High potassium to magnesium ratio affected the growth and magnesium uptake of three tomato (Solanum lycopersicum L.)cultivars
LI Hui-xia , CHEN Zhu-jun ZHOU Ting LIU Yan ZHOU Jian-bin
1 College of Natural Resource and Environment, Northwest A&F University, Yangling 712100, P.R.China
2 Key Laboratory of Plant Nutrition and the Agri-environment in Northwest China, Ministry of Agriculture, Yangling 712100, P.R.China
3 Department of Bioengineering, Yinchuan Institute of Energy, Yinchuan 750105, P.R.China
Abstract Potassium (K) and magnesium (Mg) levels and their balances are two factors affecting the growth of plant. However, the responses of different crop cultivars to K/Mg ratios are less clear. This study was aimed at assessing the different responses of tomato (Solanum Lycopersicum L.) cultivars to the different K/Mg supply ratios. Three tomato cultivars (Zhongza 9 (ZZ),Gailiangmaofen (MF), and Jinpengchaoguan (JP)) were grown in pots with three different K+/Mg2+ ratios (4:0, 4:1 and 8:1,represented by K/Mg4:0, K/Mg4:1, and K/Mg8:1, respectively). Compared with K/Mg4:1 treatment, the leaf chlorophyll content,net photosynthetic rate, and total biomass of tomato seedlings under K/Mg4:0 treatments were decreased by 69.7, 89.1,and 53.1%, respectively. The Mg deflciency symptoms were observed when the Mg content in shoot became lower than 4 mg g–1 DW. Compared with K/Mg4:1 treatment, total biomass of tomato seedlings of K/Mg8:1 treatment was decreased by 21.6%; the shoot and root Mg contents were decreased by 10.4 and 21.8%, respectively; and Mg uptake of tomato was reduced by 34.1%. There were signiflcant differences in biomass and Mg uptake for the three cultivars between the different K+/Mg2+ treatments. The Mg uptake of the three different cultivars ranked as ZZ>JP>MF under Mg deflciency and high K condition. In conclusion, the growth and Mg uptake and allocation of tomato were influenced signiflcantly by imbalance K and Mg supply. JP and ZZ were the cultivars with the highest efflciency in Mg uptake.
Keywords: tomato cultivars, potassium-magnesium, ionic interaction, magnesium uptake
1. lntroduction
Magnesium (Mg) is an essential nutrient for plant growth and development. It is the most abundant divalent cation in cytosol of plant cells, and plays a critical role in many physiological processes (Liet al.2001). Mg regulates enzyme activity, photosynthesis, protein synthesis, lipid metabolism, and carbohydrate allocation in plants (Shaul 2002; Verbruggen and Hermans 2013). Therefore, Mg deflciency restricts plant growth and dry matter partitioning between shoots and roots. Moreover, generation of highly reactive oxygen species (ROS) is also mostly associated with Mg-deflciency stress, resulting in metabolism disorders in plants (Hermans and Verbruggen 2005; Cakmak and Kirkby 2008; Guoet al.2016).
There are two reasons for Mg deflciency, i.e., absolute deflciency and cation competition (Gransee and Führs 2012). Absolute Mg deflciency can be a consequence of low Mg contents in the soil due to the excessive loss of Mg, which usually occurs in acid soils with light texture and low cation exchange capacity (Aitkenet al.1990).Imbalanced input of mineral fertilizers in intensive cropping pattern without recycling of crop residues has increased Mg depletion from soils (Hermanset al.2004). Apart from absolute deflciency, Mg competition with other cations, such as K+, Ca2+, H+,, or Al3+, also results in its deflciency(Gransee and Führs 2012).
Mg2+competition with K+are very important and widely studied. Excessive K+affects Mg2+movement towards the surface of the roots (Karley and White 2009). The presence of excessive K+in the soil reduces the bioavailability of Mg (Heijdenet al.2013). The Mg2+ions that reach the surface of the roots enter the endodermis through apoplast pathway, and then enter the cells through transmembrane transporters (Hermanset al.2013; Farhatet al.2016). The activity of Mg transporters and the number of competitive sites occupied by other cations are two major factors that affect Mg uptake by plants (Cakmak and Yazici 2010).
