Experimental study on the effect of An-pressing and Rou-kneading Huantiao (GB 30) on certain brain nuclei of pleasure circuits in rats with chronic neuralgia
Xiao Bin (肖彬), Li Zheng-yu (李征宇), Yu Zhong-yi (俞仲毅), Zhang Jin (张进), Yan Jun-jie (严俊洁), Liu Xiao (刘骁)
1 College of Acupuncture and Tuina, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
2 Research Center, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
3 Shanghai Literature Institute of Traditional Chinese Medicine, Shanghai 200020, China
4 Shanghai East Hospital, Shanghai 200120, China
5 College of Pharmacy, Fudan University, Shanghai 200032, China
Abstract Objective: To explore the central neurobiological mechanisms of pleasure effect on rats with neuralgia treated by tuina manipulations of An-pressing and Rou-kneading Huantiao (GB 30).
Keywords: Tuina; Massage; Neuralgia; Analgesia; Endorphins; Pro-opiomelanocortin; Rats
Tuina is an ancient therapy based on the fundamental theory of traditional Chinese medicine(TCM). Stimulating the Ashi points, which has previously been applied for analgesia, is commonly and widely used by the acupuncturists and tuina practitioners[1-2].Tuina therapy is effective in treating neuralgia, since it can obviously relieve pain and provide a comfortable feeling. Clinical data of tuina showed that most patients with chronic pain like to be An-pressed and Roukneaded at their Ashi points (or tender points)[3-4]. In order to preliminarily explore the mechanism of tuina for neuralgia, our research observed the analgesic effect of An-pressing and Rou-kneading Huantiao(GB 30), as well as its influence on certain brain nuclei of the pleasure circuits in rats with neuralgia.
1 Materials
1.1 Experimental animals
Sixty-four male Sprague-Dawley (SD) rats weighing 180-200 g were selected. They were bought from Sino-British SIPPR/BK Lab Animal Ltd. (Shanghai)[certification number: 152 (Shanghai)], and bred at the Experimental Animal Center, Shanghai University of Traditional Chinese Medicine [certification number:SCKX (Shanghai) 2003-0003, laboratory animal facilities:clean grade]. They were housed on a cycle of 12 h day-12 h night with food and water available ad libitum.This disposal of animals during the experiment was consistent with animal ethics.
1.2 Apparatuses
PL-200 thermal pain threshold detector (Thai Union Technology Co., Ltd., China); Reiche-Jung semiconductor slicer (Leica Biosystems, Germany);LW-200LFT optical microscope (Leica Microsystems,Germany); Canon A650 camera (Canon, China),Image-Pro Plus 6.0 professional image analysis software(Media Cybernetics, USA).
1.3 Reagents
5% BSA blocking buffer, monoclonal mouse anti-rabbit immunoglobin G (IgG) labeled with biotin,β-endorphin antibody, immunohistochemical ABC kit and DAB coloration kit (Wuhan Boster Biological Engineering Co., Ltd., China); pro-opiomelanocortin(POMC) in situ hybridization kit, methyl green-pyronine and acetylcholinesterase staining solution (Shanghai Rombo Biological Engineering Co., Ltd., China).
2 Methods
2.1 Modeling methods
The chronic constriction injury (CCI) model of neuralgia was inducted[4]. After 7-day adaptively feeding and weight balanced, in accordance with the random number table, 18 rats were randomly selected as a normal group and the other 46 rats were selected to develop neuralgia models by method of sciatic nerve ligation[5]: the 46 rats were anesthetized with sodium pentobarbital [40 mg/(kg·bw) i.p.]. The rat's left sciatic nerve was exposed. The sciatic nerve was ligated with a 4/0 surgical catgut slightly. Rats were injected with potassium penicillin G 100 000 U after the surgery.Three day after modeling, pain tolerance value was measured. Rats with obvious swollen feet accompanied with limping were considered as successful neuralgia models.
