Alleviation of arsenic toxicity by phosphate is associated with its regulation of detoxification, defense, and transport gene expression in barley
Gerald Zvobgo, Jonas Lwalaba Wa Lwalaba, Tichaona Sagonda, James Mutemachani Mapodzeke,Noor Muhammad, lmran Haider Shamsi, ZHANG Guo-ping
Department of Agronomy, College of Agriculture and Biotechnology, Zhejiang University/Zhejiang Key Laboratory of Crop Germplasm Resource, Hangzhou 310058, P.R.China
Abstract Arsenic (As) contamination in soils has posed a severe threat to safe crop production. The previous studies showed the antagonism between phosphorus (P) and As in plant growth and As uptake, while the mechanisms of alleviating As toxicity by P is not completely clear. Due to the limiting P condition, it is imperative to understand how low P addition can be used to suppress arsenate As (V) uptake and the subsequent mechanisms involved. Thus in this study we investigated the effect of P addition on As uptake, anti-oxidative enzyme activity, and anti-oxidant content, and the relative expression of transport,defense, and detoxification genes using two barley genotypes differing in As toxicity tolerance. P addition significantly reduced As concentration in plant tissues, and caused the great changes in activities of catalase and superoxide dismutase,glutathione content, and the relative expression of examined genes when the plants of the two barley genotypes were exposed to 100 μmol L–1 As, with ZDB160 (As-tolerant) being much more affected than ZDB475 (As-sensitive). The current results show that P addition can alleviate As toxicity by regulating the expression of As transport, defense, and detoxification genes to a greater extent in As tolerance of barley, suggesting the possibility of controlling As uptake and toxicity by applying low amount of P fertilizers in the As-contaminated soils.
Keywords: arsenate, phosphate, transporter, tolerance, toxicity
1. lntroduction
Over the past two decades, substantial amount of studies have been made on the detrimental effects of heavy metals on the environment, due to the natural and human-induced factors contributing to the large deposit of these pollutants into soil and water. Of late strategies to perturb the effects of heavy metals using other chemical substances have been on the rise because of the antagonistic effects between these toxic ligands and the non-toxic chemicals (Lwalabaet al. 2017). Arsenic (As), like other heavy metals, has a negative impact on plants, animals as well as humans, while conversely phosphate (P) stands out to be the most essential mineral nutrient required for plant growth. As enters the food chain through irrigation with As-containing groundwater(Awasthiet al. 2017). Of the two widely available inorganic As variants, arsenite (III) and arsenate (V), much attention has been placed on As (V) due to its availability in aerobic conditions and its chemical similarity with P (Zvobgoet al.2018a). With most countries exceeding the permissible WHO guidelines of 10 μg L–1As in drinking water and US Environmental Protection Agency (USEPA) limits of 20 mg kg–1in soil, it is perceived that a large amount of crops might be exposed to high As concentration, thus endangering the health of human beings (EPA 2012). In addition, the diminishing quantity of P in the environment is also posing a significant threat to plants due to the predominance of As (V) toxicity in the absence of P (Zvobgoet al. 2018a).Thus understanding the mechanisms by which plants can combat the toxic effects of As (V) in P limiting conditions is imperative for breeding and engineering As-tolerant crops.
It is well documented that As (V) is rapidly transported by phosphate transporters (PHTs) in the roots upon exposure of plants to As (Wanget al. 2002), resulting in the interference of glycolysis and oxidative phosphorylation and thereby inhibiting ATP synthesis. The toxicity of As (V)partly depends on the expression of these PHTs, with Astolerant genotypes suppressing the expression of PHTs to a greater extent compared to the As-sensitive ones (Zvobgoet al. 2018a). The suppression of As (V) uptake system is considered to be the most crucial mechanism of As tolerance in plants (Meharg and Macnair 1992). Following As (V)uptake, it is rapidly reduced to a more toxic form As (III)by arsenate reductase (Huanget al. 2012). As (III) binds to phytochelatins (PC, low molecular weight enzymatically synthesized thiol peptides) and sequestered into the vacuolesviatonoplast localized ABCC transporters in a process of intracellular detoxification (Leslieet al. 2004;Songet al. 2014). In addition, As can induce cell damage through reactive oxygen species (ROS) signaling pathways.On the other hand, plants have developed complex defense mechanisms to scavenge ROS, which include enzymatic antioxidants like superoxide dismutase (SOD, EC; 1.15.1.1),catalase (CAT, E.C.1.11.1.6), and ascorbate peroxidase(APX, EC; 1.11.1.11) and non-enzymatic components like glutathione and ascorbic acid (Peret al. 2016). SOD presents the first line of defense against ROS accumulation by dismutating theradicals to H2O2which is acted upon by CAT and APX, thus preventing ROS mediated oxidative damage (Harbet al. 2015). Glutathione and ascorbic acid are also crucial for protecting plants against oxidative stress and are found at high concentrations in the chloroplast and other cellular components (Mittler 2002). Earlier studies on the oxidative stress and the antioxidant activities response of plants to As stress have shown consistent findings of higher response of antioxidant activities in As-tolerant rice(Begumet al. 2016) and As-tolerant tobacco in comparison to the sensitive genotypes (Zvobgoet al. 2015). Thus the coordination of events from As (V) uptakeviaPHTs to induction of ROS scavengers and sequestration of As into the vacuole play important roles in As detoxification, but how these processes contribute to As tolerance in natural variants of barley genotypes as affected by limited P supply remains poorly understood.