Many studies have reported the strong antagonistic relationship between Mg2+and K+, i.e., higher K+concentration inhibits Mg2+uptake. The decrease has been found in the Mg content of rice (Oryza sativaL.) (Dinget al.2006),and cowpea crops (Vigna unguiculataL. Walp.) (Narwalet al.1985) at higher K+concentrations. The competition between K+and Mg2+is unidirectional, only governed by K+,the increase in K+concentrations signiflcantly reduces plant uptake of Mg2+, and this inhibition occurs mainly in plant roots(Omar and El-Kobbia 1966). However, Hannawayet al.(1982) showed that the K+concentration of nutrient solution had no signiflcant effect on Mg uptake from the solution into the roots of Kenhy tall fescue (Festuca arundinacea);nonetheless, increased K+concentration signiflcantly inhibits Mg accumulation in plant shoots. Thus, the antagonism(interaction) between K+and Mg2+occurs during the transport from roots to shoots (Ohno and Grunes 1985). The studies regarding the interaction between K and Mg mainly focused on forests, grasses, and arable crops, and less attention has been paid on vegetable crops (Tanoi and Kobayashi 2015;Guoet al.2016). The activity of Mg2+transporters depends on the genetic makeup of different species (Cui 2016). The response of different tomato cultivars to varying K/Mg ratios have not been extensively studied so far.
Tomato (Solanum LycopersicumL.) is one of the popular vegetables in the world, and also a main crop grown under greenhouse in China (Yu 2011). Compared with other grains or grass crops, tomato demands more Mg to form the same biomass (Broadley and White 2010; Gerendás and Führs 2013). The Mg requirement of the plant gradually increases with increase in the growth of tomato (Hao and Papadopoulos 2004). Mg deflciency of tomato in the solar greenhouse in North China has become quite frequent in calcareous soil (Yanet al.2016), which is usually considered as rich in available Mg (exchangeable Mg 2–4 cmol kg–1).The soils in North China are usually developed from the loess or similar parent materials, which are also rich in K.The application of K fertilizer is also high in the greenhouse(as high as 730 mg kg–1). It signiflcantly increases available K, and the imbalanced K/Mg ratio is considered as the main reason of Mg deflciency (Chenet al.2013). In this study, a hydroponic experiment was conducted to evaluate the different responses of various tomato cultivars to the different K/Mg ratios. Our hypothesis was that the different tomato cultivars had the different responses to K/Mg ratio.
2. Materials and methods
2.1. Experimental design
The experiment was conducted using the hydroponic method. Three different K and Mg ratios were set,K+/Mg2+=4:1 (control), 4:0 and 8:1, represented by K/Mg4:1,K/Mg4:0, and K/Mg8:1, respectively. Meanwhile, three different tomato (S. lycopersicumL.) cultivars were selected, Zhongza 9 (ZZ), Gailiangmaofen (MF) and Jinpengchaoguan (JP),all of which are dominant cultivars in calcareous soil in North China. Seeds were provided by the Seed Breeding Center of the Horticulture College, Northwest A&F University(Yangling, Shaanxi Province, China). Nine treatments were arranged following completely randomized design, with each treatment replicated nine times.Tomato seeds were sown in hole trays containing a seedling substrate (pH 5.5–6.5)with an organic mass fraction≥50% and a humic acid mass fraction≥20%. After sowing the seeds in the substrate, the trays were put in greenhouse at (26±3)°C during the day,and (20±3)°C at night, at air humidity of 80%; and substrate moisture was maintained by regular watering. The seedlings were transferred to experimental chamber, and grown for one week. When the flfth leaves were fully expanded,seedlings were transplanted into rectangular hydroponic containers and supplied with half-strength Yamazaki nutrient solution (Yamazaki 1981) for 7 d. Then, a full strength solution was used from 8–45 d. The nutrient solution was prepared as follows. The K+concentrations were 4, 4, and 8 mmol L–1, respectively. For each treatment, 4 mmol L–1K+was provided with KNO3; for the 8 mmol L–1K+treatment,the remaining K was provided in the form of K2SO4. The concentrations of Mg2+were 0, 1, and 1 mmol L–1, provided in the form of MgSO4·7H2O. The nutrient solution was prepared using deionized water. Other ions were formulated based on Yamazaki nutrient solution (Yamazaki 1981),prepared by dissolving analytical reagents in deionized water containing the following (in µmol L–1): Ca(NO3)2·4H2O,1 500; NH4H2PO4, 660.9; Na2Fe-EDTA, 38; H3BO3, 19.4;MnCl2·4H2O, 4.4; ZnSO4·7H2O, 3.0; CuSO4·5H2O, 2.0; and(NH4)6Mo7O12, 0.01. The pH of the nutrient solution was adjusted to 6.0–6.5 using 0.01 mol L–1NaOH and HNO3.Meanwhile, the electric conductivity of the solution was also monitored regularly (<1.2 dS m–1) in order to meet the requirement of the hydroponic culture.
