A New Species of the Asian Toad Genus Megophrys sensu lato(Amphibia: Anura: Megophryidae) from Guizhou Province, China
Shize LI, Ning XU, Jing LIU, Jianping JIANG, Gang WEI and Bin WANG,*#
1 Department of Food Science and Engineering, Maotai University, Renhuai 564500, China
2 CAS Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization and Ecological Restoration Biodiversity Conservation Key Laboratory of Sichuan Province, Chengdu Institute of Biology (CIB), Chinese Academy of Sciences (CAS), Chengdu 610041, China
3 Biodiversity Conservation Key Laboratory, Guiyang College, Guiyang 550002, China
Abstract We describe a new species of the genus Megophrys sensu lato from Guizhou Province, China. Molecular phylogenetic analyses based on mitochondrial DNA and nuclear DNA sequences all strongly supported the new species as an independent lineage in Megophrys (Panophrys) clade. The new species is distinguished from its congeners by a combination of the following morphological characteristics: (1) small body size with SVL < 38.8 mm in male and SVL< 42.3 mm in female; (2) vomerine teeth absent; (3) tongue not notched behind; (4) a small horn-like tubercle at the edge of each upper eyelid; (5) tympanum distinctly visible, rounded; (6) two metacarpal tubercles in hand; (7) relative finger lengths: II < I < V < III; (8) toes with rudimentary webbing at bases; (9) heels overlapping when thighs are positioned at right angles to the body; (10) tibiotarsal articulation reaching the level between tympanum to eye when leg stretched forward; (11) an internal single subgular vocal sac in male; (12) in breeding male, the nuptial pads with black nuptial spines on the dorsal bases of the first and second fingers.
Keywords Megophrys sensu lato, new species, taxonomy, phylogenetic analysis, Guizhou Province, China
1. Introduction
Megophryidae Bonaparte, 1850 (Amphibia: Anura) is a large group of Asian toads (Frost, 2018). Delorme et al.(2006) classified this family into three subfamilies, i.e.Leptobrachiinae Delorme, Dubois, Grosjean and Ohler,2006, Leptolalaginae Dubois, 1983 and Megophryinae Bonaparte, 1850. Frost (2018) classified all recognized species of the subfamily Megophryinae into one genus Megophrys sensu lato Kuhl and Van Hasselt, 1822. The Asian horned toad genus Megophrys sensu lato is widely distributed in the eastern and central China, throughout southeastern Asia, and extending to the islands of the Sunda Shelf and the Philippines (Frost, 2018).
Although Megophrys sensu lato has been widely recognized as a monophyletic group by a series of phylogenetic studies (e.g. Chen et al., 2017; Mahony et al., 2017; Liu et al., 2018), the generic and/or subgeneric classi fications in this group are still under intense debate(Tian and Hu, 1983; Dubois,1987 “1986”; Rao and Yang, 1997; Lathrop, 1997; Dubois and Ohler, 1998;Delorme et al., 2006; Fei and Ye, 2016; Chen et al.,2017; Mahony et al., 2017; Liu et al., 2018; Munir et al., 2018). Fei and Ye (2016) classified 36 Chinese species of the subfamily Megophryinae (members of Megophrys sensu lato) into six genera, i.e. Liuophrys Fei, Ye and Jiang, 2016, Atympanophrys Tian and Hu,1983 containing three subgenera (Atympanophrys,Borealophrys Fei, Ye and Jiang, 2016 and Gigantophrys Fei, Ye and Jiang, 2016), Boulenophrys Fei, Ye and Jiang, 2016, Xenophrys Günther, 1864 containing two subgenera (Tianophrys Fei and Ye, 2016 and Xenophrys),Ophyrophryne Boulenger, 1908 and Brachytarsophrys Tian and Hu, 1983, mainly based on morphology. Chen et al. (2017) suggested that the group contained five genera, i.e. Atympanophrys, Megophrys, Xenophrys,Ophyrophryne and Brachytarsophrys, based on molecular phylogenetics. Mahony et al. (2017) classified all members of Megophryinae into a single genus Megophrys including seven subgenera (Megophrys, Xenophrys,Panophrys Rao and Yang, 1997, Atympanophrys,Ophyrophryne, Pelobatrachus Beddard, 1908 “1907” and Brachytarsophrys) based on molecular phylogenetics and morphological comparisons. Liu et al. (2018) indicated Panophrys as a large monophyletic group through comprehensive sampling. Nevertheless, to now, it is likely that there was inadequate morphological crude to corroborate molecular phylogenetic conclusions.
Whatever, Megophrys sensu lato belongs to one of the most diverse groups of amphibians with currently 83 recognized species (Frost, 2018), and as note, 29 species of Megophrys sensu lato were described in the last decade (Deuti et al., 2017; Fei et al., 2009; Li et al.,2014; Mahony, 2011; Mahony et al., 2011; Mahony et al.,2013; Mahony et al., 2018; Mo et al., 2010; Munir et al.,2018; Nikolay et al., 2017; Orlov et al., 2015; Tapley et al., 2017; Tapley et al., 2018; Wang et al., 2012; Wang et al., 2014; Wang et al., 2017a, b; Yang et al., 2018; Zhang et al., 2017; Zhao et al., 2014). And even so, molecular phylogenetic studies still surprisingly indicated mass of cryptic species in the group, for example, 20 cryptic species were suggested by Chen et al. (2017), and 43 cryptic species were proposed just only in Megophrys(Panophrys) by Liu et al. (2018). Obviously, these cryptic species need to be veri fied and described.
During field surveys in the Leigong Mountains,Leishan County, Guizhou Province, China, some Megophrys sensu lato specimens were collected from the montane forests. This kind of specimens has been identified as M. minor (Wu et al., 1986), but previous molecular studies indicated the population as a cryptic species [Megophrys sp8 in Chen et al., 2017; Megophrys(P.) sp34 in Liu et al., 2018]. Our molecular phylogenetic analyses and morphological comparisons also supported it as a new taxon of Megophrys sensu lato and could be classified it into subgenus Megophrys (Panophrys).Therefore, we describe it herein as a new species.
2. Materials and Methods
2.1. Sampling Two adult females and ten adult males of the new taxon (for voucher information see Table S1) were collected from the mountain streams in the Leigong Mountain, Leishan County, Guizhou Province,China (Figure S1). Six tadpoles (voucher numbers:CIBLS20171101001–CIBLS20171101006) of the new taxon were also collected in a mountain stream where the new taxon was found. They were identified as the new taxon because they were almost identical in morphology and one representative of them was genetically close to the adult specimens of the new taxon (see the results).The stages of tadpoles were identi fied following Gosner(1960). All specimens were fixed in 10% buffered formalin for one day, and then later transferred to 70%ethanol. Tissue samples were taken and preserved separately in 95% ethanol prior to fixation. The specimens were deposited in Chengdu Institute of Biology, Chinese Academy of Sciences (CIB, CAS).
2.2. Molecular data and phylogenetic analyses Three adult specimens and one tadpole of the new taxon were included in molecular analyses (for voucher information see Table S2). Total DNA was extracted using a standard phenol-chloroform extraction protocol (Sambrook et al., 1989). Two fragments of the mitochondrial genes encoding16S rRNA and cytochrome oxidase subunit I(COI) were amplified using the primers in Simon et al.(1994) and Che et al. (2012), respectively. They were ampli fied under the following conditions: 35 cycles at 95°C for 4 min, 95 °C for 1 min, 52 °C (for 16S rRNA)/46°C (for COI) for 40 second, and 72 °C for 1 min followed by a 10 min extension at 72 °C. The nuclear gene fragments encoding brain-derived neurotrophic factor(BDNF) and recombination activating gene 1 (RAG1)were ampli fied using the primers and protocols in Vieites et al. (2007) and Shen et al. (2013). All primers were presented in Table S3. PCR products were puri fied with spin columns and then were sequenced with primers same used in PCR. Sequencing was conducted using an ABI3730 automated DNA sequencer in Shanghai DNA BioTechnologies Co., Ltd. (Shanghai, China). All sequences were deposited in GenBank (for GenBank Accession numbers see Table S2).
For molecular analyses, the available sequence data for all related species of the genus Megophrys sensu lato were downloaded from GenBank, primarily from previous studies (Chen et al., 2017; Liu et al., 2018). For phylogenetic analyses, corresponding sequences of one Leptolalax oshanensis and one Leptobrachium boringii were downloaded and used as outgroups according to Chen et al. (2017). GenBank Accession numbers of all sequences were shown in Table S2.