In P-limiting conditions, PHTs are regulated in a complex manner by various genes and transcription factors (TFs)(Mueheet al. 2014). TFs are sequence-specific proteins that regulate transcription (Saleh and Pagés 2003). They are responsible for selective gene regulation and are expressed in specific tissues. With the exception of a few,they all contain a DNA binding domain, an oligemerization site, a transcription regulation domain, and a nuclear localization signal. TFs can either act as repressors or activators depending on whether they stimulate or inhibit transcription of target genes (Doebley and Lukens 1998).Although some TFs such as the AP2, ERF, DREB, and WRK6 have been perceived as As-responsive TFs (Huanget al. 2012; Castrilloet al. 2013; Shuklaet al. 2015),no much information is available on the expression of these TFs upon combined As (V) and P exposure. In P-starvation conditions, the MYB and PHR TFs positively regulated some PHTs in rice, whereas the PHO TF showed negative responses on the PHTs (Huet al. 2011).The PHO transporters are involved in loading P into the xylem (Seccoet al. 2012). Mechanisms involved in P transport and the starvation response mechanisms have been largely studied, but still some insights into whether As (V) elucidates the same responses and how under As(V) stress minute concentrations of P might alter these mechanisms still needs to be highlighted in order to understand the mechanisms of As tolerance.
Although the natural variations of different plant mechanisms in response to As and other plant developmental mechanisms have been done inArabidopsis(Weigel 2012;Shuklaet al. 2015) and maize (Liet al. 2012), studies on the mechanisms of how P alleviates As (V) toxicity in barley are vague. Thus this study was done with the aims of understanding how As (V) transport, detoxification, and defense mechanisms vary in natural variants of barley genotypes under limited P levels, which will help us understand how As-tolerant barley genotypes achieve their tolerance to As.
2. Materials and methods
2.1. Plant materials and treatments
The experiment was conducted in 2017 at a greenhouse of Zhejiang University, Hangzhou, China. Two barley genotypes differing in As sensitivity,viz, ZDB160 (As-tolerant) and ZDB475 (As-sensitive), were used in this study according to our previous study (Zvobgoet al. 2018b).Healthy seeds were surface-sterilized with 2% H2O2for 30 min and immediately rinsed about 4 times in distilled water. The seeds were soaked in distilled water for 6 h at 25°C prior to germination. Germination of the seeds followed the same procedures in our previous studies(Zvobgoet al. 2018a). Ten days after germination, uniformly sized seedlings were transplanted into 1-L plastic pots for hydroponic culture. Seedlings were subjected to 1/2 strength nutrient solution (NS) and thereafter full strength solution.The NS which was renewed every 5 d, constituted: 1 mmol L–1Ca(NO3)2·4H2O, 1 mmol L–1KCl, 1 mmol L–1MgSO4,0.25 mmol L–1NH4H2PO4, 50 mmol L–1CaCl2, 20 mmol L–1Fe-citrate·nH2O, 12.5 mmol L–1H3BO3, 0.5 mmol L–1H2MoO4,0.5 mmol L–1CuSO4·5H2O, 2 mmol L–1MnCl2·4H2O, and 2 mmol L–1ZnSO4·7H2O, with pH being adjusted to 5.8±0.1.The solution was continuously aerated with air pumps. The experiment consisted of 3 treatments: control, As (100 μmol L–1) and As (100 μmol L–1)+P (50 μmol L–1). Treatments were initiated from 2 leaf stage with As and P being administered as solutions of Na2HAsO4and Na2HPO4in distilled water,respectively. In all the treatments, the NS was modi fied with equivalent moles of NH4H2PO4being replaced by NH4Cl.The pots were placed in a growth chamber in Zhejiang University (22/18°C day/night) in 2017. After 7 days of treatment, plants were harvested and separated into roots and shoots. Roots were soaked in 20 mmol L–1Na2-EDTA to remove adhering cations and thereafter rinsed 3 times with distilled water. Plant samples were kept in –80°C for further analysis, while the other samples were oven-dried for elemental analysis.
2.2. Determination of plant biomass, As, and P concentrations
Total biomass was measured after plants were oven-dried at 80°C for 72 h and approximately 100 mg of the dry weight was used for As and P elemental analysis. Dried roots and shoots were digested in 5 mL of absolute HNO3and 1 mL of 30% H2O2at 80°C on a hot plate using a microwave digester(Microwave 3000; Antoon Paar, Austria). The residue from digested samples was dissolved in 25 mL Milli-Q water. P concentration was determined using an inductively coupled plasma-optical emission spectrometer (ICP-OES; Optima 8000 DV; PerkinElmer, USA) with the reference standard of 100 mg L–1of quality control standard supplied by PerkinElmer, while As concentration was determined with an inductively coupled plasma-mass spectrometer (ICP-MS;ELAN DRC-e; PerkinElmer) with the reference standard of 1 000 mg L–1As supplied by Sigma-Aldrich (USA).
2.3. Measurement of SOD and CAT activities
Extraction for antioxidant enzymes was done using the following procedure; about 0.2 g of fresh root samples were homogenized in extraction buffer of 3 mL ice-cold of 1 mol L–1Tris buffer (pH 7.8). The samples were centrifuged at 12 000×g for 20 min at 4°C. The supernatant was preserved in a separate tube at 4°C and was used as the enzyme extract for assay.
Superoxide dismutase (SOD, E.C. 1.15.1.1) was measured by inhibiting the photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm (Beyer and Fridovich 1987)in a reaction mixture (300 μL) that contained 1 mol L–1Tris buffer (pH 7.8), 75 mmol L–1NBT, 20 mmol L–1ribo flavin,100 mmol L–1Na2EDTA, 130 mmol L–1methionine, and 2.5 μL of enzyme extract. The reaction was conducted in a 96-well microplate (COSTAR, USA) with the samples and control uniformly placed under 4 000 lx of light for 20 min.Wells without the enzyme developed maximal color. The absorbance ofcomplex formed was recorded at 560 nm against a reagent blank. 1 U of SOD activity was defined as the amount of the enzyme that inhibited photoreduction of NBT by 50% in comparison with wells lacking enzymes. Enzyme activity was expressed as units per gram of fresh root.
