, Astragalus m embranaceus and Lonicera japonica). According to the Chinese Pharmacopoeia 2015 edition (volume 1), the three decoction pieces were prepared into alkaloid extract,saponin extract and organic acid extract, respectively; and mixed to reparation of tablets. With bioactivities like lowering blood glucose, improving lipid metabolism and insulin sensitivity, JQJTT was confirmed to have definite therapeutic effect on type 2 diabetes by a double-blind placebo-controlled randomized clinical trial [4]. However,the quality standard of JQJTT still needs to be further improved.
Concept and current research on Q-marker of CMM
Q-marker of CMM is chemical constituents that are inherent in crude drug or formed during processing,closely-related to CMM attribute and indicators reflecting safety and efficacy of CMM in clinic. Q-marker should meet four basic requirements [1]: (1) Q-marker exists in crude drugs, extracts, and (or) formulations; (2) Q-marker should be qualitatively identified and quantitatively determined, and be closely related to the functional properties of CMM; (3) Q-Marker is consistent with the theory and practice of traditional Chinese medicine; (4)Q-marker has transferability and traceability characteristics. The innovative academic concept of Q-marker of CMM has important academic value and practical significance for the study of CMM quality standards. Its research idea and technical methods have become key scientific issues demanded to be improved.
Main research path on Q-marker of CMM
The main research path to find Q-marker is through identification of chemical constituent, discovery of exclusive constituent and validation of its bioactivity, by which the success was achieved in several cases [5, 6]. As a common research path in this field, such method,however, it has some inevitable defects. Chemical constituents in common CMM have been already largely explored, and it is hard and laborious to find out novel constituents. It is still unknown that whether the exclusive constituent can be found out or whether they are related to bioactivity. Moreover, it is usually difficult to confirm whether the found constituent is truly exclusive that the exclusive constituent would be non-exclusive with the development of phytochemistry. For example, coptisine is generally considered to be an exclusive constituent of C.chinensis, but it has been reported that coptisine can also be isolated from Corydalis y anhusuo W. T. Wang [7].Besides, the bioactivity of the exclusive constituent might be hard to evaluate if it is low or difficult to concentrate.Therefore, according to the principle of CMM's Q-marker,we are exploring a new research approach based on the Q-marker connotation that it should be associated with clinical efficacy and can be used to evaluate the quality of CMM, which emphasizes the basic attributes -“associated clinical efficacy, botanically inherent,traceable in processing, and qualitatively & quantitatively detectable”.
As is known to all, after a large number of constituents(potential Q-marker) are obtained through phytochemical separation, their bioactivity evaluation should be followed for confirmation. However, apart from the fact that it is impossible to evaluate the bioactivities of all constituents isolated, the correlation between research in cell or animal and clinical efficacy is poorly elucidated,leading to discovery of “pseudo-Q-marker” which is hard to control quality and associate with clinic [8-11].Therefore, the active constituents could be found out from the terminal-clinical effect. In fact, clarifying their metabolism rule in vivo, and the up-stream prototype or metabolites could be reversely analyzed, which is fundamental to explore the clinic-related potential Q-marker.