2.2 Grouping and interventions
Before modeling, according to the random number table, 18 rats were randomly selected from 64 rats as a normal group. The other 46 rats were used to duplicate CCI models. Ten rats failed in modeling, and the other 36 successful modeled rats were randomly divided into a model group and a tuina group according to the random number table, with 18 rats in each group.
Normal group: The rats in the normal group received no intervention. The thermal pain test was conducted before treatment, after one-week treatment, two-week treatment and three-week treatment. Six rats were randomly selected according to the random number table at each time point respectively for measuring pain-sensitivity score. The whole brains were taken out directly and immunohistochemistry and in situ hybridization experiments were conducted.
Model group: The rats in the model group received no intervention after modeling. The rest of the procedure was the same as that in the normal group.
Tuina group: The rats in the tuina group were treated with An-pressing and Rou-kneading Huantiao (GB 30)after modeling. Selection of tender points: after the sciatic nerve was ligated, local swelling was obvious.Slightly stimulating the local area would cause the rat to struggle and resist. According to the point-selection principle of taking the tender point as acupoint,Huantiao (GB 30) was just in the sciatic nerve ligation area, so it was selected as the treatment point. The rats in the tuina group were fixed. The top of stimulating stick was applied to the left Huantiao (GB 30) (where had received the operation) with An-pressing and Rou-kneading manipulations for 1 min, with stress of 0.1 kg and frequency of 50 times/min (measured by detector technique in vivo), once a day, for three weeks. The rest of the procedure was the same as that in the normal group.
All groups were fed with normal food and water.
2.3 Observation items and testing methods
2.3.1 Thermal pain test for measuring pain-sensitivity score
In quiet environment, with room temperature at(20±2) ℃, PL-200 thermal pain threshold detector was applied to measure the latency period of lifting feet.The difference value between the two legs (the operation side and the control side) was taken as the pain-sensitivity score (s).
2.3.2 Immunohistochemistry for assaying β-endorphin
Preparation of tissue slices: For each rat, under anesthesia with pentobarbital sodium [40 mg/(kg·bw),i.p.], inserted a tube into the left ventricle, and infused 0.9% sodium chloride 200 mL and 4%paraformaldehyde in 0.1 mol/L sodium phosphate buffer (PBS) 400 mL in turn. After that, took the brain out and put it into 20% sucrose solution (with 4%paraformaldehyde, 4 ℃). After 4-6 h, put the brain into 30% sucrose solution (4 ℃). After the specimen sinking to the bottom of the container, used semiconductor refrigeration slicer to make 30 μm thick frozen coronal sections and put them into 0.05 mol/L PBS with 30%sucrose and 30% ethylene glycol. Kept them at -20 ℃.
Location of brain nuclei: The brain nuclei were located according to the Stereotactic Atlas of Rat's Brain[6-7]. Because the apex line was difficult to find while the median line of sagittal suture was clear, and the exposed area of amygdaloid nucleus, accumbens nucleus and arcuate nucleus in sagittal slices were larger than those in coronal slices, we sliced the brains sagittally, from the middle to the sides.
ABC method[8]: Douched the sections with 0.01 mol/L PBS; then put them into 10% goat serum incubating liquid for 30 min (37 ℃); added anti-β-endorphin antibody; after 4 h, continued incubating for 48 h at 4 ℃. The sections were washed in 0.01 mol/L PBS and then put into biotin labeled goat anti-rabbit IgG for 1 h.Washed the sections with 0.01 mol/L PBS and then put them into ABC liquid for DAB coloration 1 h later.