Sequences were assembled and aligned using the Clustalw module in BioEdit 7.0.9.0 (Hall, 1999) with default settings. Alignments were checked by eye and revised manually if necessary. To avoid bias in alignments, GBLOCKS 0.91.b (Castresana, 2000) with default settings was used to extract regions of defined sequence conservation from the length-variable 16S gene fragments. Non-sequenced fragments were defined as missing loci.
Gene trees were reconstructed for the mitochondrial genes concatenated data and nuclear genes concatenated data, respectively. Phylogenetic analyses were conducted using maximum likelihood (ML) and Bayesian Inference (BI) methods, implemented in PhyML 3.0(Guindon et al., 2010) and MrBayes 3.12 (Ronquist and Huelsenbeck, 2003), respectively. To avoid under- or over-parameterization (Lemmon and Moriarty, 2004;McGuire et al., 2007), the best partition scheme and the best evolutionary model for each partition were chosen for the phylogenetic analyses using PARTITIONFINDER 1.1.1 (Robert et al., 2012). In the analyses, 16S, each codon position of the protein-coding genes (COI, RAG1 and BNDF) were de fined, and Bayesian Inference Criteria(BIC) was used. As a result, the analyses selected the best partition scheme (i.e. 16S gene/each codon position of COI gene) and the TrN+I+G model for each partition for mitochondrial DNA dataset, and as well, selected the best partition scheme (i.e. each codon position of RAG1 and BNDF genes) and the TrN+I+G as the best model for the second codon position of nuclear genes and the GTR+G+I model as the best model for the other two codon position of RAG1 and BNDF genes. For the ML tree,branch supports were drawn from 10000 non-parametric bootstrap replicates. In BI analyses, two runs each with four Markov chains were run for 60 million generations with sampling every 1000 generations. The first 25% of generations were removed as the “burn-in” stage followed by calculation of Bayesian posterior probabilities and the 50% majority-rule consensus of the post burn-in trees sampled at stationarity. Finally, pairwise COI gene sequence divergence with uncorrected p-distance model was estimated using MEGA 6.06 (Tamura et al., 2011) to determine the genetic distance between Megophrys sensu lato species.
2.3. Morphological comparisons All twelve adult specimens and six tadpole specimens of the new taxon collected in this work were measured. The terminology and methods followed Fei et al. (2009). Measurements were taken with a dial caliper to 0.1 mm. Nineteen morphometric characters of adult specimens were measured: SVL, snout-vent length (distance from the tip of the snout to the posterior edge of the vent); HDL,head length (distance from the tip of the snout to the articulation of jaw); HDW, maximum head width (greatest width between the left and right articulations of jaw);SL, snout length (distance from the tip of the snout to the anterior corner of the eye); ED, eye diameter (distance from the anterior corner to the posterior corner of the eye);IOD, interorbital distance (minimum distance between the inner edges of the upper eyelids); IND, internasal distance (minimum distance between the inner margins of the external nares); TYD, maximal tympanum diameter;LAL, length of lower arm and hand (distance from the elbow to the distal end of the Finger IV); LW, lower arm width (maximum width of the lower arm); FIL, first finger length (distance from base to tip of finger I); FIIL,second finger length (distance from base to tip of finger II); FIIIL, third finger length (distance from base to tip of finger III); FIVL, fourth finger length(distance from base to tip of finger IV); THL, thigh length (distance from vent to knee); TL, tibia length (distance from knee to tarsus);TW, maximal tibia width; TFL, length of foot and tarsus(distance from the tibiotarsal articulation to the distal end of the Toe IV); and FL, foot length (distance from tarsus to the tip of fourth toe). A total of 10 morphometric characters of larvae were measured: TOL, total length;SVL, snout-vent length; BH, maximum body height; BW,maximum body width; SL, snout length (distance from the anterior corner of the eye to the tip of the snout); SS,snout to spiraculum (distance from spiraculum to the tip of the snout); MW, mouth width (distance between two corners of mouth); TBW, maximum width of tail base;TAL, tail length (distance from base of vent to the tip of tail); and TH, tail height (maximum height between upper and lower edges of tail).
We compared morphological characters of the new taxon with other Megophrys sensu lato species.Comparative data were obtained from the literature for M. aceras (Boulenger, 1903), M. actuta (Li et al.,2014), M. ancrae (Mahony et al., 2013), M. auralensis(Ohler et al., 2002), M. baluensis (Boulenger, 1899a), M.baolongensis (Ye et al., 2007), M. binchuanensis (Ye and Fei, 1995), M. binlingensis (Fei et al., 2009), M. boettgeri(Boulenger, 1899b), M. brachykolos (Inger and Romer,1961), M. carinense (Boulenger, 1889), M. caudoprocta(Shen, 1994), M. cheni (Wang and Liu, 2014), M.chuananensis (Fei et al., 2001), M. damrei (Mahony,2011), M. daweimontis (Rao and Yang, 1997), M. dringi(Inger et al., 1995), M. edwardinae (Inger, 1989), M.el fina (Poyarkov et al., 2017), M. fansipanensis (Tapley et al., 2018), M. feae (Boulenger, 1887), M. feii (Yang
et al., 2018), M. flavipunctata (Mahony et al., 2018),M. gerti (Ohler, 2003), M. gigantica (Liu et al., 1960),M. glandulosa (Fei et al.,1990), M. hansi (Ohler, 2003),M.himalayana (Mahony et al., 2018), M.hoanglienensis(Tapley et al., 2018), M. huangshanensis (Fei and Ye,2005), M. insularis (Wang et al., 2017a), M. intermedia(Smith, 1921), M. jingdongensis (Fei and Ye, 1983),M. jinggangensis (Wang et al., 2012), M. kobayashii(Malkmus and Matsui, 1997), M. koui (Mahony et al.,2017), M. kuatunensis (Pope, 1929), M. lancip (Munir et al., 2018), M. latidactyla (Orlov et al., 2015), M.lekaguli (Stuart et al., 2006), M. liboensis (Zhang et al.,2017), M. lini (Wang et al., 2014), M. lishuiensis (Wang et al., 2017b), M. longipes (Boulenger,1886), M. major(Boulenger, 1908), M. mangshanensis (Fei et al.,1990),M. maosonensis (Bourret, 1937), M. medogensis (Fei et al., 1983), M. megacephala (Mahony et al., 2011), M.microstoma (Boulenger, 1903), M. minor (Stejneger,1926), M. montana (Kuhl and Van Hasselt, 1822),M.monticola (Günther, 1864), M. nasuta (Schlegel,1858), M. nankiangensis (Hu et al.,1966), M. obesa (Li et al., 2014), M. omeimontis (Liu, 1950), M.oreocrypta(Mahony et al., 2018), M. oropedion (Mahony et al.,2013), M. pachyproctus (Huang and Fei, 1981), M.palpebralespinosa (Bourret, 1937), M. parallela (Inger and Iskandar, 2005), M. parva (Boulenger, 1893), M.periosa (Mahony et al., 2018), M. popei (Zhao et al.,2014), M. robusta (Boulenger, 1893), M. rubrimera(Tapley et al., 2017), M. sangzhiensis (Jiang et al., 2008),M. serchhipii (Mathew and Sen, 2007), M. shapingensis(Liu, 1950), M. shuichengensis (Tian and Sun, 1995), M.spinata (Hu et al., 1973), M. stejnegeri (Taylor, 1920), M.synoria (Stuart et al., 2006), M. takensis (Mahony, 2011),M. tuberogranulata (Mo et al., 2010), M. vegrandis(Mahony et al., 2013), M. wawuensis (Fei et al., 2001),M. wuliangshanensis (Ye and Fei, 1995), M. wushanensis(Ye and Fei, 1995), M. zhangi (Ye and Fei, 1992) and M.zunhebotoensis (Mathew and Sen, 2007).
2.4. Bioacoustics analyses The advertisement calls of the new taxon from Leigong Mountain, Guizhou Province, China, were recorded in the field. SONY PCM-D50 digital sound recorder was used to record within 20 cm of the calling individuals. The sound files in wave format were resampled at 48 kHz with sampling depth 24 bits. The sonograms and waveforms were generated by WaveSurfer software (Sjöander and Beskow,2000) from which all parameters and characters were measured. Ambient temperature was taken by a digital hygrothermograph.