Intrinsic property and bioactivity association of secondary metabolites provide an important clue for exploring Q-marker
Secondary metabolites in plant biosynthetic pathways are the main source of active constituents in CMM, which are affected by soil, climate, symbiotic organisms, cultivation methods, and other factors during the growth and development of plants, forming their own unique biosynthetic pathways [12]. That is to say, although the chemical constituents (precursors, intermediates, and secondary metabolites) of plants from the same source have various structures, they are mostly composed of a certain basic structural unit and form into different intrinsic constituents. Moreover, adequate achievements have been made in the biosynthetic pathways of active constituents (secondary metabolites) of common CMM[13-15]. For example, the biosynthesis pathway of berberine, one of the active constituents of C. chinensis,belongs to the benzylisoquinoline alkaloid synthesis pathway. Benzylisoquinoline alkaloid synthesis starts from L-tyrosine, which forms two key intermediates including (S)-scoulerine and (S)-tetrahydrocolumbamine via a multi-step enzymatic reaction.(S)-tetrahydrocolumbamine is then transformed into berberine (Figure 1), as well as structurally similar active constituents like coptisine, palmatine, columbamine, and magnoflorine [16], leading to a scientific issue that whether the quality control of C. chinensis can be realized by controlling (S)-tetrahydrocolumbamine or(S)-scoulerine, the common up-stream key intermediate of known active constituents of C. chinensis. If it does, it is possible to correlate and control several downstream active constituents with similar structure and effect to evaluate the quality of CMM. Therefore, the new approach strategy – “active constituents are discovered from clinic, upstream prototypes or metabolites of active constituents are reversely analyzed by metabolic rule in vivo, and these intrinsic constituents serve as potential Q-marker”, is an innovative development to expand current research paths of CMM's Q-marker (Figure 2).
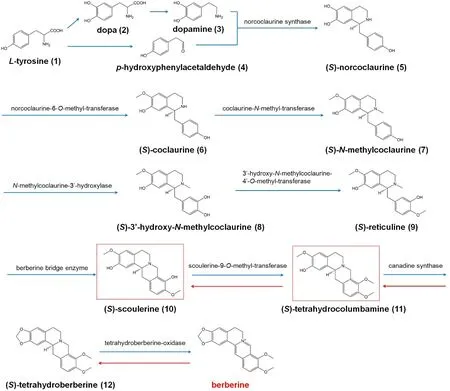
Figure 1 The biosynthetic pathway of berberine from Coptis chinensis.
Q-marker of JQJTT can be explored by integration strategy – “Discovery of clinical active constituents, Reverse analysis of metabolic transformations and Traceability of biosynthesis pathways”
Currently, the quality of JQJTT is controlled by qualitation and quantitation of partial constituents and general requirements for preparations, which are insufficient to reflect integral quality of formula, and are difficult to connect the quality transmit and trace of crude drugs, decoction pieces and preparations. With its relatively simple composition, largely explored metabolism of bioactive constituents in v ivo and botanic biosynthetic pathways, JQJTT can be a model promoting the research about discovery and validation of Q-marker of CMM.
The production and quality control of representative sample of JQJTT, and the serum collection of patients administered with JQJTT
The JQJTT preparation is produced with qualified Coptis chinensis, A stragalus m embranaceus and Lonicera japonica following the technique in the Chinese Pharmacopoeia 2015 edition (volume 1). Based on national statutory standard, the multi-constituent determination and chemical fingerprint can be applied to more integrally evaluate the quality of crude drugs,decoction pieces and preparations, which ensures that the subsequent research result can be comparable and traceable. Besides, following the medical ethics and criteria of inclusion and exclusion, the patients with type 2 diabetes are given with JQJTT, and their serum samples are collected.
The identification of absorbed constituents and metabolic products of JQJTT and their reverse analysis according to transformations
Based on the established methodology, the absorbed constituents and their metabolic products of JQJTT are determined by UPLC-QTOF/MSEor UPLC-Q-Orbitrap MS/MS, and the unknown compound structure and discrepant constituents can be speculated. Next, following the strategy of reverse analysis, the constituents of JQJTT absorbed in blood and their transformation rule can be speculated and elucidated, according to the structural metabolic characteristics of constituents and their products (Figure 3).