2.3.3 Assaying POMC with in situ hybridization[9]
Fixed the sections in 4% paraformaldehyde-0.1 mol/L PBS stationary liquid (containing 1/1 000 DEPC) for 20-30 min at room temperature; washed it with distilled water for 30 min; mixed 30% hydrogen peroxide and pure methanol together at a proportion of 1:50, and soaked the sections in for 30 min at room temperature to inactivate endogenous peroxidase; for in situ hybridization, applied multiphase 3'-endian digoxin labeled probes, and biotinylated rat anti-digoxin antibody, followed by biotin-streptoavidin peroxidase staining method; stained the sections at room temperature and controlled the reaction time under microscope; terminated the coloration by washing the sections with distilled water after the process was completed; smeared with methyl green-pyronin staining solution; then dehydrated the sections, and cleared them with xylene, and mounted them with neutral gum at last.
2.3.4 Nissl staining and locating brain nuclei
With nissl staining, the brain nuclei could be found easily. According to the Stereotactic Atlas of Rat's Brain[6-7], the brain nuclei could be located accurately.Found amygdaloid nucleus, accumbens nucleus and arcuate nucleus under microscopes with 4×magnification. Took photos for amygdaloid nucleus,accumbens nucleus and arcuate nucleus under 40×magnification, and analyzed them with Image-Pro Plus 6.0 professional image analysis software. Under the counting menu, we chose the positive cells first, and then counted the number of positive cells of each nucleus in a certain area automatically in these photos.
2.4 Statistical analysis
All values were analyzed by the SPSS version 18.0 software. The measurement data were presented as mean ± standard deviation ( x ±s), and the differences between the two groups were evaluated by one-way ANOVA if the data were in normal distribution.Differences among each group were evaluated by least significant difference (LSD) test if the data had homogeneity of variance. Between-group differences were evaluated by non-parametric test if the data were not in normal distribution. P<0.05 (bilateral) was considered as statistically significant.
3 Results
3.1 Effect of tuina therapy on pain-sensitivity score of rats with neuralgia
Before treatment, the scores of the tuina group and the model group were (-0.831±0.812) and(-0.828±0.547), and there was no significant difference(P>0.05). The statistical difference between the tuina group and the model group appeared after one-week treatment (P<0.05), after two-week treatment (P<0.05)and after three-week treatment (P<0.01). With the increase of time, the pain-sensitivity score gradually decreased. The difference between the tuina group and the normal group had no statistical significance (P>0.05)from the 15th day of treatment. So, the tuina therapy of An-pressing and Rou-kneading Huantiao (GB 30) could obviously decrease the pain-sensitivity scores of rats with neuralgia, and it had significant accumulative analgesic effect (Figure 1).

Figure 1. Influence of tuina therapy on pain-sensitivity score of rats with neuralgia after different time of treatmentNote: Compared with the normal group, 1) P<0.05; 2) P<0.01; compared with the model group, 3) P<0.01
3.2 Changes of the number of Nissl's body in different brain nuclei at different time points in each group
The result analyzed by the software showed that the numbers of Nissl's body of the model group in arcuate nucleus, accumbens nucleus and amygdaloid nucleus were reduced, which indicated that the function of these nuclei got weakened. After treated respectively for one week, two weeks and three weeks, the numbers of Nissl's body of the tuina group in these nuclei were all much higher than those of the model group, with significant differences (all P<0.01), while the differences between the tuina group and the normal group were not statistically significant (all P>0.05), which indicated that tuina therapy of An-pressing and Rou-kneading Huantiao (GB 30) could accelerate the recovery of the function of these nuclei (Figure 2, Figure 3 and Figure 4).