3. Results
3.1. Phylogenetic analyses Aligned sequence matrix of 16S+COI and RAG1+BNDF contained 1095 bp and 1672 bp, respectively. ML and BI trees of the mitochondrial DNA dataset presented almost consistent topology (Figure 1), and as well, ML and BI trees of the nuclear DNA dataset showed almost identical topology (Figure 2), though relationships of some lineages were unresolved (Figures 1 and 2). Megophrys sensu lato was strongly supported as a monophyly by all analyses. In mitochondrial DNA trees, subgenera(Panophrys, Xenophrys, Ophyrophryne, Atympanophrys,Brachytarsophrys, Megophrys and Pelobatrachus) of Megophrys sensu lato suggested by Mahony et al. (2017)were resolved as monophyletic groups, respectively,except subgenera Xenophrys and Megophrys. In nuclear DNA trees, all of them were also resolved as monophyletic groups. However, relationships between the subgenera were not resolved in all analyses. All samples of the new taxon were strongly clustered into one independent clade which was deeply nested into the Megophrys (Panophrys) clade. In mitochondrial DNA trees, the new taxon clade was weakly clustered into a clade including M. baolongensis, M. wushanensis and M. tuberogranulata, but the relationships between them were not resolved except the weakly-supported sister relationship of M. baolongensis and M. wushanensis.In nuclear DNA trees, the new taxon was weakly supported as the sister of M. baolongensis but with almost unresolved relationships with other species in Megophrys(Panophrys) clade.
Genetic distances on COI gene with uncorrected p-distance model between specimens of the new taxon were < 0.5%, much lower than interspecific genetic distances in the genus Megophrys (1.4%–33%; Table S4).Genetic distances between the new taxon and its closelyrelated species, M. wushanensis, M. tuberogranulata and M. baolongensis, were 5.9%–9.3%, at the same level with or even higher than that between some substantial species, for example, M. huangshanensis vs. M. boettgeri(1.4%), M. sangzhiensis vs. M. spinata (4.0%), M.baolongensis vs. M. wushanensis (5.9%), M. sangzhiensis vs. M. binlingensis (6.7%) and M. wushanensis vs. M.tuberogranulata (7.1%).
3.2. Description of the new species
Megophrys (Panophrys) leishanensis sp. nov.
Holotype: CIBLS20160610002 (Figure 3 A, B and C), adult male, from Leigong Mountain (26.35888° N,108.19055° E, 1571 m a. s. l.), Leishan County, Guizhou Province, China, collected by Shize LI on 10 June 2016.
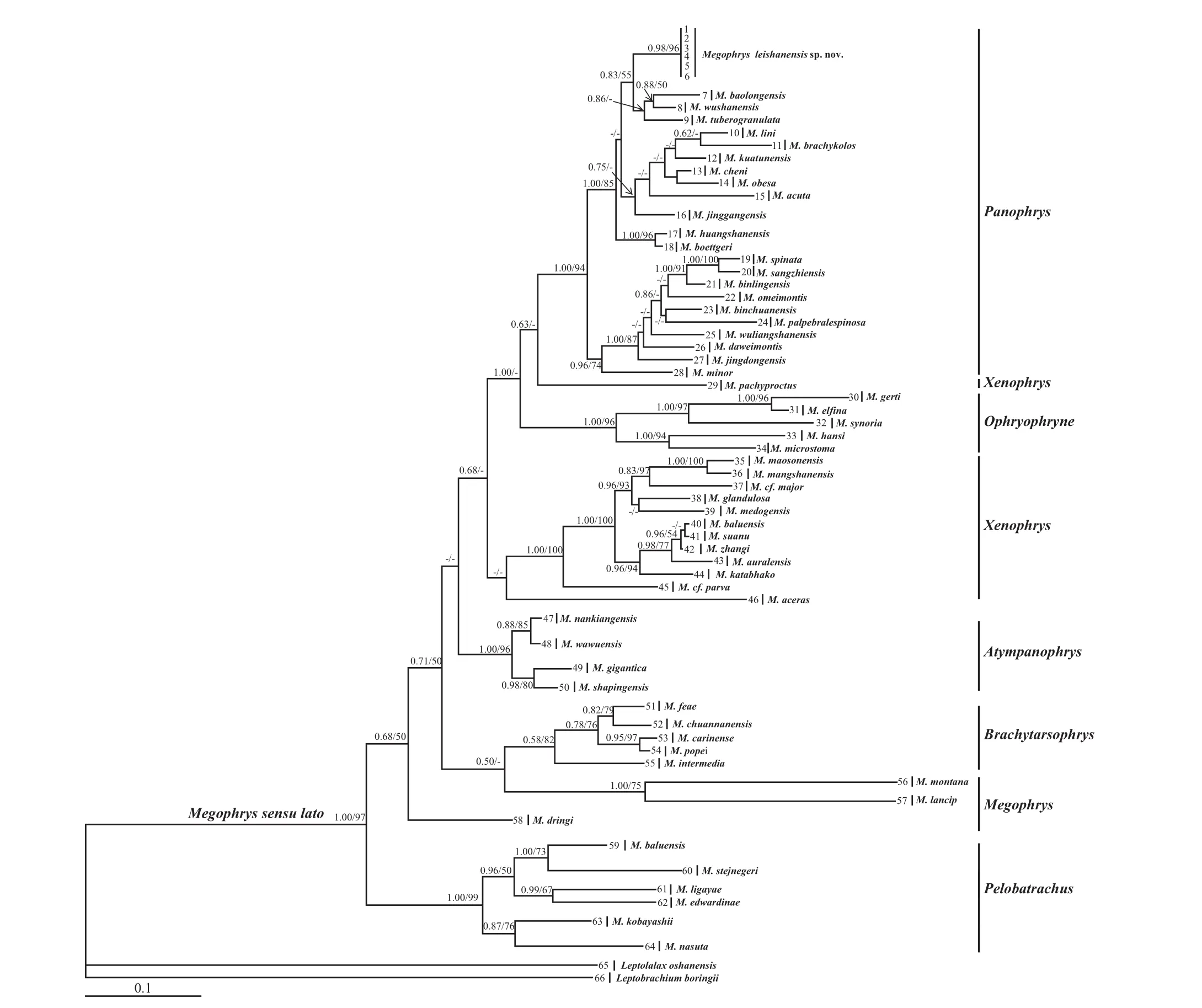
Figure 1 Maximum likelihood (ML) tree of the genus Megophrys sensu lato reconstructed based on the 16S rRNA and COI gene sequences.Bayesian posterior probability/ ML bootstrap support were denoted beside each node. Samples 1-66 refer to Table S2. According to Mahony et al. (2017), seven subgenera (Panophrys, Xenophrys, Ophyrophryne, Atympanophrys, Brachytarsophrys, Megophrys and Pelobatrachus) of Megophrys sensu lato were denoted.
Paratype: nine adult males and two adult females from Leigong Mountain, Leishan County, Guizhou Province, China, collected by Shize LI. Nine males: CIBLS20141004004 collected on 4 October 2014, CIBLS20160610001, CIB LS20160610003,CIBLS20160610004 and CIBLS20160610006 collected on 10 June 2016, CIBLS20171001001,CIBLS20171001003, CIBLS20171001004 and CIBLS20171001005 collected on 1 October 2017; two adult females CIBLS20160610005 and CIBLS20171001002 collected on 10 June 2016 and 1 October 2017, respectively.
Diagnosis: Megophrys leishanensis sp. nov. is assigned to the genus Megophrys sensu lato based on molecular phylogenetic analyses and the following generic diagnostic characters: snout shield-like; projecting beyond the lower jaw; canthus rostralis distinct; chest gland small and round, closer to the axilla than to midventral line;femoral gland on rear of thigh; vertical pupils (Fei et al.,2009).

Figure 2 Maximum likelihood (ML) tree of the genus Megophrys sensu lato reconstructed based on the nuclear DNA sequences of RAG1 and BNDF genes. Bayesian posterior probability/ML bootstrap support were denoted beside each node. Samples 1-66 refer to Table S2.According to Mahony et al. (2017), seven subgenera (Panophrys, Xenophrys, Ophyrophryne, Atympanophrys, Brachytarsophrys, Megophrys and Pelobatrachus) of Megophrys sensu lato were denoted.
