Optical and Electrical Properties of Organic Semiconductor Thin Films for Optoelectronic Devices
, , ,
(1 College of Electronics Information Engineering, South-Central University for Nationalities, Wuhan 430074, China; 2 Hubei Key Laboratory of Intelligent Wireless Communications, South-Central University for Nationalities, Wuhan 430074, China)
Abstract Organic semiconductor thin films of tris(8-hydroxy-quinoline)aluminum (AlQ), aluminum (III) bis(2-methyl-8-quninolinato)-4-phenylphenolate (BAlQ), and α-naphthylphenylbiphenyl amine (NPB) were deposited by vacuum sublimation. The transmission, abosorption and the optical properties of the thin films were investigated. In addition, the thin film devices of sandwich structure were fabricated and the current-voltage characteristics were studied. The results showed that the organic thin films were highly transparent. AlQ and BAlQ demonstrated almost the same direct energy bandgap (4.46 eV), which was larger than that of NPB (3.11 eV). The internal free carrier density of AlQ, BAlQ and NPB were of the same order of magnitude (1022 m-3), whereas NPB exhibited the highest zero-field mobility (1.7510-8 cm2V-1s-1) and electrical conductivity (1.4510-10 Scm-1).
Keywords sorganic semiconductor, thin film, optical and electrical properties
In recent years, a considerable amount of research has been carried out on the organic semiconductors which is becoming an attractive alternative to inorganic semiconductors for applications in optoelectronic devices, for instance, photovoltaic solar cells[1-3], electroluminescent diodes[4-6], electrochromic devices[7,8], light-emitting electrochemical cells[9-11]and organic semiconductor lasers[12,13]. In parallel with these activities, many researchers have made much progress in the understanding of the underlying physics that controls the properties of these devices. In comparison with inorganic semiconductors, however, much less is known about the optical and electrical properties of the organic semiconductors which are crucial to the structure design and performance improvement of the optoelectronic devices. In this study, the thin films of tris(8-hydroxy-quinoline) aluminum (AlQ), aluminum (III) bis(2-methyl-8-quninolinato)-4-phenylphenolate (BAlQ), andα-naphthylphenylbiphenyl amine (NPB) were prepared by the vacuum sublimation technique. The optical properties of the thin films were investigated by optical transmittance, absorbance spectra and energy bandgap. Furthermore, single-layer thin film devices were fabricated, and the electrical properties of the materials were studied through the measurements of current-voltage characteristics, electrical conductivity and charge carrier mobility.
1 Experimental procedure
1.1 Materials
Organic semiconductor materials of AlQ, BAlQ and NPB were purchased from Sigma-Aldrich Chemical Company and used as-received. The molecular structures of the materials are shown in Fig.1. Commercially quartz glass was used as substrates to prepare organic semiconductor thin films and single-layer devices in this experiment.

Fig.1 Molecular structures of organic semiconductor materials of AlQ, BAlQ and NPB图1 有机半导体材料AlQ, BAlQ和NPB的分子结构图
1.2 Substrate cleaning
Substrate cleaning plays an important role in the deposition of thin films. The quartz glass was cut into 3 cm×3 cm plates in this experiment. Prior to their use, it was routinely cleaned by rubbing first with detergent and then with the mixture of aether and alcohol (the volume ratio of aether to alcohol is 1∶1), followed by rinsing in deionized water, and finally dried in a flow of nitrogen.
1.3 Thin film deposition
Using the previously cleaned quartz glass as substrates, the organic semiconductor thin films of AlQ, BAlQ and NPB were prepared by vacuum evaporation, respectively. The thermal evaporation process was performed by a suitable quartz crucible with a multifunctional deposition system of OLED-V at a base pressure of about 2×10-5Pa. During the deposition process, the substrate was kept at about 310 K. The glass substrate was also rotated during the deposition process by means of a rotary working holder at a speed of 20 r/min so as to obtain a uniform thickness of thin films. The deposition rate was controlled to be about 0.1 nm·s-1, and it was continuously measured by a quartz oscillating thickness monitor. The thickness of AlQ, BAlQ and NPB thin films are all approximately 100 nm.
1.4 Devices fabrication
In order to study the electrical properties of organic semiconductor thin films, the single-layer devices with the structure of glass/Al (200 nm)/organic layer (100 nm)/Al (200 nm) were fabricated by the vacuum sublimation technique. The effective area was defined by a shadow mask with 0.6 cm×0.6 cm patterns. All layers were continuously deposited under the base pressure of about 2×10-5Pa without breaking the vacuum. The deposition rates of aluminium and organic materials are about 0.3 and 0.1 nms-1, respectively.
1.5 Characterization methods
The transmittance and absorbance of the thin films were recorded by a double beam spectrophotometer of Perkin Elmer Lambda 900 UV/VIS/NIR, respectively. The current-voltage (J-V) characteristics of the single-layer devices were measured using a Keithley-4200 source measure unit. All measurements were carried out at room temperature (about 300 K) under ambient conditions.
2 Results and discussion
2.1 Optical properties
The optical transmission spectra of all the thin films are depicted in Fig.2. Inspection of Fig.2 shows that these thin films are highly transparent in the spectral range from 460 to 800 nm. The high transparency indicates the small surface roughness and the better homogeneity of the thin films. The transmission of the thin films decreases obviously when the wavelength is reduced to around 460 nm. As can be seen, the fundamental absorption edge is closely related to the used materials. The fundamental absorption edge (λ0) of thin films AlQ, BAlQ and NPB are located at 270-300, 260-320 and 350-440 nm, respectively. The absorption edge of these materials gives the energy bandgap (Eg), and the relationship betweenEgand the position of the normally sharp absorption edge is described as[14]:
(1)
wherehis Planck’s constant,cvelocity of light, andλ0is wavelength of absorption edge. At high absorption levels, the relationship between absorption coefficient (α) andEgis expressed to calculate energy bandgap of the thin films by the following formula[15]:
αhv=C(hν-Eg)p,
(2)
whereCis an energy-independent constant,pis an exponent which can assume values of 1/2, 3/2, 2 and 3 depending on the nature of the electronic transitions responsible for optical absorption.p=1/2 and 3/2 for direct allowed and forbidden transitions, respectively,p=2 and 3 for indirect allowed and forbidden transition, respectively[16].