Figure 2. Number of Nissl's Body in each nucleus after treatment for one week (×40)Note: Compared with the normal group, 1) P<0.05, 2) P<0.01; compared with the model group, 3) P<0.01

Figure 3. Number of Nissl's Body in each nucleus after treatment for two weeks (×40)Note: Compared with the normal group, 1) P<0.01; compared with the model group, 2) P<0.01

Figure 4. Number of Nissl's Body in each nucleus after treatment for three weeks (×40)Note: Compared with the normal group, 1) P<0.05, 2) P<0.01; compared with the model group, 3) P<0.01
3.3 Changes of β-endorphin immune positive cells in accumbens nucleus and amygdaloid nucleus of rats in each group
β-endorphin immune positive substances were distributed in accumbens nucleus, amygdaloid nucleus,etc. The result showed that the numbers of β-endorphin immune positive cells of the model group in accumbens nucleus and amygdaloid nucleus were reduced, and the differences between the model group and the normal group were statistically significant (all P<0.01) after 1-week treatment. After treated respectively for one week, two weeks and three weeks,the numbers of β-endorphin immune positive cells of the tuina group in these nuclei were all much higher than those of the model group with significant differences (all P<0.01), and the differences between the tuina group and the normal group were not statistically significant (all P>0.05). The result indicated that tuina therapy of An-pressing and Rou-kneading Huantiao (GB 30) could increase the number of β-endorphin immune positive cells in accumbens nucleus and amygdaloid nucleus in rats with neuralgia after treating respectively for one week, two weeks and three weeks, which might be related to its analgesic and pleasure effect (Table 1, Table 2 and Figure 5-Figure 10).

Table 1. Changes of β-endorphin immune positive cells in accumbens nucleus of rats in each group ( x ±s)