The new species could be identi fied from its congeners by a combination of the following morphological characters: (1) small body size (SVL in male < 38.8 mm and SVL in female < 42.3 mm); (2) vomerine teeth absent; (3) tongue not notched behind; (4) a small horn-like tubercle at the edge of each upper eyelid; (5)tympanum distinctly visible, rounded; (6) two metacarpal tubercles in hand; (7) relative finger lengths: II < I < V <III; (8) toes with rudimentary webbing at bases; (9) heels overlapped when thighs are positioned at right angles to the body; (10) tibiotarsal articulation reaching the level between tympanum to eye when leg stretched forward;(11) an internal single subgular vocal sac in male; (12) in breeding male, the nuptial pads with black nuptial spines on the dorsal bases of the first and second fingers.
Description of holotype: SVL 37.5 mm; head width slightly larger than head length (HDW/HDL ratio about 1.1); snout obtusely pointed, protruding well beyond the margin of the lower jaw; loreal region vertical and concave; canthus rostralis well-developed; top of head flat; an small horn-like tubercle at the edge of the upper eyelid; eye large and convex, eye diameter 40.5% of head length; pupils vertical; nostril orientated laterally, closer to snout than eye; tympanum distinct, TMP/EYE ratio 0.60; vomerine ridges and vomerine teeth absent; margin of tongue smooth, not notched behind.
Forelimbs slender, the length of lower arm and hand 39.6% of SVL; fingers slender, relative finger lengths: II< I < V < III; tips of digits globular, and digits without lateral fringes in fingers; subarticular tubercle distinct at the base of each fingers; two metacarpal tubercles, inner palmar tubercle moderate and elliptical and outer palmar tubercle smaller (Figure 3 D).
Hindlimbs slender (TL/SVL = 0.47); heels overlapped when thighs are positioned at right angles to the body,tibiotarsal articulation reaching the level between tympanum to eye when leg stretched forward; tibia length longer than thigh length; relative toe lengths I < II < V <III < IV; tips of toes round, slightly dilated; subarticular tubercle absent; toes with rudimentary webbing at bases,without lateral fringes; inner metatarsal tubercle elliptical;no outer metatarsal tubercle (Figure 3 E).
Dorsal skin rough, with numerous granules; several large warts scattered on flanks; an small horn-like tubercle at the edge of each upper eyelid; tubercles on the dorsum forming an X-shaped weak ridge and two discontinuous dorsolateral parallel ridges on either side of the X-shaped ridge; a dark brown butter fly-shaped marking on dorsum of head and between the eyes; several tubercles on the flanks and dorsal surface of thighs and tibias and forming four transverse tubercle rows; supratympanic fold distinct(Figure 3 A).

Figure 3 The holotype specimen (CIBLS20160610002) of Megophrys leishanensis sp. nov. A, dorsal view. B, ventral view. C, lateral view. D,ventral view of hand. E, ventral view of foot. Scale bar equals to 10 mm.
Ventral surface smooth; chest gland small and round,closer to the axilla than to midventral line; femoral gland on rear of thigh; posterior end of the body protruding distinctly and appearing as an arc-shaped swelling, above the anal region (Figure 3 B).
An internal single subgular vocal sac (Figure 4 C); in breeding season, presence of nuptial pads (Figure 4 F)with black nuptial spines (Figure 4 G) dorsally on the base of the first and second fingers.
Coloration of holotype in life: A dark brown butter flyshaped marking on dorsum of head and between the eyes;an X-shaped marking on the dorsum, four dark transverse bands on the dorsal surface of the thigh and shank; six dark brown and five white vertical bars on the lower and upper lip; ventral surface of body olive with brown and white spots; lower lip grey; several white blotches on the belly; ventral surface of limbs dark brown with white spots; palms and soles uniform pinkish, tip of digits pale grey; inner metatarsal tubercle and two metacarpal tubercles pinkish; pectoral and femoral glands white(Figure 4 A, B and C).
Coloration of holotype in preservation: Dorsal surface fades to greyish-brown; the dark brown butter fly-shaped marking and X-shaped markings on dorsum and dark brown transverse bands on limbs and digits become more distinct; ventral surface yellowish; creamy-white substitutes the pinkish in the anterior surface of the thighs and lateral surface of the trunk and hand palm (Figure 3).
Variation: Morphometric variations of adult specimens of the new species were presented in Tables 1 and S1.An inverted triangular brown speckle between two upper eyelids with X-shaped marking on back of trunk in some adult individuals (Figure 5 A) and in some adult individuals with V- shaped marking on back of trunk(Figure 5 B); in some adult individuals a brown Y-shaped marking on the dorsum of head and disconnected with a V- shaped marking on back (Figure 5 C); in some adult individuals the inverted triangular brown speckle is connected to the X-shape marking (Figure 5 D); in some adult individuals the ventral part of posterior limb with gray-purple and on the belly the white and brown patches are well demarcated (Figure 5 E); in some adult individuals the white spots on ventral part of belly are less numerous and some brick-red spots are mixed with the white spots or brown spots on ventral part of belly(Figure 5 F).

Figure 4 Photos of the holotype CIBLS20160610002 of Megophrys leishanensis sp. nov. in life. A, dorsal view. B, ventral view. C, lateral view showing internal single subgular vocal sac (1). D, ventral view of hand. E, ventral view of foot. F, nuptial pads on the first and second fingers (2). G, black nuptial spines (3).
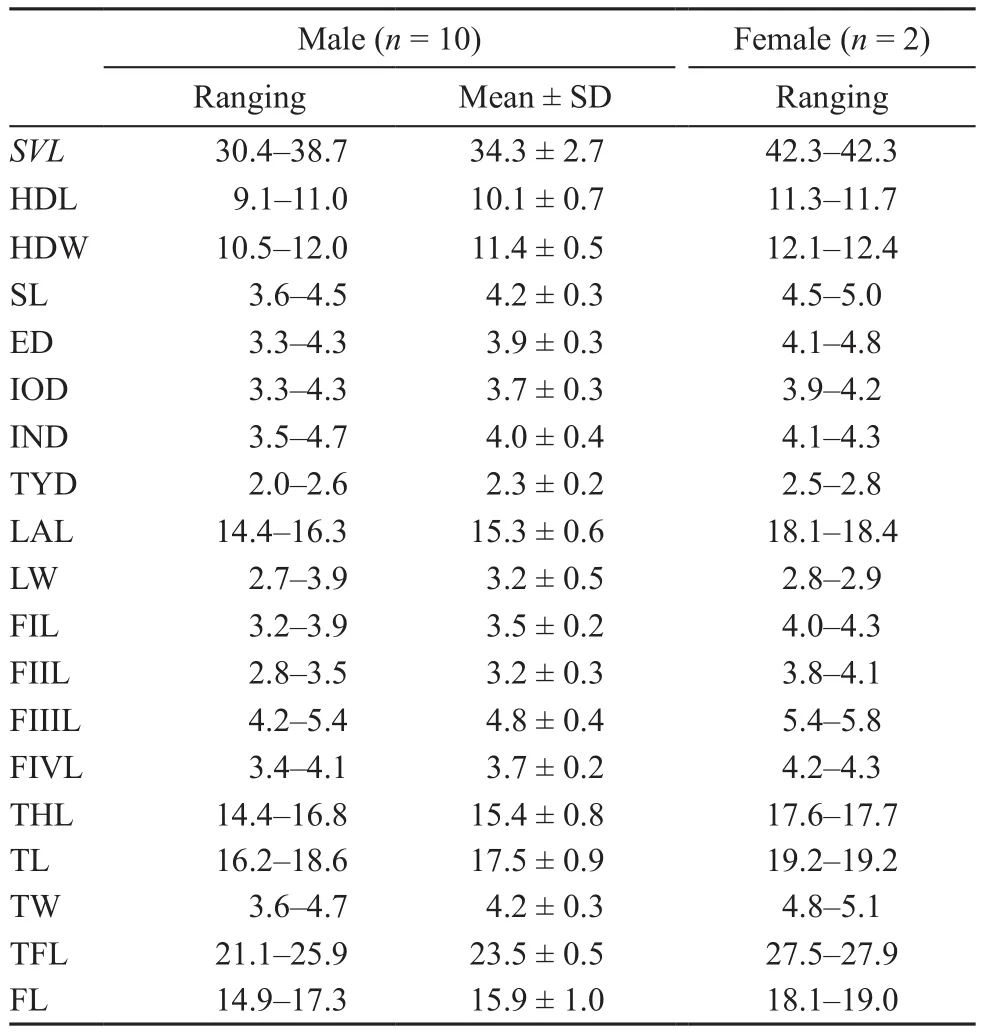
Table 1 Basic statistics for measurements of the adult specimens of Megophrys leishanensis sp. nov.