Catalase (CAT, E.C.1.11.1.6) activity was measured by degrading H2O2(E=0.0436 mmol L–1cm–1) with the decrease in the absorbance followed spectrophotometrically at 240 nm for 30 s (Aebi 1984). The samples were homogenized in a reaction mixture (300 μL) that contained 1 mol L–1Tris buffer (pH 7.8), 300 mmol L–1H2O2, and 10 μL of enzyme extract. 1 U of CAT activity was defined as the amount of enzyme that catalyzes the decomposition of 1 mmol L–1of H2O2mL–1min–1and the specific activity was expressed as mmol L–1per gram fresh root.
2.4. Quantification of glutathione and reduced glutathione
Determination of total glutathione, glutathione (GSH), and reduced glutathione (GSSG) was done using 5,5´-dithio-bis(2-nitrobenzoic acid) (DTNB) and glutathione reductase(GR) recycling method (Tietze 1969). The method involves a sequential oxidation and reduction of GSH respectively by DTNB and GR under the presence of NADPH. Extraction for GSH was done by homogenizing about 0.2 g fresh leaves in 2 mL of 5% (v/v) trichloroacetic acid (TCA). Then, the homogenates were centrifuged at 4 000×g for 10 min and the supernatants were used for assay. For total glutathione,the samples were homogenized in a reaction mixture(250 μL) on a 96-well microplate (COSTAR, USA) that contained 30 μL standard sample, 120 μL assay buffer(0.1 mol L–1Na-phosphate buffer (pH 7) with 1 mmol L–1EDTA), 50 μL of reaction mixture 1 (4.38 mL assay buffer,313 μL of 10 mmol L–1DTNB and 50 μL GR) and 50 μL reaction mixture 2 (5 mg NADPH in 5 mL assay buffer). Into 1-well of the test samples, 1 μL of scavenger was added for GSSG assay, while the other well without scavenger was used for total glutathione assay. The change in absorbance was recorded spectrophotometrically at 412 nm over a 3-min interval, data were collected every 0.5 min. The total glutathione and GSSG of the samples were calculated based on the following eqs. (1) and (2), respectively:

Where, A is the gradient of the standard curve, and B is the Y intercept.
GSH/GSSG ratio was calculated as:

2.5. RNA isolation, cDNA preparation, and gene expression analysis
Total RNA was isolated from roots after 7 days of treatment using the RNAprep Kit (Tiangen, China) following manufacturers’instructions with residual DNA being eliminated by DNase 1.RNA abundance and purity were determined using a Nanodrop and on 1% agarose gel, respectively. The cDNA was synthesized following instructions from PrimeScript RT Reagent Kit (Perfect Real Time, TaKaRa, Japan).Three biological and 2 technical replicates per treatment of cDNA sample were assayed using SYBR Green PCR Mastermix (Applied Biosystem, China) by quantitative real-time PCR (qRT-PCR) in the Lightcycler96 (Roche International Diagnostics Systems, Switzerland). The PCR pro files were as follows: initial denaturation 95°C for 30 s,denaturation 95°C for 5 s for 40 cycles, and annealing at 60°C for 30 s, followed by steps for Melt-Curve analysis(60-95°C, 0.5°C increment for 5 s per step). Barley specific primers for As transport, detoxification, defense, and TFs genes were designed (Table 1) using Primer-BLAST (www.ncbi.nlm.nih.gov) and were supplied by Sangon Biotech(Shanghai, China). Threshold value (CT) was calculated using LightCycler96 SW1.1 Software and mRNA was quanti fied according to Schmittgen and Livak (2008). CTvalue of reference genes, barleyactin(Rapaczet al. 2012)(GenBank ID: AY145451) was subtracted from the gene of interest, and 2–ΔΔCT was calculated as follows:
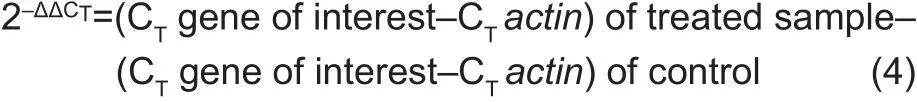
2.6. Statistical analysis
All data were presented as the mean values for each treatment. Statistical analysis was performed using the PROC ANOVA and PROC GLM procedures available in SAS 9.1 for Windows software (SAS Institute Inc., Cary,NC, USA). An analysis of variance (ANOVA) was conducted to determine differences among treatments. Significance of differences between barley genotypes and treatments was evaluated using Fisher’s least significant difference(LSD) test.
3. Results
3.1. Barley growth and dry weight
After 7 days of As and P treatments, the difference in plant growth between treatments and genotypes could be detected (Fig. 1). Exposure of barley plants to As-containing solution in the absence of P caused a significant reduction in tissue dry weight of all genotypes (Table 2). However,ZDB475 was more severely affected by As treatment than ZDB160, with shoot dry weight being reduced by 42 and 39%, respectively. The same was true for roots, with root dry weight being reduced by 56 and 44% for ZDB475 and ZDB160, respectively. Although P addition had no much effect on shoot dry weight, it alleviated the reduction of root weight caused by As toxicity, with ZDB160 and ZDB475 having 40 and 30% more root dry weight in the As+P treatment than that in As treatment alone, respectively.ZDB160 had lower root/shoot ratio than ZDB475 when exposed to As treatment, indicating that ZDB160 maintains relatively normal shoot growth under As stress in comparison with ZDB475 as indicated by the large significant difference in shoot dry weight in comparison to the root dry weight of the 2 genotypes under As stress conditions.
3.2. As and P concentrations in plant tissues
Concentrations and translocation factors (TF) of As and P affected by different treatments are shown in Table 3.As concentration was basically similar in the roots of both genotypes, however, the significant difference was noted in the shoots between the 2 genotypes, with ZDB475 having approximately 9 and 4 times as high as in ZDB160 in As and As+P treatments, respectively. Also As TF in ZDB475 was 7 and 4 folds higher than that in ZDB160 in As and As+P treatments. For P concentration, ZDB475 was significantly higher than ZDB160, respectively of plant tissue and As or P treatments. As treatment caused the significant reduction of P concentration in plant tissues of both genotypes, while P addition did not alter the reduction largely. In addition,As treatment (As alone and As+P treatments) caused the significant increase in the TF of P. The increase in P translocation was significant in ZDB160 with the highesttranslocation being noted in ZDB160 under combined addition of As and P. The increase in P translocation was inversely proportion to As translocation in ZDB160 while it was not well pronounced in ZDB475. The difference in As and P uptake is indicative of genotypic differences as explained by the high significant difference between genotypes in regards to As and P concentrations.