Table 2. Changes of β-endorphin immune positive cells in amygdaloid nucleus of rats in each group ( x ±s)
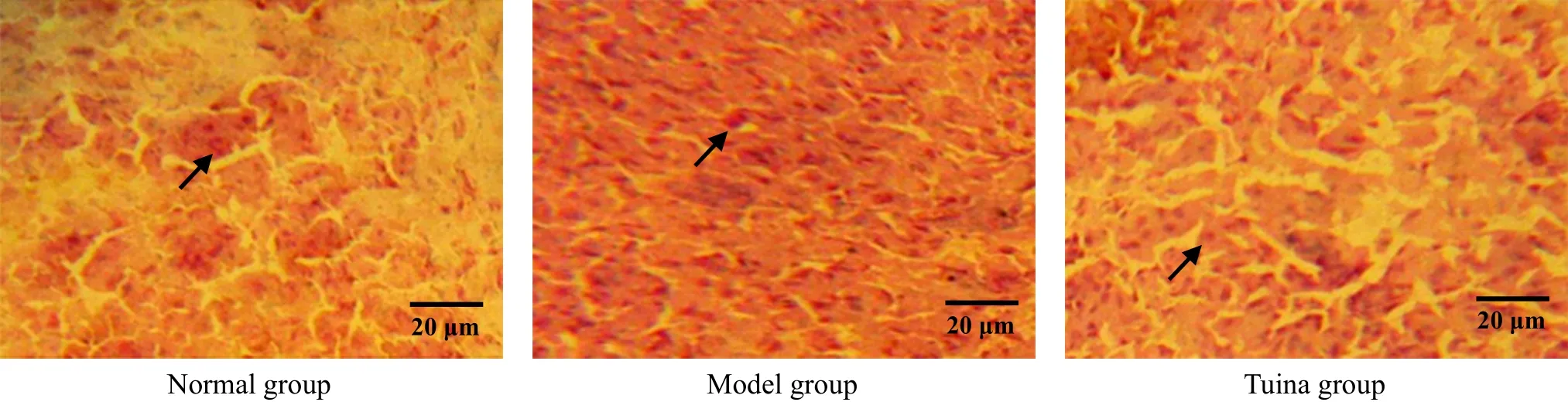
Figure 5. β-endorphin in accumbens nucleus by immunohistochemistry after 1-week treatment (methyl-green-pyronin, ×400)
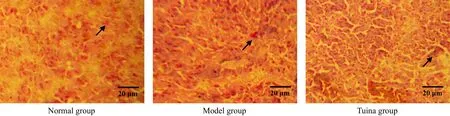
Figure 6. β-endorphin in accumbens nucleus by immunohistochemistry after 2-week treatment (methyl-green-pyronin, ×400)

Figure 7. β-endorphin in accumbens nucleus by immunohistochemistry after 3-week treatment (methyl-green-pyronin, ×400)
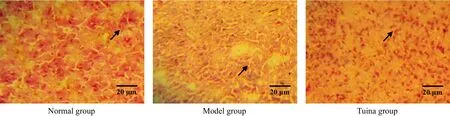
Figure 8. β-endorphin in amygdaloid nucleus by immunohistochemistry after 1-week treatment (methyl-green-pyronin, ×400)
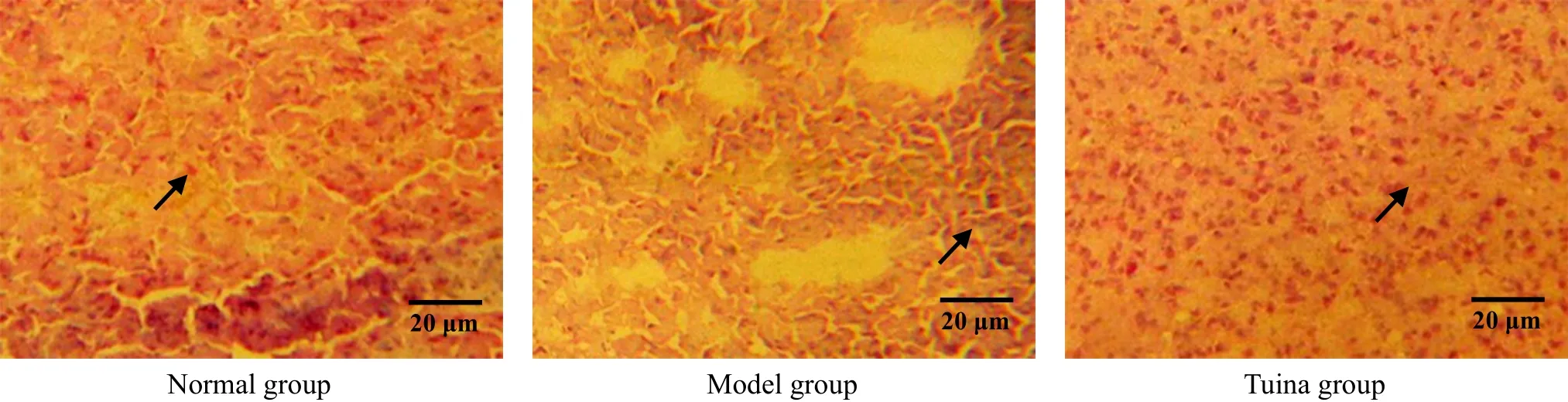
Figure 9. β-endorphin in amygdaloid nucleus by immunohistochemistry after 2-week treatment (methyl-green-pyronin, ×400)

Figure 10. β-endorphin in amygdaloid nucleus by immunohistochemistry after 3-week treatment (methyl-green-pyronin, ×400)
3.4 Changes of POMC positive cells in arcuate nucleus in each group
POMC was distributed in arcuate nucleus. The result showed that the number of POMC positive cells of the model group in arcuate nucleus was reduced(Figure 11-Figure 13), and the difference between the model group and the normal group was statistically significant (all P<0.01) at different time points. After treated respectively for one week, two weeks and three weeks, the numbers of POMC positive cells in arcuate nucleus of the tuina group were much higher than those of the model group, with significant differences(all P<0.01), and the differences between the tuina group and the normal group were not statistically significant (all P>0.05). The result indicated that tuina therapy of An-pressing and Rou-kneading Huantiao(GB 30) could increase the number of POMC positive cells in arcuate nucleus after treating respectively for one week, two weeks and three weeks (Figure 11-Figure 13, Table 3).