Tadpole description: The following tadpole description is based on a single specimen (CIBLS20171101001)at Stage 26, the tadpole was confirmed as Megophrys leishanensis sp. nov. by molecular analyses. Body slender,body and tail yellow-brown; tail height greater than body height; dorsal fin arising, behind the origin of the tail,height near mid-length, tapering gradually to narrow, tip pointed; tail 1.9 times as long as snout-vent length; tail height 21.8% of tail length; body width slightly longer than body height(BW/ BH = 1.1); tail fins lightly colored,tail muscles with small black spots; eyes large, lateral,nostril near eyes; spiracle on the left side of the body and not distinct; oral disk terminal, lips expanded and directed upwardly into a umbelliform oral disk; transverse width of expanded funnel 34.3% of snout-vent length (Figure 6).
Variation: Measurements of tadpoles were presented in Table 2. All specimens of tadpole were similar in morphology and color pattern, but different from mouth width at different Gosner’s stage, the mouth width is decrease gradually from stage 25 to 27. At stage 25 the MW/SVL is 43.6%, at stage 26 is 4.3–36.9% and at stage 26 is 32.1%.
Secondary sexual characteristics: Adult females with SVL 42.3 mm, larger than adult males with SVL 30.4–38.7 mm (Table 1). Adult males have a single subgular vocal sac (Figure 3 C). In breeding male, the nuptial pads on the dorsal bases of the first finger and second fingers with black nuptial spines (Figure 3 F and G).
Advertisement calls: The advertisement call of Megophrys leishanensis sp. nov. was recorded from the holotype CIBLS20160610002 from the banks of the streamlet, and the ambient air temperature was 18.9 °C.The sonograms and waveforms are shown in Figure 7.One strophe consists of a series of syllables of a pulsed structure. The call consists of a few strophes of 5.450–7.210 s duration (mean ± SD: 6.303 ± 0.881, n = 5). Each strophe contains 12–14 syllables (mean ± SD: 13 ± 0.816,n = 5). Each syllable has a duration of 0.100–0.109 s(mean ± SD: 0.105 ± 0.003, n = 37). The interval between syllables has a duration of 0.309–0.692 s (mean ± SD:0.409 ± 0.075, n = 36). Syllables are repeated in series at a rate of 1.15–3.24 times (mean ± SD: 2.60 ± 0.383, n =36) per second. Syllable intervals gradually increase from the beginning to the middle of the strophe then decrease to end. Amplitude modulation within strophe is apparent,beginning with moderately high energy pulses, increasing slightly to a maximum by approximately mid strophe, andsubsequently decreasing towards the end of each strophe.Calls have a broad frequency range of 1180–7660 kHz.

Table 2 Measurements of the tadpoles of Megophrys leishanensis sp. nov.

Figure 5 Color variation in Megophrys leishanensis sp. nov. in life. A, dorsal view of the male specimen CIBLS20141004004. B, dorsal view of the male specimen CIBLS20160610001. C, dorsolateral view of the male specimen CIBLS20171001001. D, dorsal view of the female specimen CIBLS20160610005. E, ventral view of the male specimen CIBLS20141004004. F, ventral view of the female specimen CIBLS20171001002.
Morphological comparisons
By having small body size, Megophrys leishanensis sp. nov. differs from M. aceras, M. auralensis, M.binlingensis, M. carinense, M. caudoprocta, M.chuananensis, M. damrei, M. feae, M. flavipunctata, M.gigantica, M. glandulosa, M. himalayana, M. intermedia,M. jingdongensis, M. lekaguli, M. liboensis, M. longipes,M. major, M. mangshanensis, M. maosonensis, M.medogensis, M. megacephala, M. omeimontis, M.oreocrypta, M. periosa, M. popei, M. sangzhiensis, M.shapingensis, M. shuichengensis, M. spinata and M.takensis (maximum SVL < 42.3 mm in the new species vs. minimum SVL > 45 mm in the latter).

Figure 6 Tadpole stage of Megophrys leishanensis sp. nov. Dorsal view (A) and ventral view (B) of specimen CIBLS20171101001 in life.
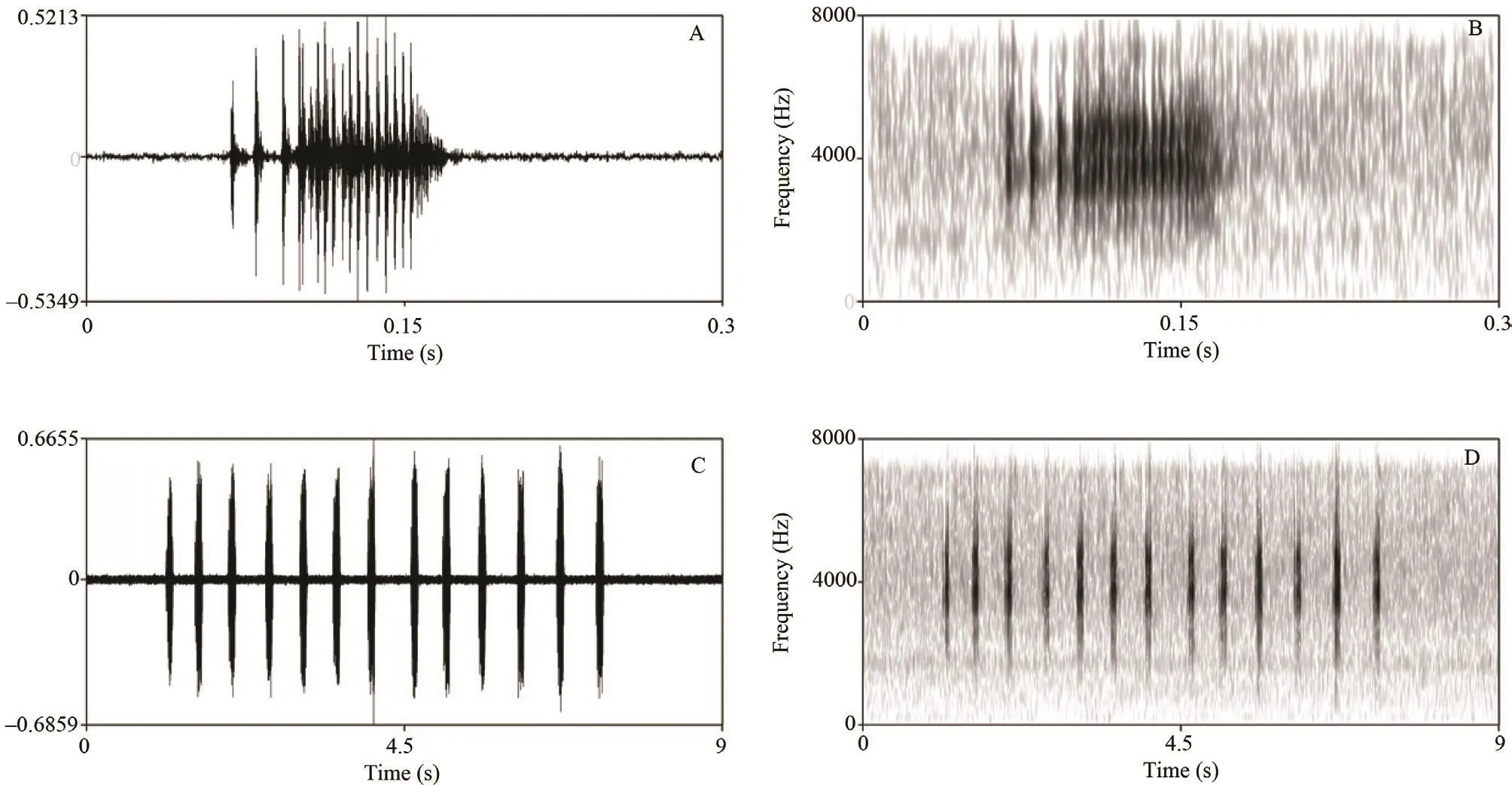
Figure 7 Advertisement call of male Megophrys leishanensis sp. nov., CIBLS20160610002, holotype. A, waveform showing one syllable. B,sonogram showing one syllable. C, waveform showing 13 syllables of one strophe. D, sonogram showing 13 syllables of one strophe.