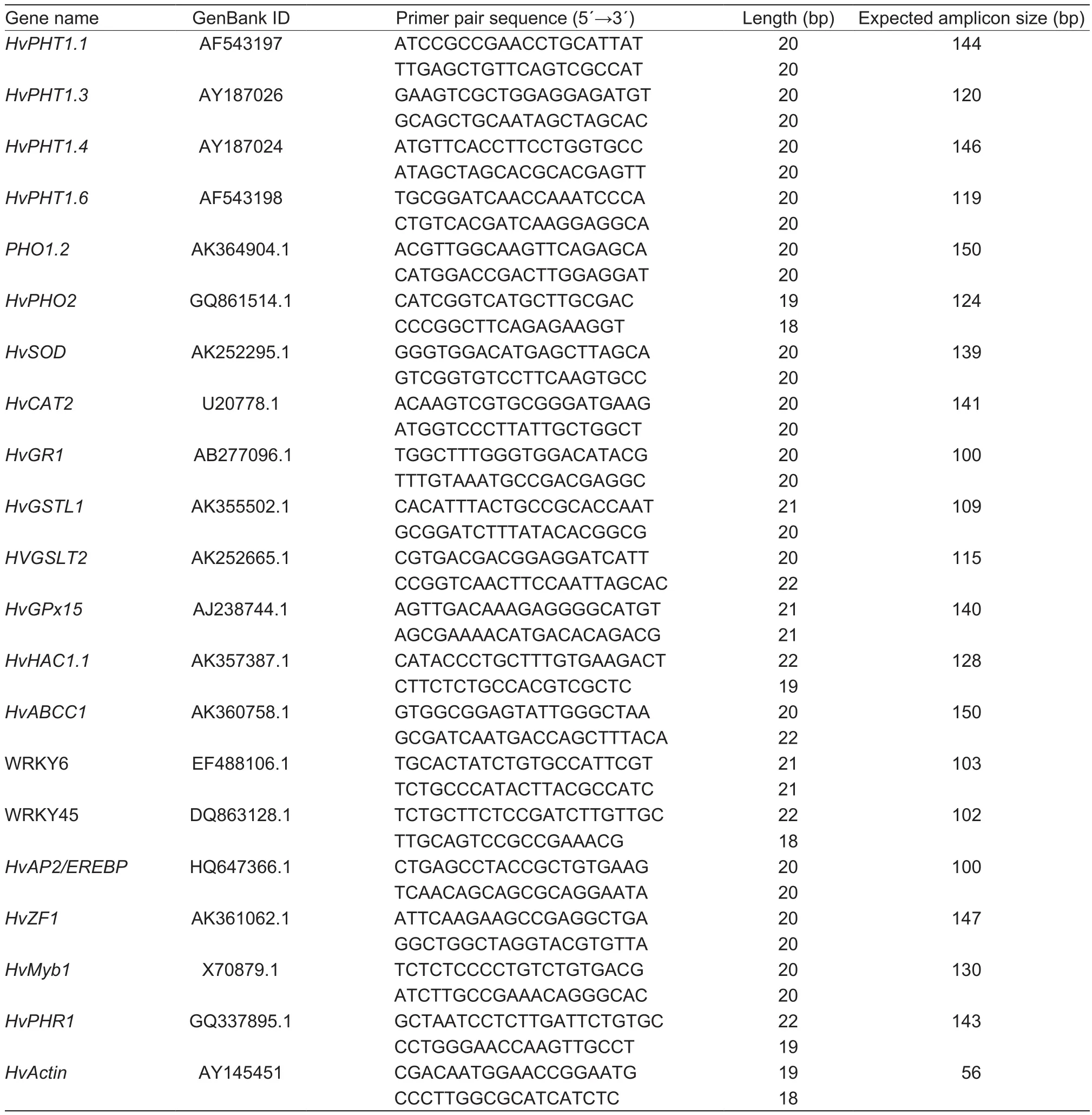
Table 1 Barley specific primer sequence used it this study
3.3. Antioxidant enzyme activity
The activities of SOD and CAT in the two barley genotypes as affected by different treatments are shown in Table 4. In general, there were significant differences in the activities of the 2 enzymes, with ZDB475 having higher SOD activity and ZDB160 having higher CAT activity. As treatment caused the significant increase in the activities of both anti-oxidative enzymes, with ZDB160 having more increase than ZDB475.Hence, SOD and CAT activities of ZDB160 increased by 1.62 and 1.75 folds for As treatment in comparison to control,respectively, while those of ZDB475 increased by 1.21 and 6.1 folds, respectively. No significant difference was detected in SOD activity between As and As+P treatments for ZDB475, while ZDB160 decreased by 1.39 folds. Similarly, P addition also reduced CAT activity, with ZDB475 being more affected than ZDB160. The significant differences between genotypes were much more pronounced in CAT in contrast to SOD signifying a difference in the mechanisms between the genotypes in their responses to cope with As and P.

Fig. 1 Phenotypic differences between ZDB160 and ZDB475 under arsenic (As) (100 μmol L–1) and As (100 μmol L–1)+phosphorus(P) (50 μmol L–1) after 7 days of treatment.