The transformation rule of constituents in JQJTT is traceable following “crude drugs,decoction pieces and preparations”
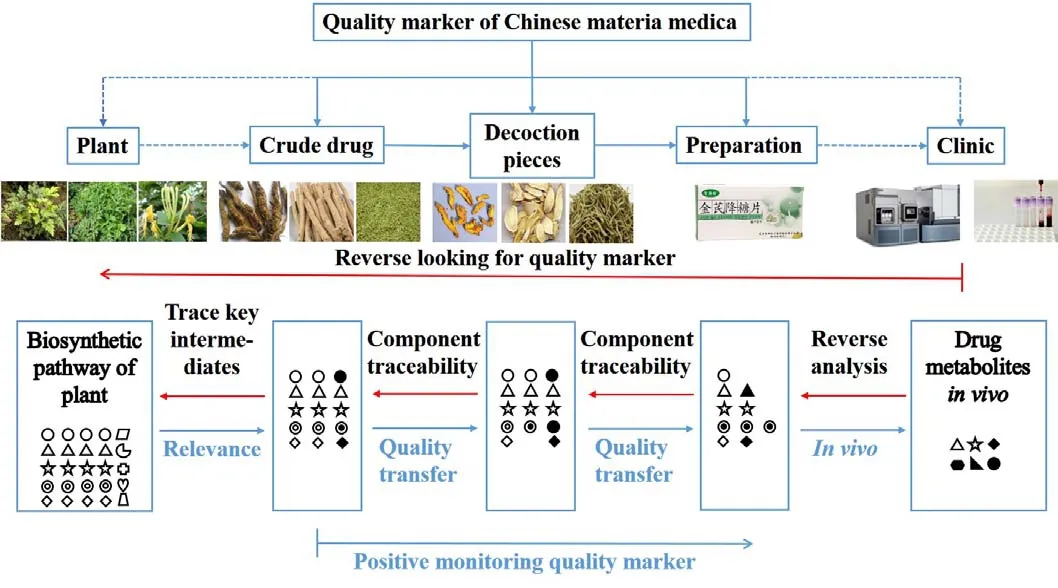
Figure 2 The new approach strategy for quality marker of Chinese materia medica.
With the clarification of constituents absorbed in blood and their metabolites, these constituents in crude drugs,decoction pieces and preparations related to JQJTT are detected by UPLC or UPLC-MS/MS to reversely trace and analyze and eventually ensure what secondary metabolites should be focused in crude drugs or plants.
The discovery of potential Q-marker of JQJTT by botanic biosynthetic pathways
With the clarified biosynthetic pathways as clue, the absorbed constituents with quality transmit capability and traceable characteristics are selected to analyze their up-stream secondary metabolites or key intermediates,which can be determined as potential Q-markers if they are bioactive, inherent and able to transmit quality and can be traced and detected.
The validation of Q-marker of JQJTT by classical evaluation model related to therapeutic effect
The potential Q-markers of JQJTT are qualitatively and quantitatively analyzed by UPLC or UPLC-MS/MS.Following the Chinese Pharmacopeia, the bioassays on different batches of JQJTT are conducted in established hypoglycemic model in cells (insulin resistance cell model established using HepG2 cells) [17] and animals(rats fed with high fat diet followed by an intraperitoneal injection of streptozocin) [18]. Using multivariate statistical analysis, the integral relevance between Q-marker and hypoglycemic activity is assessed and the Q-marker of JQJTT is finally confirmed. Retrospective clinical observation can be applied to verify when necessary.
For all above, we combine the common ideas and techniques to form a new approach of CMM’s Q-marker,which is based on “Discovery of clinical active constituents, Reverse analysis of metabolic transformations and Traceability of biosynthesis pathways”, so as to enrich and improve the theory and technical methods of CMM’ Q-marker (Figure 4).
Discussion
The proposal of innovative conception, Q-marker, makes up for the deficiency of current quality control, which has important academic values and practical significances.And to promote the revolution and development of quality control in CMM with Q-marker as a core, the research idea and technique needs to be expanded and improved.
In this article, in terms of the fact that the CMM quality standard should be correlated with clinical therapeutic effect, we propose a novel ideology that the discovery of Q-marker can start from the identification of bioactive constituents from clinical observation, followed by reverse analysis about drug metabolism in vi vo. This strategy overcomes the defect that the conventional research about quality control is hard to associate with clinical safety and efficacy, and prevents the conventional research from being blind and accidental. Our proposal promotes the current quality control of CMM to be ideally-innovated, with definite therapeutic effect and clarified biosynthesis pathway to be prerequisites.