By lacking vomerine teeth, Megophrys leishanensis sp.nov. differs from M. aceras, M. ancrae, M. carinense, M.baluensis, M. caudoprocta, M. chuananensis, M. damrei,M. daweimontis, M. fansipanensis, M. flavipunctata,M. glandulosa, M. hoanglienensis, M. himalayana,M. insularis, M. intermedia, M. jingdongensis, M.jinggangensis, M. kobayashii, M. lancip, M. latidactyla,M. lekaguli, M. liboensis, M. major, M. mangshanensis,M. maosonensis, M. medogensis, M. megacephala, M.montana, M. nasuta, M. omeimontis, M. oreocrypta, M.oropedion, M. palpebralespinosa, M. parallela, M. parva,M. periosa, M. popei, M. robusta, M. rubrimera, M.sangzhiensis, M. stejnegeri, M. takensis, M. zhangi and M. zunhebotoensis (vomerine teeth present in the latter).
By having a small horn-like tubercle at the edge of each upper eyelid, Megophrys leishanensis sp. nov.differs from M. binchuanensis, M. binlingensis, M.damrei, M. gigantica, M. minor, M. nankiangensis, M.oropedion, M. pachyproctus, M. spinata, M. takensis, M.wuliangshanensis, M. wushanensis, M. zhangi and M.zunhebotoensis (tubercle lacking in the latter).

Figure 8 Habitats of Megophrys leishanensis sp. nov. in the type locality Leigong Mountain, Leishan County, Guizhou Province, China. A,landscape of montane forests in the type locality. B, a mountain stream in the type locality.
By having a small horn-like tubercle at the edge of each upper eyelid, Megophrys leishanensis sp. nov.differs from M. carinense, M. feae, M. gerti, M. hansi,M.intermedia, M. koui, M. latidactyla, M. liboensis,M. microstoma, M. palpebralespinosa, M. popei, M.shuichengensis and M. synoria (vs. having a prominent and elongated tubercle at the edge of each upper eyelid in the latter).
By a tongue not notched behind, Megophrys leishanensis sp. nov. differs from M. ancrae, M.baolongensis, M. binlingensis, M. boettgeri, M. carinense,M. cheni, M. chuananensis, M. damrei, M. fansipanensis,M. feae, M. feii, M. flavipunctata, M. gerti, M.glandulosa, M. hoanglienensis, M. huangshanensis, M.insularis, M. jingdongensis, M. kuatunensis, M. liboensis,M. mangshanensis, M. maosonensis, M. medogensis, M.minor, M. nankiangensis, M. omeimontis, M. oropedion,M. pachyproctus, M. parallela, M. popei, M. robusta, M.sangzhiensis, M. shapingensis, M. shuichengensis, M.spinata, M. vegrandis, M. wawuensis, M. zhangi and M.zunhebotoensis (vs. tongue notched behind in the latter).
By lacking lateral fringe in toes, Megophrys leishanensis sp. nov. differs from M. actuta, M.auralensis, M. baolongensis, M. binchuanensis, M.boettgeri, M. carinense, M. cheni, M. chuananensis, M.el fina, M. feae, M. feii, M. gigantica, M. glandulosa, M.hansi, M. intermedia, M. jingdongensis, M. jinggangensis,M. kuatunensis, M. latidactyla, M. lini, M. major,M. maosonensis, M. nankiangensis, M. omeimontis,M. palpebralespinosa, M. popei, M. rubrimera, M.sangzhiensis, M. serchhipii, M. shapingensis, M.shuichengensis, M. spinata, M. vegrandis, M. zhangi and M. zunhebotoensis (vs. lateral fringe present in the latter).
By toes with rudimentary webs at bases, Megophrys leishanensis sp. nov. differs from M. brachykolos,M. carinense, M. jingdongensis, M. jinggangensis,M. lini, M. major, M. palpebralespinosa, M. popei,M. shuichengensis, M. spinata (vs. at least one-fourth webbed in the latter).
By heels overlapping when thighs are positioned at right angles to the body, Megophrys leishanensis sp. nov.differs from M. acuta, M. brachykolos and M. obesa (vs.heels not meeting when thighs are positioned at right angles to the body in the latter).
By having an single internal subgular vocal sac in male, Megophrys leishanensis sp. nov. differs from M.caudoprocta, M. shapingensis, M. shuichengensis (vs.vocal sac absent in the latter).
By having nuptial pads and nuptial spines on the dorsal base of the first and second fingers in breeding male,Megophrys leishanensis sp. nov. differs from M. acuta,M. feii, M. shapingensis and M. shuichengensis (vs.nuptial pads and nuptial spines lacking in the latter).
We give more detailed compassion with the phylogenetically close species:
Megophrys leishanensis sp. nov. differs from M. minor by having a small horn-like tubercle at the edge of each upper eyelid (vs. absent in the latter), tongue not notched behind (vs. notched in the latter), tibiotarsal articulation reaching the level between tympanum to eye when leg stretched forward (vs. tibiotarsal articulation reaching the level between eye and tip of snout in the latter), having two metatarsal tubercles in each hand (vs. absent of metatarsal tubercle in hand in the latter).
Megophrys leishanensis sp. nov. differs from M.baolongensis by SVL in males < 38.8 mm (vs. SVL in males > 42 mm in the latter), tongue not notched behind(vs. tongue feebly notched behind in the latter), lacking lateral fringes on toes (vs. having narrow lateral fringes on toes in the latter), toes with rudimentary webs at bases(vs. toes without web in the latter), heels overlapping when thighs are positioned at right angles to the body (vs.just meeting in the latter).
Megophrys leishanensis sp. nov. differs from M.wushanensis by having a small horn-like tubercle at the edge of each upper eyelid (vs. absent in the latter), lacking lateral fringes on toes (vs. lateral fringes on toes wide in the latter), heels overlapped when thighs are positioned at right angles to the body (vs. just meeting in the latter),having two metatarsal tubercles in each hand (vs. absent of metatarsal tubercle in hand in the latter).
Megophrys leishanensis sp. nov. differs from M.tuberogranulata by the SVL in females < 42.3 mm (vs.SVL in females > 50 mm in the latter), tympanum about 0.7 times of eye diameter (vs. tympanum 0.5 times of eye diameter in the latter), having a small horn-like tubercle at the edge of each upper eyelid (vs. absent in the latter),the fourth finger significantly longer than the first (vs.first and fourth fingers with almost same length in the latter); outer metatarsal tubercle absent in soles (vs. outer metatarsal tubercle flattened in soles).
Distribution and habitats: Megophrys leishanensis sp. nov. is only known from the type locality, Leigong Mountain, Leishan County, Guizhou Province, China. It inhabits high mountain forest (Figure 8 A) at elevations between 1200–1750 m a. s. l., and is more frequently found in bamboo forest and encountered in forest nearby streams (Figure 8 B). Six sympatric amphibian species,i.e. Megophrys spinata Liu and Hu, 1973, Odorrana lungshengensis Liu and Hu, 1962, Leptolalax oshanensis Liu, 1950, Paramesotriton caudopunctatus Liu and Hu,1973, Rana zhenhaiensis Ye, Fei, et Matsui, 1995 and Vibrissaphora leishanensis Liu and Hu, 1973, were found in the type locality.
Etymology: This speci fic name leishanensis is a Latinize toponymic adjective that refers to Leigong Mountains,Leishan County, Guizhou Province, China, where the new species was collected. For the common name, we suggest Leishan horned toad (English) and Leishan Jiao Chan(Chinese).
4. Discussion
Most cryptic congeners in the genus Megophrys sensu lato are dif ficult to be distinguished from each other due to the superficial similarities in morphology: drab colorations,complicated markings and even changeable colorations and skin markings of the same individual under different environmental conditions (Fei et al., 2012). These result in considerable challenges in field identification, which in turn cause ambiguities in taxonomy and distributions(Wang et al., 2014). Megophrys minor has the widest distribution in Megophrys sensu lato, including the provinces of Sichuan, Guizhou, Chongqin, Yunnan,Guangxi, Jiangxi and northern Vietnam (Fei et al., 2012),and several new species has been separated from M.minor (Chen et al., 2017; Mahony et al., 2017; Liu et al., 2018), but most of which are not described. In this study, based on molecular phylogenetic analyses and morphological comparisons, a new species Megophrys leishanensis sp. nov. was described which was also initially identified as M. minor. Phylogenetic analyses based on mitochondrial DNA and nuclear DNA all suggested that the new species belonged to Megophrys(Panophrys) but was separated from its congeners.Genetic distance on COI gene between the new species and its closely-related species (M. baolongensis, M.wushanensis and M. tuberogranulata) was 5.9%–9.3%,matching the level about interspecific divergences in amphibians 3.1%—28.2% (Che et al., 2012) and being much higher than that between many sister species (of which, most species have been completely recognized as valid species) in Megophrys sensu lato (Table S4).Finally, it was different from its congeners on a series of morphological characters. At all, multiple evidences support the validity of the new species.