Figure 11. POMC in situ hybridization in arcuate nucleus after 1-week treatment (methyl-green-pyronin, ×400)
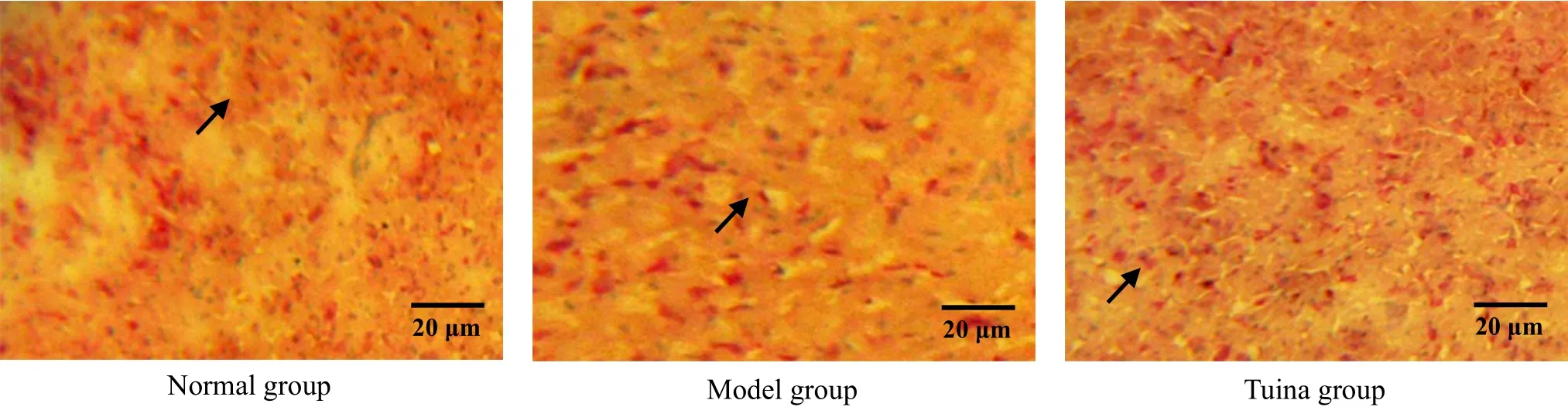
Figure 12. POMC in situ hybridization in arcuate nucleus after 2-week treatment (methyl-green-pyronin, ×400)
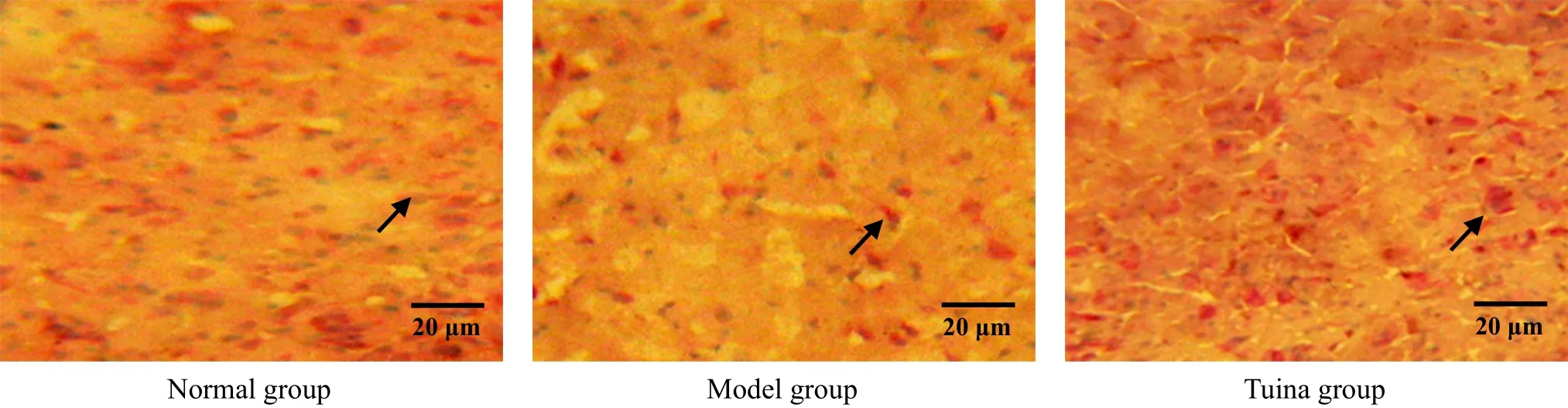
Figure 13. POMC in situ hybridization in arcuate nucleus after 3-week treatment (methyl-green-pyronin, ×400)

Table 3. Changes of POMC in situ hybridization in arcuate nucleus of rats in each group ( x ±s)
4 Discussion
Neuralgia is a common type of chronic pain and is characterized by excessive sensitivity in the damaged area and adjacent tissues[10]. The pain will last for a long time after the damage is cured, which brings on great suffering to the patients. So, it is a significant problem for us to solve in clinic[11]. Currently, the methods of treatment include drug, nerve block, physical therapy,etc. Tuina therapy has been used for analgesia for a long time. ‘An-pressing the tender point can relieve pain',recorded in Huang Di Nei Jing (Yellow Emperor's Classic of Internal Medicine), indicating that people touched and pressed tender points when they felt painful in ancient times. Nowadays, tuina therapy of An-pressing and Rou-kneading the tender points is a common and effective method for relieving neuralgia[12-13].
In this research, the method of a slight sciatic nerve ligation was used to make a neuralgia model, and the thermal pain test was used to evaluate the pain severity.Huantiao (GB 30), where had operation, was An-pressed and Rou-kneaded, which imitated the tuina therapy of An-pressing and Rou-kneading Huantiao(GB 30). The pain-sensitivity score as well as pain behaviors were observed to explore the effect of the therapy on rats with neuralgia. The result showed that the therapy could improve pain threshold and reduce pain-sensitivity score, and the differences between the tuina group and the model group were statistically significant (P<0.05) after one-week treatment, which indicated that tuina therapy of An-pressing and Rou-kneading Huantiao (GB 30) had significant analgesic effect, and the effect was accumulative. The outcome was the same as that of our previous experiments[14].