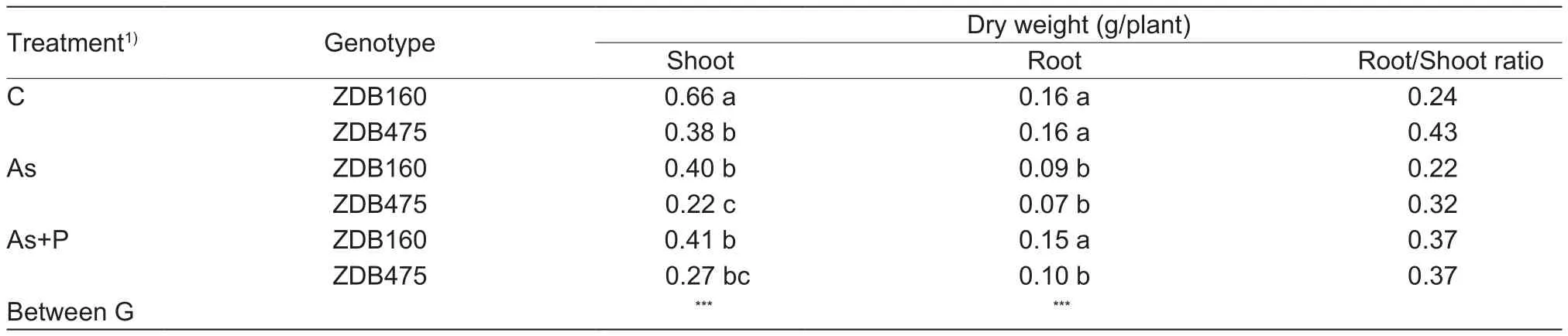
Table 2 Effects of arsenic (As) and As+phosphorus (P) treatments on plant biomass of 2 barley genotypes

Table 3 Effects of arsenic (As) and As+phosphorus (P) treatments on As and P contents of tissues in 2 barley genotypes
3.4. Total and reduced glutathione contents
The contents of GSSG, total GSH, and the GSH/GSSG ratio are presented in Table 5. ZDB475 had higher GSSG content than ZDB160 when both genotypes were exposed to As treatment, while exogenous application of P causedreduction of these parameter values in both genotypes,with ZDB475 being much more affected than ZDB160.As treatment had little effect on the total GSH content in ZDB475, but increased by 1.44-fold in ZDB160 in comparison with the control. Although P addition induced a more increase of total GSH content in ZDB160 than in ZDB475, the increase was comparable compared to As treatment alone. As treatment induced a drastic reduction in the GSH/GSSG ratio in ZDB475, while it had relatively small effect on ZDB160. Remarkable increase in the GSH/GSSG ratio was detected upon P addition, with ZDB160 having much more increase than ZDB475.

Table 4 Effects of arsenic (As) and As+phosphorus (P) treatments on superoxide dismutase (SOD) and catalase (CAT) contents of root in 2 barley genotypes
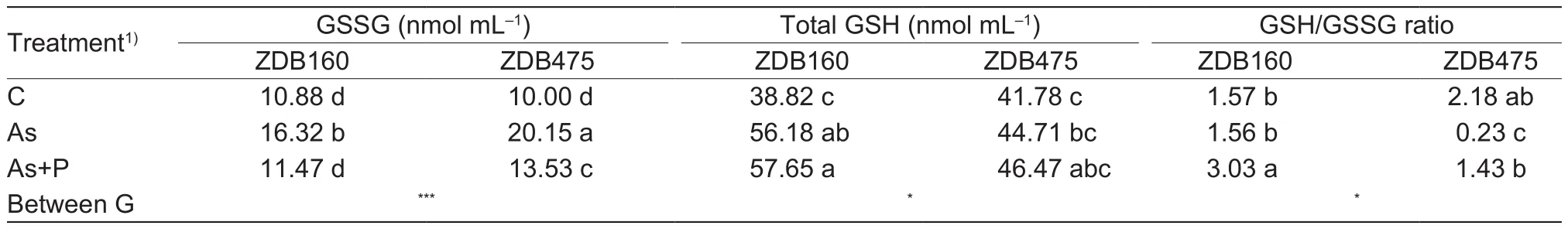
Table 5 Effects of arsenic (As) and As+phosphorus (P) treatments on root reduced glutathione (GSSG), total glutathione (total GSH) and GSH/GSS ratio in 2 barley genotypes
3.5. Expression of transporter genes
The relative expression of transporter genes are presented in Fig. 2. There was a significant difference between genotypes under different treatments in the expression of all the transporter genes examined in this study. In comparison to the control, the relative expression ofHvPHT1.1,HvPHT1.3,HvPHT1.4, andHvPHT1.6was significantly down-regulated in ZDB160 exposed to As treatment in the absence of P, while a significant up-regulation was observed in ZDB475 under the same conditions. The fold of reduction in the expression ofHvPHT1.1,HvPHT1.3,HvPHT1.4,andHvPHT1.6in ZDB160 under As treatment was 1.6,1.9, 1.8, and 3.1, respectively, while the fold increase in ZDB475 was 4.1, 2.1, 1.9, and 3.5, respectively. In regards toHvPHO2transporters, no significant difference in the relative expression was observed in ZDB160 between As treatment and control, while ZDB475 showed high up-regulation. However, the contrasting results were observed in the relative expression of PHO1.2 in regards to ZDB160 while in ZDB475 they were the same. P addition elucidated varying responses in the relative expression of the transporter genes. There was an exponential increase in the relative expression of the PHTs in ZDB160 exposed to the simultaneous additions of As and P, with the highest relative expression being observed inHvPHT1.1. The fold increase in the relative expression ofHvPHT1.1,HvPHT1.3,HvPHT1.4, andHvPHT1.6was 8.3, 1.9, 2.5, and 4.3,respectively in comparison to the control. However, in ZDB475, different responses for the relative expression of the same PHTs were observed. Although there was a significant reduction in the relative expression of the PHTs for the As+P treatment in comparison to As treatment alone,no much significant difference was detected between As+P treatment and control. In addition, P addition lowered the relative expression of the PHO transporter genes in comparison to that of alone As treatment, with the effects being much more pronounced in ZDB160 (HvPHO1.2) and ZDB475 (HvPHO2).