Megophrys leishanensis sp. nov. was only found in Leigong Mountain, Leishan County, Gauizhou Province,China. It restrictedly inhabits the forest floor, nearby slowly flowing mountainous streams surrounded by moist evergreen at elevations between 1200 m and 1750 m.These areas are being threatened by tourism development,and the population of Megophrys leishanensis sp. nov.is declining based on the monitoring from 2014 to 2018,so future research should focus on determining the distribution and population status of the species.
AcknowledgementsWe are grateful to editors and reviewers for their working on the manuscript.Collections in field were permitted by Administration of Leigong Mountain Nantioanl Nature Reserve (No.LGS20040348). This study was approved by the animal ethical committee of Chengdu Institute of Biology,Chinese Academy of Sciences, and animal experiments were carried out following the institutional guidelines(No. 2017CIBAEC0344). This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB31000000),National Natural Sciences Foundation of China (NSFC-31201702), Biodiversity Conservation Key Laboratory of Guizhou province Education Department, Guiyang College, The laboratory on biodiversity conservation and applied ecology of Guiyang college, Ocean Park Conservation Foundation of Hong Kong (No. PR 1030001252), Biodiversity Conservation Program of Ministry of Ecology and Environment of China(#2110404), Guizhou Provincial Department of Education Youth Science and Technology Talents Growth Project(Nos. KY[2018]455, KY[2018]468 and KY[2018]453),The Specialty Food Resource Utilization Talent Base of Moutai University.
Appendix
Figure S1 Geographical location of the type locality of Megophrys leishanensis sp. nov., Leigong Mountain, Leishan County, Guizhou Province,China.
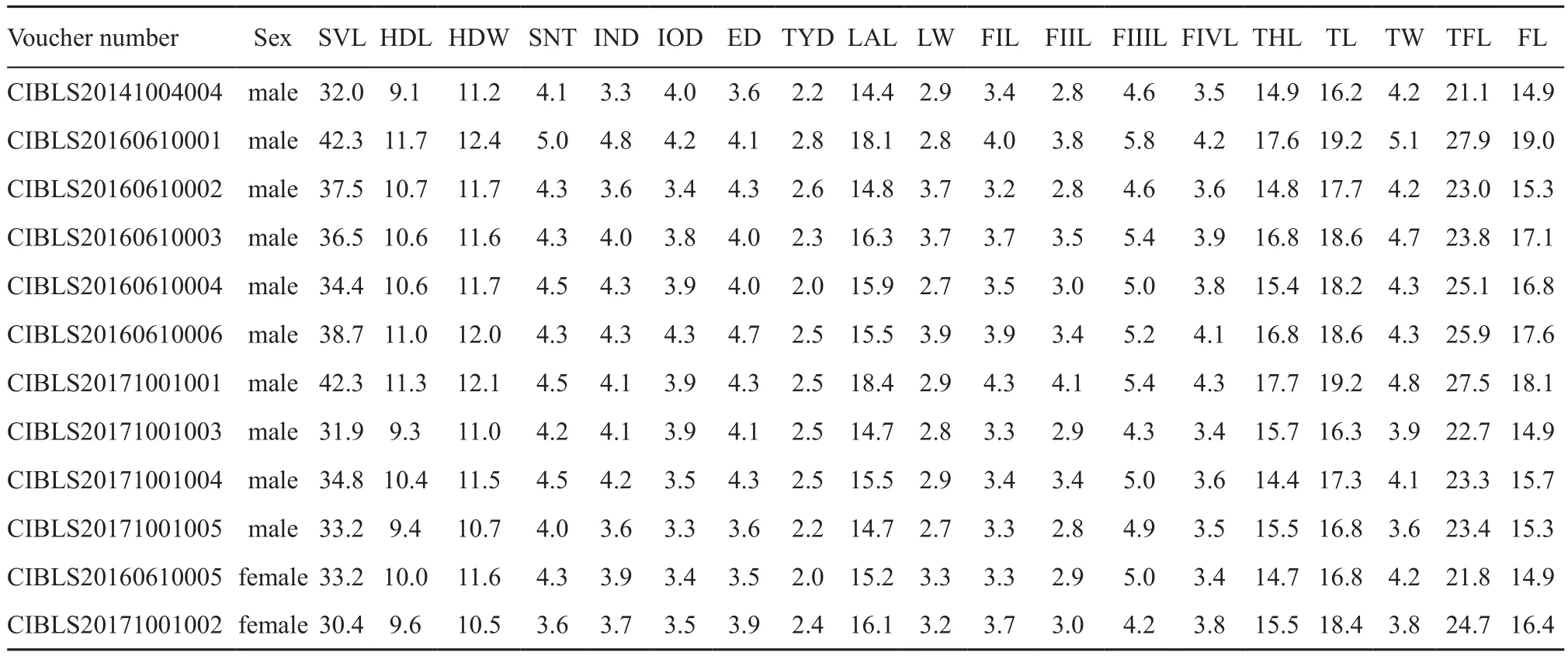
Table S1 Measurements of the adult specimens of Megophrys leishanensis sp. nov.
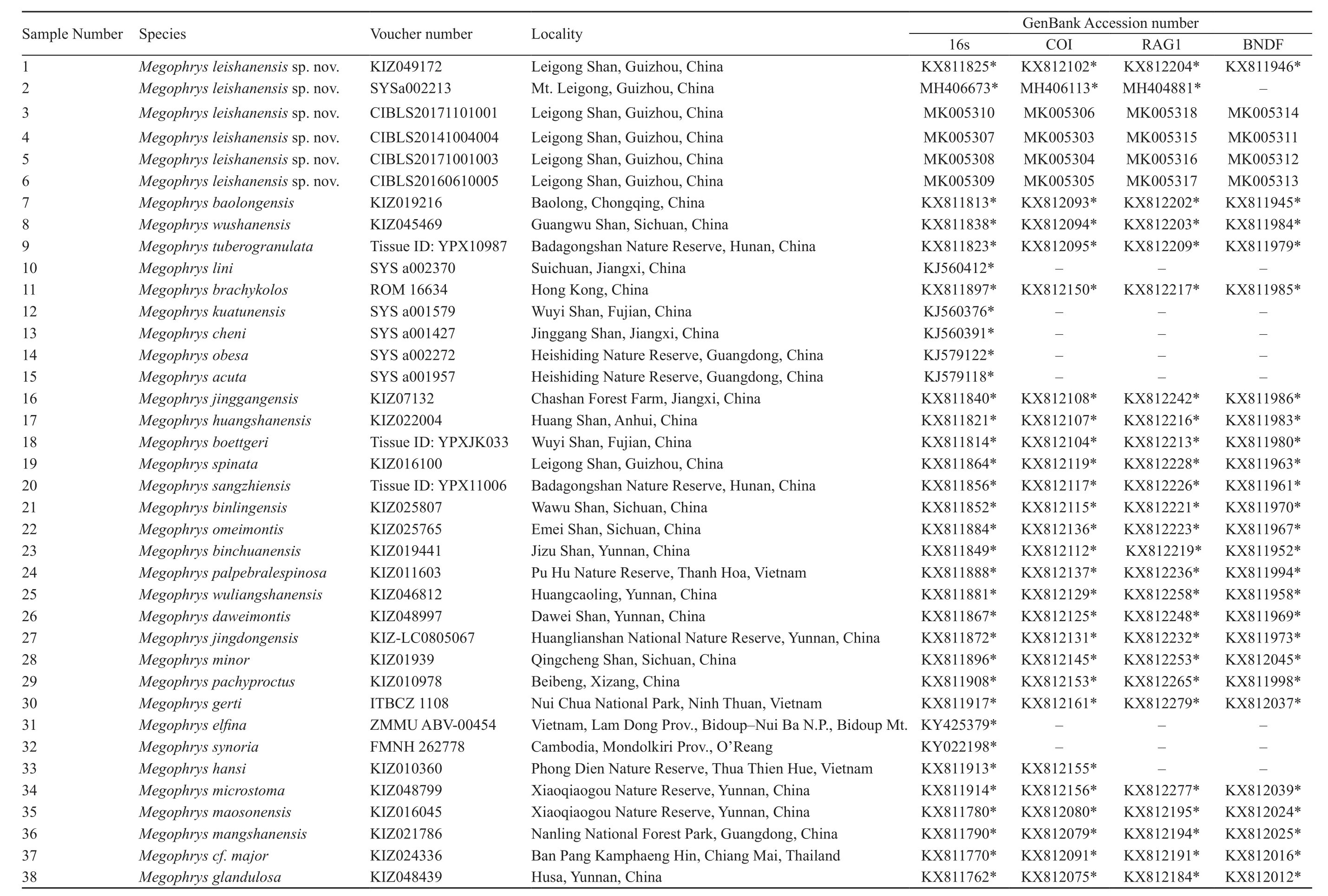
Table S2 Informationof samples used in the molecular analyses in this study. *sequence was downloaded from GenBank.