Fig.2 Transmission spectra of the thin films AlQ, BAlQ and NPB图2 薄膜AlQ, BAlQ和NPB的透射谱
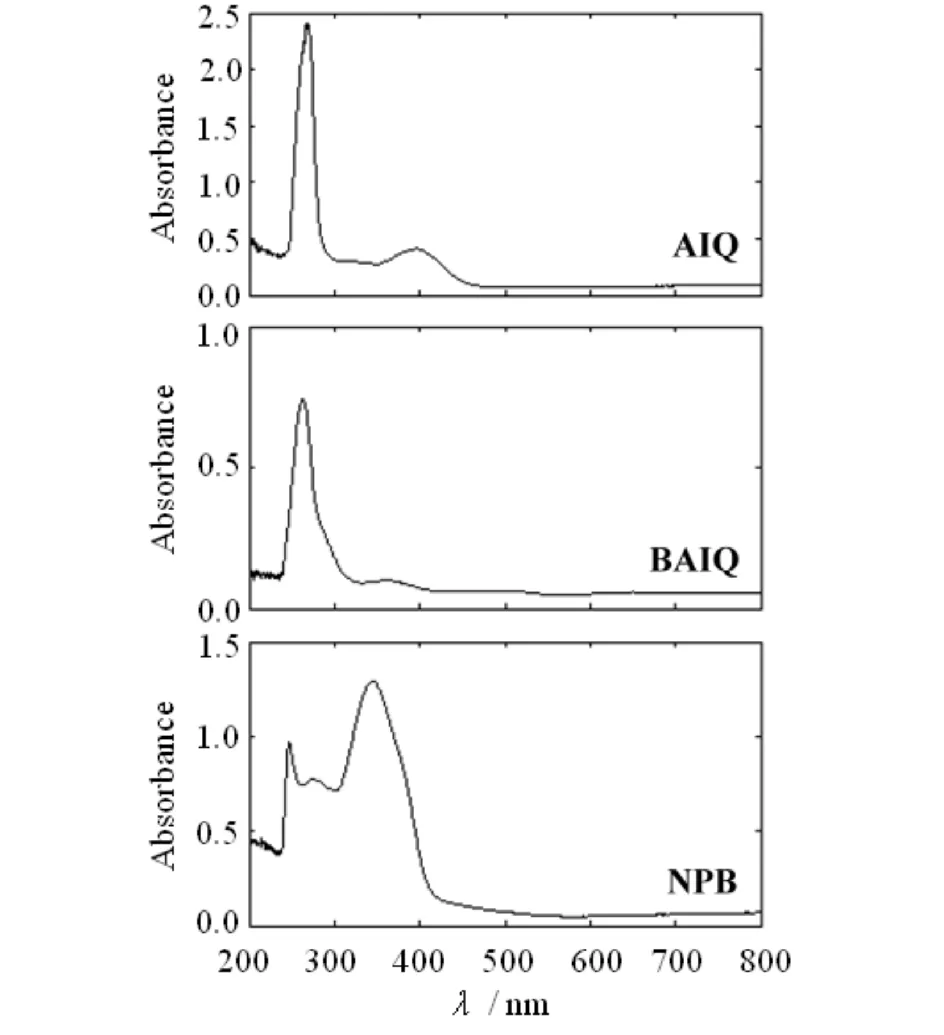
Fig.3 Absorbance of the thin films AlQ, BAlQ and NPB图3 薄膜AlQ, BAlQ和NPB的吸光度
Fig.3 shows the optical absorbance spectra of all the thin films as a function of wavelength. The absorption coefficientαwas calculated using the following equation:
(3)
whereAis the measured absorbance anddthe thickness of the films. Fig.4 shows the typical curves of (αhν)2versushνfor all the thin films at room temperature. The values ofEgwere obtained by extrapolation of the linear portion of the graph to (αhν)2=0[17]. A good straight line is obtained withp=1/2 for all the thin films. The observed valuesEg(direct transitions) for the prepared thin films are tabulated in Tab.1. Note that theEgvalues of thin films AlQ, BAlQ and NPB are 4.46, 4.47 and 3.11 eV, respectively. Clearly, AlQ and BAlQ share the almost sameEgvalue, and theEgvalues of AlQ and BAlQ are larger than that of NPB.