Besides, we have to point out that the new strategy is a new exploration, not a denial of the current CMM’s Q-marker research method. Certainly, our research methods of CMM’s Q-marker have inevitable limitations.For example, this research strategy can be applied to botanical, not animal or mineral medicines. And the botanical biosynthetic pathways and chemical substances of the subjects should be partially elucidated. In addition,obtaining clinical samples might be inconvenient for some labs. Nonetheless, complying with the concept,principle and original intention of CMM’s Q-marker, the research path should be diverse. Each research strategy has a certain scope of application. It should be selected and changed according to different research objects, so as to jointly promote the innovation and development of theory and technical methods of CMM’s Q-marker.
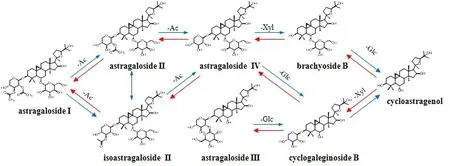
Figure 3 Metabolites of astragalus saponins in Jinqi Jiangtang Tablet from patients’ serums
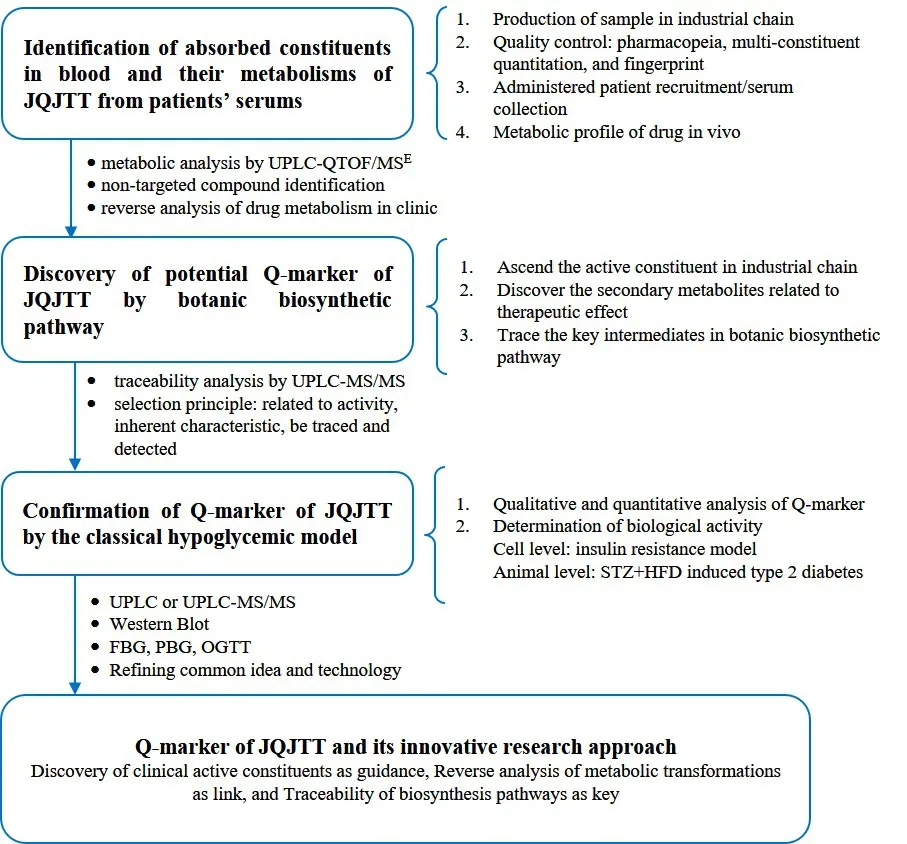
Figure 4 The Q-marker of JQJTT and its innovative research approach
1. Liu CX, Cheng YY, Guo DA, et al. A new concept on quality marker for quality assessment and process control of Chinese medicines. Chin Herb Med, 2017.9: 3-13.