*****DF 43*38*39*33*35***BN 812017–– –– – – – ––––––– ––KX 812026 KX 812006 KX 812030 KX 812041 KX 8119 KX 8119 KX 8119 KX 8119 KX 8119 KX 812048 KX 812046 KX ber um G1 k Accession n RA 812197*––2200*2262*–2271**2274*2269*–2268*––2169*81 81 81 81––2181*81 81 81 405011 81 81 81–––––8 1––2171*2284*2282*KX KX KX KX KX MH KX KX KX KX KX KX KX an GenB CO 2082*I 35*81––2084*2159*–2062*2060*2056*–2057*–2052*2051*2050*–2054*2166*2164*KX 81––2072*KX 81 81 KX KX 81 4062 KX MH 81 81 KX KX 81––2163*KX 81––KX 81 81 81 KX KX KX 81 81 81 KX KX KX 16s 811767*1310*79*69*11 50*91*1317*1310*13 *KX K K XJ83 8946 811765*KX 811807*KX 8946 KX 811797*KX 811925*KX 811900*KX 811902**KX 406775 MH 811904*KX 811810**KX 504261**KM 8118 KX 504251 KM 5889 HQ 811927*KX 6798 KY KJ83 KJ83 811922*KX 811919*KX 811918*KX KJ8313 811921*KX 811928*KX 811930*KX ogopan ark: K d hailan ogopan es in arat, T nal P ark ark: K nal P ark hilipp hina hamm atio nal P ity, P P alu N i T ational P atio atio ang N dia n S ga C inab ulu N unung M inab akho boan alu N ichuan, C inab hina ung K dong e B bo am alu N hina o., S, C hina ha-K Speu ark, N un hina am ak: G unuang onesia ung K hina nan, C hina hina ichuan ark, Z alaysia, C ichuan, C unnan, C g, C izang, C hina hina ejiang C ng N: G un uangxi, C R, G ho pong ung K: G ak, M ichuan atural P izan: G nan, C an, C eserve, S hina an, C ling abah Natu ichu ihuai, H Bo, P ational P ichu–Malaysia: S abah u, X am han, G aling N eng, X abah, Y e, Yun, S hilippines Nanjiang araw cality mi, Java, Ind nanca N han, S Wawu S Malaysia: S han, S han, S uliang, Y re R un Vietnam: U Mt. W araw–Zh Lo Beib Trail angm Aural, K Liziping–Mengyu Nan N Huan Khao gcao China: Z Dayao S China: N kabu Su Malaysia: S Trail Paso Palawan, P Bintulu, S Malaysia: S Malaysia, no exact locality ei S Em ei S Em ber 5 I11393 p:2223 2 6 081 PX 3 015 9 3753 2045 Voucher num 8 599 43 13125 1916 P-05 PX KIZ01427 89 79 KIZ06621 YC 8/ZS 87596 03 Z 8 NA 3 86194 48 27 AS 9 8 IA ZMH A NC 3143 SM 11799 8 7 KIZ04850 K519 ZS 7 CIB Z KIZ02579 KIZ02546 51 9 Sa003933 SY KIZ01451 KIZ04670 CIB20050 Tissue ID: Y 89 S a0005 SY MK ZF UM B:Am AS LS MZ IM UN H A13125 ZM KU MU ZM NH FM IM UN KIZ01941 KIZ02577 Tissue ID: Y hako sis edogen Megophrys baluensis Megophrys zha Megophrys aceras ananen u ngi Megophrys katab onta ayae sis Megophrys cf. parva Megophrys sha Megophrys auralensis sis ardinae Megophrys nankiangensis awuensis ayashii Megophrys carinense shan Megophrys m Megophrys popei m boringii Megophrys feae Megophrys intermedia rachiu Megophrys w na pecies berS Megophrys chu Megophrys san Megophrys gigantica pingen Megophrys m Megophrys lancip i Megophrys dring Megophrys baluensis Megophrys stejnegeri Megophrys lig Megophrys edw Megophrys kob Megophrys nasuta ensis Leptolalax o Leptob Sample Num 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66

Table S3 Primers used in this study.
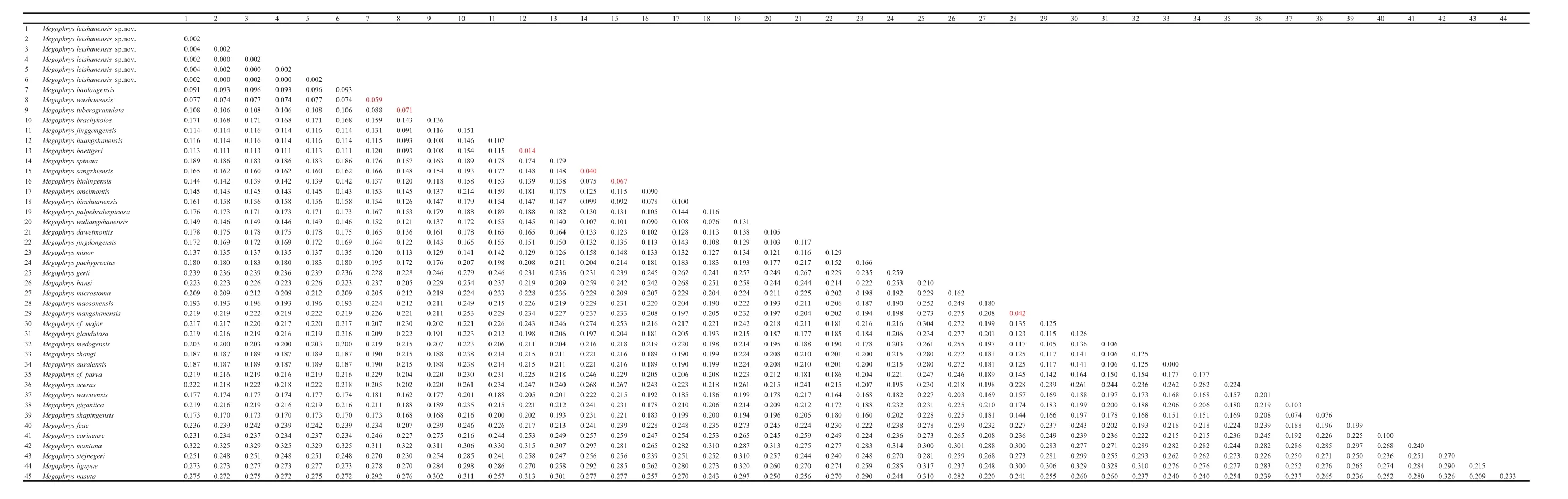
Table S4 Uncorrectedp-distance between Megophrys sensu lato species based on COI gene sequences.
 Asian Herpetological Research2018年4期
Asian Herpetological Research2018年4期
- Asian Herpetological Research的其它文章
- Amphibians and Reptiles of Luzon Island, Philippines: the Herpetofauna of Pantabangan-Carranglan Watershed, Nueva Ecija Province, Caraballo Mountain Range
- Call Characteristics of Two Sympatric and Morphologically Similar Tree Frogs Species, Polypedates megacephalus and Polypedates mutus (Anura: Rhacophoridae), from Hainan, China
- Maternal Thermal Effects on Female Reproduction and Hatchling Phenotype in the Chinese Skink (Plestiodon chinensis)
- Function of Lactate Dehydrogenase in Cardiac and Skeletal Muscle of Phrynocephalus Lizard in Relation to High-Altitude Adaptation