3.6. Expression of defense and detoxification genes

Fig. 2 Relative expression of barley. PHT1.1 (A), PHT1.3 (B), PHT1.4 (C), PHT1.6 (D), PHO1.2 (E), and HvPHO2 (F) in roots of 2 barley genotypes (ZDB160 and ZDB475) under different arsenic (As) and phosphorus (P) treatments. C, control; As, As(100 μmol L–1); As+P, As (100 μmol L–1)+P (50 μmol L–1). All values are means of 3 replicates (±SE). Different letters indicate significant difference at P<0.05 probability level.
The relative expression of various defense and detoxification genes was assayed, and significant differences between genotypes and among treatments were observed (Fig. 3).In the absence of P, As increased the expression ofHvSOD(5.0, 1.68),HvCAT2(48.0, 32.8),HvGR1(2.04, 2.09),HvGSLT1(1.2, 2.19),HvGPx15(1.02, 1.52),HvHAC1.1(1.72, 1.78), andHvABCC1(2.24, 5.26) in ZDB160 and ZDB475, respectively in comparison with the control. The relative expression of antioxidant enzymes-related genes(HvSODandHvCAT) was higher in As-tolerant genotype(ZDB160) than the sensitive genotype (ZDB475). However for the glutathione-related genes (HvGR1,HvGSTL1,andHvGPx), the relative expression between ZDB160 and ZDB475 was comparable. The effects of P addition in alleviating As toxicity were clearly visible such that it repressed the genes which were up-regulated in the alone As treatment. The effect was more pronounced in ZDB160,and the decreased folds of relative expression were:HvSOD(3.31),HvCAT(22.2),HvGR1(1.52),HvGPx15(0.61),andHvABCC1(0.22) relatively to the alone As treatment.While, for ZDB475, the decreased folds of the relative expression wereHvCAT2(16.8),HvGR1(1.76),HvGSLT2(0.72), andHvGPx15(0.89) relatively to the alone As treatment. Interestingly, the relative expression ofHvABCC1significantly increased in the As+P treatment in comparison with alone As treatment alone.
3.7. Expression of As and P responsive TFs
The expression pattern of As-responsive TF was analyzed and significant differences in the expression patterns of the TFs between genotypes and treatments were observed(Fig. 4). Among all the TFs analyzed in this study, fold decrease in the relative expression of the P responsive TF,HvMyb1, was noted regardless of the genotype and treatment. WRKY TFs responded differently to As and P treatment. As treatment increased the relative expression ofWRKY6in both ZDB160 (2.13) and ZDB475 (1.61), and conversely reduced the relative expression ofWRKY45in both ZDB160 (3.32) and ZDB475 (2.13). Interestingly,relative expression ofWRKY6andWRKY45were obviously increased in As+P treatment, with ZDB475 being more affected relative to ZDB475.HvAP2/ERF, being an Asresponsive TF, was strongly induced under As treatment in ZDB475, an As-sensitive genotype and subsequently repressed by P addition.
4. Discussion

Fig. 3 Relative expression of barley. HvSOD (A), HvCAT2 (B), HvGR1 (C), HvGSLT1 (D), HvGSLT2 (E), HvGPx15 (F), HvHAC1(G), and HvABCC1 (H) in roots of 2 barley genotypes (ZDB160 and ZDB475) under different arsenic (As) and phosphorus (P)treatments. C, control; As, As (100 μmol L–1); As+P, As (100 μmol L–1)+P (50 μmol L–1). All values are means of 3 replicates (±SE).Different letters indicate significant differences at P<0.05 probability level.
Plants have evolved various mechanisms to combat the adverse effects of heavy metals, such as restriction of uptake or transport, compartmentalization within the cells and alteration of cellular metabolism including more production of enzymatic and non-enzymatic antioxidants.Upon exposure to As, plants also restrict As (V) uptake in As-tolerant genotypes (Zvobgoet al. 2018a), increase the production of enzymes (Harbet al. 2015) and sequestration by phytochelatins (Hayashiet al. 2017). However, these responses are more pronounced in P-limiting conditions(Vrommanet al. 2017) because of the antagonistic effects between As and P. In this study, we aimed at investigating the mechanisms of barley in As tolerance under the different P levels. Under As and As+P stress, a significant difference in plant biomass was observed between tolerant and sensitive barley genotypes. This corroborated with the shoot As concentrations and TF, with ZDB475 being higher than ZDB160. It is true that As non-hyperaccumulaters incorporate the strategies of restricting As translocation from root to shoot as one of their defense mechanism against As(Zvobgoet al. 2015). It is clear from Fig. 1 that the shoots of ZDB475 showed stunted growth under As exposure,indicating its sensitiveness to As toxicity and lesser effect of P in alleviating As toxicity.

Fig. 4 Relative expression of barley. WRKY6 (A), WRKY45 (B), HvAP2/ERF (C), HvZF1 (D), HvMYb1 (E), and HvPHR1 (F) in roots of 2 barley genotypes (ZDB160 and ZDB475) under different arsenic (As) and phosphorus (P) treatments. C, control; As,As (100 μmol L–1); As+P, As (100 μmol L–1)+P (50 μmol L–1). All values are means of 3 replicates (±SE). Different letters indicate significant differences at P<0.05.