2. Yue SJ, Liu J, Feng WW, et al. System pharmacology-based dissection of the synergistic mechanism of Huangqi and Huanglian for diabetes mellitus. Front Pharmacol 2017. 8: 694.
3. Gao LH, Liu Q, Liu SN, et al . A refined-JinQi-JiangTang tablet ameliorates prediabetes by reducing insulin resistance and improving beta cell function in mice. J Ethnopharmacol 2014. 151: 675-685.
4. Chao M, Zou D, Zhang Y, et al. Improving insulin resistance with traditional Chinese medicine in type 2 diabetic patients. Endocr, 2009. 36(2): 268.
5. Zhong Y, Zhu J, Yang Z, et al. Q-marker based strategy for CMC research of Chinese medicine: A case study of Panax Notoginseng saponins.Phytomedicine, 2018. 44: 129-137.
6. Liao M, Shang H, Li Y, et al. An integrated approach to uncover quality marker underlying the effects of Alisma orientale on lipid metabolism, using chemical analysis and network pharmacology.Phytomedicine, 2018. 45: 93-104.
7. Shi JM, Han WL, Ye WC, et al. Study on chemical constituents of Corydalis yanhusuo W.T.Wang. Nat Prod Res Dev (Chin), 2011. 23: 647-651.
8. Feng WW, Zhang Y, Tang JF, et al. Combination of chemical fingerprinting with bioassay, a preferable approach for quality control of Safflower Injection.Anal Chim Acta, 2018. 1003:56-63.
9. Yan D, Xiong Y, Ma LN, et al. Proposal on establishment of quality evaluation pattern and proper mode for Chinese materia medica based on clinical efficacy. Chin Tradit Herbal Drugs (Chin),2013. 44(1): 1-5.
10. Reardon S. A mouse’s house may ruin experiments:Environmental factors lie behind many irreproducible rodent experiments. Nature, 2016.530: 264.
11. Yan D, Liu J, Wang AT, et al. Exploring research ideas of mechanism of dominant diseases in traditional Chinese medicine based on evidence-based medicine. Chin J Chin Mater Med(Chin), 2018. 13: 2633-2638.
12. Zi J, Mafu S, Peters RJ. To gibberellins and beyond!Surveying the evolution of (Di) terpenoid metabolism. Annu Rev Plant Biol, 2014. 65(1):259-286.
13. He SM, Liang YL, Cong K, et al. Identification and characterization of genes involved in benzylisoquinoline alkaloid biosynthesis in coptis species. Front Plant Sci, 2018. 9: 731.
14. Kojima M, Uritani I. Studies on chlorogenic acid biosynthesis in sweet potato root tissue in special reference to the isolation of a chlorogenic acid intermediate. Plant Physiol, 1973. 51: 768-771.
15. Moses T, Papadopoulou KK, Osbourn A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit Rev Biochem Mol Biol, 2014. 49: 439-462.
16. Ma Y, Guo J, Mao YP, et al. Analysis for biosynthetic pathways of active constituents in medicinal plants and its availability. Chin J Tradit Chin Med Pharm, 2017. 32: 2079-2083.
17. Song JJ, Wang Q, Du M, et a l. Casein glycomacropeptide-derived peptide IPPKKNQDKTE ameliorates high glucose-induced insulin resistance in HepG2 cells via activation of AMPK signaling. Mol Nutr Food Res, 2017. 61:1600301.
18. Mansor LS, Gonzalez ER, Cole MA, et al. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin.Cardiovasc Diabetol, 2013. 12:136-136.
 TMR Modern Herbal Medicine2019年1期
TMR Modern Herbal Medicine2019年1期
- TMR Modern Herbal Medicine的其它文章
- Traditional Chinese Medicine in the New Era
——New Year Message 2019 - Effects of Dingjifumai Decoction on Electrocardiogram and sodium potassium pump of rats with ventricular arrhythmia
- Carboxylesterases mediated herb-drug interactions:a systematic review
- An introduction of the experience in treating Ventricular premature beat with stasis heat