Research has shown that analgesic effect is related to certain brain nuclei[15]. In the past two decades, the pain research was focused on pain circuits and pleasure circuits. Pleasure circuits include accumbens nucleus,amygdaloid nucleus, etc. Amygdaloid nucleus, which contains a large amount of opioid receptors,dopaminergic nerve fibers and cholinergic nerve fibers,is the key part for regulating pleasure emotion[16-17].Accumbens nucleus, which contains a large amount of endogenous opioid peptides, has the function of behavior regulation and analgesia, and also participates in the reward effect[18]. Experiments have shown that neurons in the central nervous system transmit signal and produce physiological effect through various kinds of neurotransmitters and active substances. Therefore,the variation of neurotransmitters and active substances in certain brain nuclei of pleasure circuits can reflect the active state of these nuclei. Nissl's body and β-endorphin are commonly used in researches.Nissl's body is a kind of special structure in neurons, ant it is very sensitive to injury. If a neuron is injured, the number and location of Nissl's body will change accordingly, i.e. dissolving gradually until dissipation. So,the variation of the structure and number of Nissl's body can indicate the injury of a neuron[19]. After the sciatic nerve is injured, the structure of Nissl's body will change and its number will reduce[20]. So with nissl staining, we can not only locate the nucleus but also assess the functional and active state of the neuron.Our experiment found that the numbers of Nissl's body in accumbens nucleus and amygdaloid nucleus in rats of the tuina group were increased, and the differences between the tuina group and the model group were statistically significant (all P<0.01). It's indicated that the tuina therapy of An-pressing and Rou-kneading tender points could activate the expression of Nissl's body in accumbens nucleus and amygdaloid nucleus, and enhance its activity, which might reflect the active state of the two nuclei to a certain degree.