As and P are co-transported from root to shootviaroot localized PHTs (Tu and Ma 2005). In our previous study, we observed some As-responsive PHTs. In order to understand the genotypic difference in the expression of the PHTs affected by As and P levels, we investigated mRNA transcript abundance in ZDB160 and ZDB475 upon As and As+P addition. Analysis of tissue As concentration clearly showed that despite the reduced As uptake, As-tolerant plants still assimilate As even though at lower rates compared to nontolerant plants. It is interesting that regardless of the PHT,fold increase in the mRNA transcripts was observed in the As-sensitive genotype. We presume that this response of PHTs to As in ZDB475 is preserved across all the detected PHTs. The rapid up-regulation of PHT expression in ZDB160 upon limited P addition indicates the af finity of ZDB160 to P,while in ZDB475 the change was not pronounced. Similar results were also reported inArabidopsis(Shuklaet al.2015). It is assumed that P deficiency can be bene ficial to As uptake by inducing competition for binding sites and also by inducing P starvation responses (Mueheet al. 2014). In tobacco, As-tolerant genotypes were restored to their normal metabolism upon low P additions while As-sensitive genotypes required P concentration of 500 μmol L–1for the same responses (Zvobgoet al. 2015).Although the suppressed P/As transport system of As tolerance is a well established fact (Meharg and Macnair 1992), this study further illustrated that upon low P addition,As-tolerant genotype increased PHT mRNA transcripts,enabling them to uptake more P and thereby restricting As uptake by competitive inhibition. This finding can be implemented in As prone areas where low quantities of P can be used to restrict As uptake. In addition we also investigated how some selected PHO transporters are regulated by As and P addition. The PHO transporters are involved in the loading of P into the xylem and may also be involved in P signaling (Bariet al. 2006). In rice plants,moreHvPHO1.2andHvPHO2transcripts were detected in roots upon addition of As (Mueheet al. 2014). Our results also showed that upon alone addition of As, the expression of PHO transporters was higher while supplementation with P lowered the expression. These results were in line with the report by Shuklaet al. (2015). The PHO transporters are negative regulators of P movementviathe xylem, thus the increased relative expression of the PHO transporters under As stress results in lesser movement of P, while inversely, the decreased expression upon P addition increased P transportation. Upon comparison of TF of P and expression of PHO transporters, it is clear that the lower relative expression ofHvPHO1.2andHvPHO2under limited P addition in ZDB160 could have resulted in the greater translocation of P to the shoots. Thus the inverse relationship of the PHTs with the PHO relative expression in ZDB160 could contribute to the tolerance strategy employed by this genotype in response to P addition.
It is widely known that regulatory factors controlling expression of PHTs under As and P conditions are different(Wanget al. 2014), which could be related to the genotypic difference in TFs (Shuklaet al. 2015). In this study, we studied how some As and P related TFs are expressed under As and As+P limiting conditions. WRKY6 is an As-responsive TF which rapidly repress the expression ofPHT1.1(Robatzek and Somssich 2002). Functional analysis of theWRKY6usingArabidopsismutants, revealed the repression of the As/P transporterPHT1.1upon As addition(Castrilloet al. 2013), being consistent with the results reported by Shuklaet al. (2015) thatWRKY6andHvPHT1.1expression increased and decreased respectively, under P deficient+As stress condition. Our current results also showed that with the increased expression ofWRKY6in ZDB160, all the studied PHTs were down-regulated, while the contrast was true in ZDB475. Although P was added in this study, the dosages were still deficient which might explain why slight change was observed in the relative expression of theWRKY6TF. Clearly the induction ofWRKY6in ZDB160 upon As+P addition could be accounted for the down-regulation of theHvPHT1.1transporters. Thus As toxicity could be ascribed to the expression ofWRKY6TF, which inhibited PHT mRNA transcripts. However, further functional studies in barley PHT transporters in response to As transport are still required in order to obtain concrete information on As transport.
WhileWRKY6is an As-responsive TF,WRKY45is a P-responsive TF, localized in the nucleus and expressed in the roots and will be induced under P starvation. Under P starvation, plants adopt 2 strategies to monitor P availability,which are local and systematic signaling (Mueheet al. 2014)with the former depending on external P concentration while the latter takes into account for P status of the whole plant.P itself acts as a systematic signal, thus As-exposed plants may respond in 2 ways, either they can perfectly replace P with As or some P utilizing molecules like ATP may fail to recognize As due to some physical difference. Because WRKY45 is a P-responsive TF, we aimed at studying how As availability and P deficiency would regulate its expression.Earlier reports by Wanget al. (2014) showed that WRKY45 was induced by P deficiency. Surprisingly in our study, alone As addition caused a repression of the WRKY45 expression,with ZDB160 showing more reduction than ZDB475. We assumed that the lower relative expression in ZDB160 could have repressed the expression of the PHTs thus resulting in lower P and As uptake, while the contrast could be true for the As-sensitive genotype, which had conversely higher tissue P and As concentrations. We postulated that the induction ofWRKY45upon As addition would directly affect PHT mRNA transcripts through binding to the 2 W-boxes on the PHTs, resulting in enhanced As and P uptake, while the repression ofWRKY45would result in prevention of As toxicity through the same mechanism. It was also found thatWRKY45expression was induced under P deficient+As condition (Mueheet al. 2014). InArabidopsis,WRKY45positively regulated P uptake, which may explain the up-regulation ofHvPHT1.2in ZDB160 under the same treatment. The regulation of PHTs is not only controlled by a single TF, but also by a multiple of them in a coordinated way. Thus, more As and P WRKY TFs need to be further studied so as to reveal the whole mechanism.
In rice, about 231 As-responsive TFs were detected through whole genome transcriptomics studies (Huanget al.2012). In this study, we aimed at determining how some of these As-TFs were differentially expressed in the barley genotypes differing in As tolerance under As and As+P conditions and how they might bring about As tolerance.AP2/ERF TFs are important in plant development and respond to biotic and abiotic stresses. Of the 5 AP2 TF subfamilies, DREB and ERF contain proteins that bind to deficientcis-regulatory sequences. Our studies con firmed the function ofHvAP2/ERFTFs acting as As-responsive TFs, which is in line with the results reported by Huanget al.(2012). It could be assumed that the As-sensitive genotype stimulated the synthesis of AP2/ERF transcripts to cope with more As uptake. However, under P supplementation, the As-tolerant genotype had the similarHvAP2/ERFrelative expression with the control, suggesting the role of P in inhibiting As uptake and expression of related genes to a greater extent in the As-tolerant genotypes.