Fig.4 (αhν)2-hν curves for thin films of AlQ, BAlQ and NPB图4 薄膜AlQ, BAlQ和NPB的(αhν)2-hν曲线

SampleEg /eVVt /Vμ0 /cm2〠V-1〠s-1β /cm1/2〠V-1/2n0 /m-3σ /S〠cm-1AlQ4.463.91.63挠10-112.94挠10-48.49挠10221.98挠10-13BAlQ4.474.45.58挠10-95.87挠10-49.58挠10227.58挠10-11NPB3.112.81.75挠10-85.65挠10-46.10挠10221.45挠10-10
2.2 Electrical properties
Fig.5 gives the current-voltage (J-V) characteristics of all the thin films at room temperature. From the double logarithmic plot, it can be seen that theJ-Vcurves follow a power law of the formJVmwith different slopes in the lower and higher voltage regions, wheremis a power index. At low voltages there is am=1 region corresponding to an ohmic region where the current densityJis proportional to the voltageV. At higher voltages, there is a transition to am=2 region, corresponding to the onset of space-charge limited conduction (SCLC). At still higher voltages there is a transition to a region of even highermvalue, corresponding to a region where the traps within the material become filled, resulting in a sharp increase in current densityJ.

Fig.5 J-V characteristics curves for thin films of AlQ, BAlQ and NPB图5 薄膜AlQ, BAlQ和NPB的J-V曲线图
In theohmic region ofm=1, theJ-Vcharacteristics obey the Ohm’s law and the current densityJcan be expressed as[18,19]:
(4)
whereσis the electrical conductivity, anddthe film thickness.
In the SCLC region ofm=2, theJ-Vcharacteristics follow the Mott-Gurney equation[20,21]:
(5)
whereμis the carrier mobility,ε=ε0εr=3.1×10-11Fm-1is the permittivity of organic layer. According to the Poole-Frenkel law, the relation betweenμandEcan be represented as[22,23]:
(6)
whereμ0is the zero-field mobility,βthe Poole-Frenkel coefficient, andE=V/dis the applied electric field. According to the equations (4) and (5), theJ-Vcharacteristics can be described by the following equation:
(7)
From Eq.(6), we get:
(8)

When combining Eq.(4) and (5) with the formulaσ=n0qμ, the free carrier densityn0can be determined by the following relation:
(9)
whereqis the magnitude of the electron charge, andVtis the threshold voltage at which the transition occurs from ohmic conduction to SCLC conduction. Using the values ofε,q,dandVt(see Fig.5), then0are calculated out to be about 8.491022, 9.581022and 6.101022m-3for the thin films AlQ, BAlQ and NPB, respectively. As can be seen, the free carrier densityn0of AlQ, NPB and BAlQ share the same order of magnitude (1022m-3).
In addition, theσvalues were evaluated by fitting the experimental data in the ohmic region of Fig.5 according to Eq. (4), and the results were given in the Tab.1. Note that the measured values ofσcan be observed to decrease in the sequence of NPB, BAlQ, AlQ. NPB exhibits the highestσvalue (1.4510-10Scm-1), while AlQ gives the lowestσvalue (1.9810-13Scm-1). The result indicates that electrical conductivity of NPB is superior to those of AlQ and BAlQ.
3 Conclusion
Organic semiconductor thin films AlQ, BAlQ and NPB were prepared, and the optical properties in the UV-visible region of the thin films were investigated at room temperature in terms of the optical transmittance and absorbance spectra measurements. The energy bandgaps of the deposited thin films were calculated. In addition, the single-layer structure devices were fabricated with these organic semiconductor materials, and the electrical properties of the thin films were evaluated through the measurement and analysis of current-voltage characteristics of the devices. Experimental results indicate that the free carrier density of AlQ, NPB and BAlQ share the same order of magnitude, and the zero-field mobility and electrical conductivity of NPB is, respectively, about three orders of magnitude greater than those of AlQ at room temperature.