The experiment was conducted in the growing chamber for 45 d, Northwest A&F University. The containers used were plastic pots (containing 7 L nutrient solution), and covered with the opaque bubble plate. Seedlings with similar plant height,stem diameter, and petiole number were selected; their roots were washed with deionized water and surface-dried with fllter paper repeatedly. The plants were then weighed and those with relatively uniform weight were selected for experiment,with three plants per pot. The experiment was conducted in an artiflcial growth chamber at (24±2)°C during the day,and (18±2)°C at night. Supplementary light was provided using a LED light for 8 h per day, with darkness at night. The chamber was ventilated using an oxygen pump for 4 h each day. The nutrient solution was changed after every 5 d. The plants were cultured for a total of 15, 30, or 45 d. At the end of each culture stage, the whole plant was taken from each pot for further analysis.
2.2. Sample collection and analysis
Sample collectionThe tomato seedling was collected from each pot after 15, 30, or 45 d of culture. The roots were repeatedly washed with deionized water and surface-dried using fllter paper. The plant material for determination of dry weight (DW) and K and Mg solution analysis was divided into shoots and roots, then deactivated at 105°C in an oven for 30 min and then dried at 70°C for 48 h to constant weight.The DW was recorded and the samples were crushed,passed through a 60-mesh (0.25 mm) sieve, and stored in sample vials before use.
K and Mg analysisThe method of Bao (2005) was used to measure total Mg and K concentrations in shoots and roots. Briefly, a 0.5-g sample was weighed into a crucible and completely carbonized in an electric stove with low heat.Thereafter, the crucible was placed into a muffle furnace for 6 h of ashing at 550°C. The ash was dissolved with 1:1(v/v) nitric acid to a constant volume, followed by addition of a masking agent (LaCl3). For Mg, a stock solution of 10 µg mL–1was prepared, which was then used to prepare series of working standards of 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 µg mL–1.The samples were analyzed using an atomic absorption spectrophotometer (Z-2000, AAS, burner height=7.5 mm,wavelength=285.2 nm). For K, a stock solution of 100 µg mL–1was used to prepare working standards of various concentrations (0, 4, 8, 12, 16, 20 and 24 µg mL–1), and K analysis was performed using flame photometer (Taomsun-6400A, Shanghai, China). The concentrations of Mg and K in the samples were calculated with preparing standard curves.
Soil and plant analyzer development (SPAD)SPAD contents were measured using a chlorophyll meter and were measured on the third fully expanded leaves on the top of plant.
Chlorophyll determinationThe leaf chlorophyll content was extracted with 80% (v/v) acetone using Arnon’s method(1949) after 45 d of incubation. Briefly, a 0.2-g fresh sample was taken from newly expanded leaves on the top of the plant.Three replicate samples were separately placed in a mortar,followed by addition of a small amount of quartz sand and calcium carbonate powder as well as 2–3 mL of 80% acetone.The samples were ground to homogenates and 10 mL of acetone was then added. The homogenates were further ground until the tissue turned white and allowed to stand for 3–5 min. Then, the homogenates were flltered into a 25-mL volumetric flask and diluted with acetone to constant volume.The chlorophyll extracts were poured into a cuvette, with 80%acetone as the blank. The absorbance of cleared extract was measured at 663, 645, and 652 nm for chlorophylla, chlorophyllb, and total chlorophyll, respectively. Total concentrations (g kg–1FW) were calculated according to Arnon’s equations(Arnon 1949).
Photosynthetic rate measurementThe photosynthetic rate was measured at 9:00–11:30 on the third fully expanded leaves at the top of the plant using a LI-6400 portable photosynthesis system (LI-6400, LI-COR; Lincoln, NE,USA) before harvest (45 d). The conditions of infrared gas analyzer were set as follows: light intensity, 1 000 µmol m–2s–1CO2concentration, 380 µmol mol–1; relative humidity,60%; and temperature, 24°C.