In addition, we also found thatHvMYB1TF was significantly down-regulated in both barley genotypes exposed to As or As+P treatment. The results were inconsistent with those reported by Huanget al. (2012).The possible reason is due to higher As concentration employed in this study, which caused severe damage to plants. However, other studies focused on P starvation responses revealed that over-expression of MYB TF induced the expression of PHT, resulting in increased P uptake (Daiet al. 2012). The results could explain down-regulation ofHvMYB1TF, causing a dramatic impact on some PHTs, and resulting in less As and P uptake in the As-tolerant genotype under As condition. P addition reduced relative expression ofHvMYB1TF, which caused up-regulation of other PHTs,resulting in more P uptake. Obviously, it is imperative to make further studies on responses of MYB TFs to As and P.
PHR1-miR399-PHO2 pathway is a core component in the P starvation pathway. Thus we aimed at studying how As would in fluence the relative expression of some TFs involved in the pathway. P starvation response 1 (PHR1)is associated with a MYB TF which is a key regulator of P starvation (Daiet al. 2012). PHR1 targets miR399 and is induced by P starvation and they negatively regulatePHO2expression (Chiouet al. 2006). However, in our study there was no significant difference in the relative expression ofHvPHR1between As and As+P treatments. This could be due to the replacement of P with As which plants could not differentiate or due to the treatment conditions which did not completely starve plants of P as shown in P concentration measurement. It is also imperative to understand whether the PHR1-miR399-PHO2 pathway is also responsible for the response of plants to As.
It is well known that As is an oxidative stress agent that can induce cell damage through excessive reactive oxygen species (ROS) production in plants (Begumet al.2016). ROS are toxic to plants and can inhibit cell growth,even resulting in cell death. Excess ROS in plants may be eliminated by ROS scavengers, both anti-oxidative enzymes and antioxidants. The rapid production of ROS scavengers is crucial for As tolerance in plants (Huanget al. 2012).The greater stimulation of SOD and CAT activities together with the higher relative expression of the related genes in ZDB160 could explain its higher As tolerance, which is in agreement with other studies (Harbet al. 2015; Zvobgoet al. 2015; Begumet al. 2016). In addition, we wanted to understand how P addition could affect these ROS scavengers and their relative mRNA transcript level. Our results showed that P addition caused the dramatic reduction ofHvSODandHvCATmRNA transcript level. Obviously, it is due to the reduced As uptake in the As-tolerant genotype.
Glutathione, as an important antioxidant in plants, exists in either reduced (GSH) or oxidized form (GSSG) (Štepigováet al. 2007). Glutathione peroxidase catalyzes oxidation of GSH into GSSG, with glutathione reductase recycling the reduction of GSSG to GSH (Huanget al. 2012). Under As stress we observed high level of GSSG, total GSH and low level of GSH/GSSG ratio. The smaller GSH/GSSG ratio in the As-sensitive genotype under As and As+P conditions was an indication of oxidative stress. While the smaller ratio in ZDB475 even upon P addition could explain its sensitivity to As toxicity. However, analysis of the relative expression ofHvGRandHvHAC1showed no significant difference between the 2 barley genotypes under As or As+P treatments. HAC is an arsenate reductase which was observed as a novel locus by GWA mapping (Sánchez-Bermejoet al. 2014). In another study onArabidopsis,no significant difference was found in the expression of HAC upon As stress and P conditions (Shuklaet al. 2015).Furthermore, ZDB475 had higher relative expression ofHvGSLT1,HvGSLT2, andHvGPxthan ZDB160. This could be due to a different mechanism conferring As tolerance in ZDB160 apart from the glutathione system. GSTs are defense enzymes which reduce peroxidases with the help of GSH while GPx also detoxify H2O2with GSH acting as a reductant (Huanget al. 2012). Meharg and Macnair (1992)postulated that As tolerance was due to slow As uptake which could account for the results observed in ZDB160.Upon As uptake, As (V) is reduced to As (III) which is sequestrated into vacuoles by phytochelatins complexviaABCC transporters (Hayashiet al. 2017). P addition significantly reduced the expression ofHvABCC1in the As-tolerant genotype which was an indication of As stress alleviation by P. The significant upregulation ofHvABCC1under As and As+P conditions could suggest that ZDB475 may accumulate more As resulting in increased sensitivity toward As stress, which was also reported in As-sensitiveArabidopsis(Shuklaet al. 2015).
5. Conclusion
As tolerance in plants is a complex mechanism that involves various processes. In this study, we unraveled how various plant transport, defense, and detoxification mechanisms are regulated by As and limited P additions.The phyto-toxicity caused by As stress differed between the 2 barley genotypes, with ZDB160 being less affected than ZDB475. The results showed that P addition could alleviate As toxicity by regulating the expression of As transport, defense and detoxification genes, resulting in less As accumulation in shoots, which is in turn associated with less growth inhibition. Therefore, we recommend whole genome transcriptomics under As and P conditions in order to deepen our understanding of the mechanisms underlying As tolerance under limited P conditions. This will help in the precise modifications of plants to alleviate As toxicity under low P conditions.
Acknowledgements
The authors wish to thank the College of Life Science of Zhejiang University for elemental analysis and the financial support from the National Natural Science Foundation of China (31330055), the earmarked fund for China Agriculture Research System (CARS-05-02A-01) and the Jiangsu Collaborative Innovation Center for Modern Crop Production, China (JCIC-MCP).
 Journal of Integrative Agriculture2019年2期
Journal of Integrative Agriculture2019年2期
- Journal of Integrative Agriculture的其它文章
- Modelling and mapping soil erosion potential in China
- Updating conventional soil maps by mining soil–environment relationships from individual soil polygons
- Spatial variability of soil total nitrogen, phosphorus and potassium in Renshou County of Sichuan Basin, China
- Spatial variability of soil bulk density and its controlling factors in an agricultural intensive area of Chengdu Plain, Southwest China
- An integrated method of selecting environmental covariates for predictive soil depth mapping
- Remotely sensed estimation and mapping of soil moisture by eliminating the effect of vegetation cover