In the research on the activation of pleasure circuits,scientists have found that some neurotransmitters play an important role, for example, opioid peptide.β-endorphin received extensive attention for its strong pleasure effect[21-22]. We used opium to activate dopaminergic neurons in the ventral tegmental area, to make the nerve endings in accumbens nucleus to release dopamine, which aroused the reward effect and produced sense of euphoria. Our previous research also showed that An-pressing and Rou-kneading Huantiao(GB 30) could produce not only analgesic effect but also pleasure effect in rats with chronic neuralgia[14].Through monitoring brain activity by functional magnetic resonance imaging (fMRI), Li ZY, et al[23]found that tuina therapy of An-pressing and Rou-kneading the tender points could activate brain nuclei such as accumbens nucleus and amygdaloid nucleus in patients with lumbar disc herniation. Our experiment found that the expressions of β-endorphin in accumbens nucleus and amygdaloid nucleus in rats of the tuina group were increased, and the differences between the tuina group and the model group were statistically significant (all P<0.01), which indicated that the tuina therapy of An-pressing and Rou-kneading Huantiao (GB 30) could activate the expression of β-endorphin in accumbens nucleus and amygdaloid nucleus and enhance its activity.
POMC is the precursor gene of β-endorphin, and is mainly expressed in pituitary and hypothalamus. Hence,the expression of POMC in arcuate nucleus in hypothalamus can reflect the speed of opioid peptide synthesized at molecular level, and also the active degree of endogenous opioid peptides[24]. Our experiment found that the expression of POMC in arcuate nucleus in rats of the tuina group was increased,and the difference between the tuina group and the model group was statistically significant (P<0.01), which indicated that the tuina therapy of An-pressing and Rou-kneading Huantiao (GB 30) could activate the expression of POMC in arcuate nucleus and enhance its activity. It matched with the increased expression of β-endorphin in accumbens nucleus and amygdaloid nucleus, and then led to the pleasure effect. In the experiment, we also found that rats in the tuina group were stable in mood with a good appetite, but those in the model group were irritable in mood, always fighting with each other, and with a poor appetite. The findings matched with the phenomenon that patients not only get relief from pain but also feel comfortable after tuina treatment in clinic[25].
The experiment showed that tuina therapy of An-pressing and Rou-kneading Huantiao (GB 30) could increase the levels of Nissl's body and β-endorphin in accumbens nucleus and amygdaloid nucleus as well as the expression of POMC in rats with chronic neuralgia,which indicated that the analgesic effect of tuina therapy of An-pressing and Rou-kneading Huantiao(GB 30) was related to the activation and participation of the two nuclei. It's also indicated that the analgesia effect of tuina therapy might correlate with pleasure effect, which also revealed a part of the neurobiological mechanisms and explained why patients with chronic pain like to be An-pressed at their tender points. As to the influence of tuina therapy on the receptors in brain nuclei of pressure circuits in rats with neuralgia, it requires further study.
Conflict of Interest
The authors declared that there was no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Fund of Shanghai University of Traditional Chinese Medicine (上海中医药大学预算内课题, No. 2015YSN13); The Three-year Development Project for Traditional Chinese Medicine of Shanghai [上海市进一步加快中医药事业发展三年行动计划项目, No. ZY(2018-2020)-CCCX-2001-05].
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria.
Received: 18 June 2018/Accepted: 29 July 2018
 Journal of Acupuncture and Tuina Science2018年6期
Journal of Acupuncture and Tuina Science2018年6期
- Journal of Acupuncture and Tuina Science的其它文章
- Analysis of acupoint-selection patterns in acupuncture-moxibustion treatment of polycystic ovarian syndrome based on data mining
- Clinical observation of needling gluteus medius muscle trigger point plus chiropractic for sacroiliac joint subluxation
- Clinical observation on tuina plus foot bath with Chinese medicine for diabetic foot in early stage
- Effect of tuina exercise on simple obesity in college students
- Therapeutic effect observation on acupuncture plus umbilicus application with Chinese medicine in treating detrusor underactivity
- Memory response to manual acupuncture in chronic insomniacs: evidence from event-related potentials