2.3. Calculation and statistical analysis
Total Mg accumulation and uptake in plants were calculated with the following equations:

Total K accumulation and uptake were calculated using the same formula used for Mg.
Data were analyzed using SAS 8.1 (SAS Institute, Cary,NC, USA) and tested for one-way and two-way analysis of variance by Duncan’s method at the 5% level. Graphs were drawn using Excel 2007 (Microsoft Corp., Redmond,WA, USA).
3. Results
3.1. Growth of tomato
The plant height and stem diameter of tomato seedlings were signiflcantly affected by the duration of plant growth at all K/Mg ratios in all cultivars (Table 1). Compared with K/Mg4:1treatment, the plant height and stem diameter of tomato seedlings, measured 45 d after transplantation were decreased by 40.4 and 21.4%, respectively under K/Mg4:0treatment. Similarly for K/Mg8:1treatment, the above mentioned parameters were decreased by 15.8 and 15.2%, respectively. Under K/Mg4:0, the plant heights of ZZ, MF, and JP were decreased by 42.9, 57.6, and 20.2%,respectively. Moreover, the plant heights of three cultivars of K/Mg8:1treatment were decreased by 15.7, 21.2, and 10.7%, respectively.
Compared with K/Mg4:1treatment, the total biomass of shoots and roots of tomato seedlings decreased signiflcantly in K/Mg4:0(60.7 and 12.4%) and K/Mg8:1(23.6 and 12.5%)treatments (Table 2). The differences in the total and shoot biomass between different K/Mg ratios reached the signiflcant level. The effects of Mg deflciency or high K on biomass were greater in shoots than in roots. The total biomass of ZZ, MF, and JP cultivars in K/Mg4:0treatments were decreased by 48.9, 62.2, and 50.0%, respectively.The total biomass of ZZ, MF, and JP seedlings in K/Mg8:1treatment was decreased by 12.6, 29.5, and 24.7%,respectively.
3.2. Leaf SPAD, chlorophyll content and photosynthetic rate of tomato
After 30 d growth, signiflcant difference in the leaf SPAD values was observed in the different K/Mg ratios. After 45 d growth, the leaf SPAD values in K/Mg4:0treatments were decreased compared with K/Mg4:1and K/Mg8:1treatments(Fig. 1-A). The mean leaf chlorophyll contents in three tomato cultivars after 45 d were 0.84, 2.78, and 2.64 g kg–1,respectively for K/Mg4:0, K/Mg4:1, and K/Mg8:1treatments(Fig. 1-B). Compared with the K/Mg4:1treatment, leaf chlorophyll contents of K/Mg4:0treatment were decreased signiflcantly. At the K/Mg4:0condition, the leaf chlorophyll contents of MF was decreased signiflcantly than that of ZZ and JP. For net photosynthetic rates, the change was similar to leaf chlorophyll contents among the different K/Mg ratios(Fig. 1-C). Under K/Mg4:0condition, net photosynthetic rates of MF was signiflcantly lower than that of ZZ and JP.

Table 1 Effects of different K/Mg supply ratios on plant heights and stem diameters of three tomato cultivars

Table 2 Effects of different K/Mg supply ratios on shoot and root dry biomass accumulation of three tomato cultivars
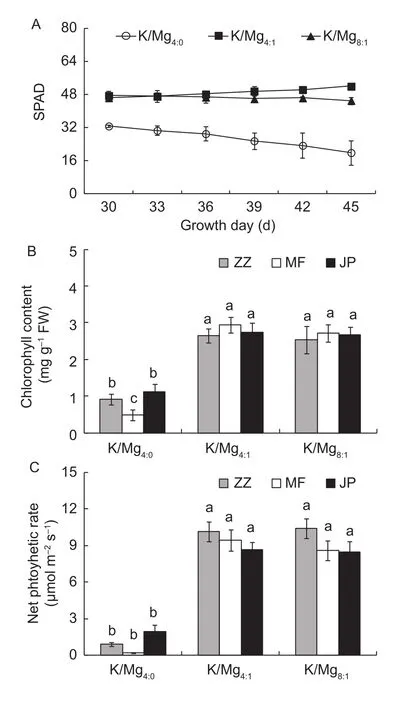
Fig. 1 Effects of K/Mg supply ratios and tomato cultivars on leaf soil and plant analyzer development (SPAD, A), chlorophyll content (B), and net photosynthetic rate (C) of tomato after incubation 45 d. Different letters indicate signiflcant differences among the treatments by Duncan’s method at the 5% level.Error bars indicate SD.
3.3. Mg and K concentrations of tomato
Both the shoot and root Mg concentrations of tomato seedlings were decreased with prolonged growth time(Table 3). The root Mg concentration was signiflcantly lower than the shoot Mg concentration; the former was approximately 1/3–1/2 of the latter. When the lower leaves showed yellowing symptoms due to Mg deflciency (28–30 d),the shoot Mg concentration was 4 mg g–1DW. Different K/Mg ratios markedly affected the shoot and root Mg concentrations in tomato seedlings. After 45 d growth under K/Mg4:0treatment, the shoot and root Mg concentrations were decreased by 54.4 and 66.5%, respectively. The decrease under K/Mg8:1was 10.4 and 21.8%, respectively,as compared with the K/Mg4:1treatment. The results indicate that Mg deflciency or high K resulted in lower Mg contents in shoots and roots of tomato. Under K/Mg4:0, the shoot Mg concentrations of ZZ, MF, and JP were decreased by 56.1, 47.2, and 59.2%, respectively. The decrease in root Mg concentration of the three cultivars was 66.5, 67.8,and 64.9%, respectively, as compared with the K/Mg4:1treatment. The shoot Mg concentrations of ZZ, MF, and JP of K/Mg8:1treatment were decreased by 12.2, 7.10,and 11.8%, respectively; the root Mg concentrations of the three cultivars were decreased by 17.8, 27.7, and 18.1%,respectively. The shoot K concentration of tomato seedlings was substantially lower with extension in the growth time(Table 4). Compared with K/Mg4:1treatment, the shoot and root K concentrations were less under K/Mg4:0. High K increased root K concentrations without affecting shoot K concentration. The differences in K concentration between various K/Mg ratios reached the signiflcant level.
3.4. Mg uptake and allocation in tomato
Compared with the K/Mg4:1treatment, Mg accumulation in tomato seedlings of K/Mg4:0treatment after 45 d was decreased (Table 5). Under K/Mg4:0, 82.3% of the total Mg was allocated to shoots, which was signiflcantly lower than the K/Mg4:1treatment. The total Mg uptake of tomato seedlings was decreased by 34.0% under K/Mg8:1, including 33.7% lower in shoots and 36.1% lower in roots. There were signiflcant differences among various cultivars under Mg deflciency and high K condition. Under K/Mg4:0, the total Mg uptakes of ZZ, MF, and JP were decreased by 83.8,91.9, and 87.2%, respectively. For K/Mg8:1treatment, the total Mg uptakes of ZZ, MF, and JP seedlings were 20.1,40.2, and 36.7% lower, respectively. The largest reduction was found in MF under Mg deflciency and high K condition.The Mg allocated to shoots was also signiflcant between various cultivars.
4. Discussion
4.1. Effects of K/Mg ratio on growth and Mg uptake of tomato
In this study, balanced application of K and Mg (control)
(Yamazaki 1981) resulted in the highest growth and Mg uptake by different tomato cultivars after 45 d of incubation.But when Mg was deflcient (K/Mg4:0treatment), the growth of different tomato cultivars was inhibited signiflcantly (Table 2).Similar results were obtained from wheat (Triticum aestivumL.) (Mengutayet al.2013), where a 21% decrease occurred in the total dry biomass at 23 d after the Mg supply was decreased from 0.45 to 0.015 mmol L–1. It is related to the key role of Mg for plant growth. Mg is the central atom of the chlorophyll molecule (Shaul 2002). Our results showed that Mg deflciency decreased the chlorophyll contents,and photosynthetic rate of tomato leaves (Fig. 1-C). Mg is essential for the function of many enzymes, including RNA polymerases, protein kinases, triphosphatases (ATPases),etc (Marschner and Cakmak 1989). Therefore, Mg plays important roles in a series of physiological processes in the plant (Cakmak 2013). For tomato, when the leaf color gradually turned from green to yellow, the shoot Mg content was 4 mg g–1DW, which was higher than the critical value of other vegetables (1.5–3 mg g–1) (Shaul 2002). It conflrms that the Mg demand of tomato is higher than other crops.Therefore, Mg nutrition of tomato, including Mg uptake and translocation in plant, and optimal K/Mg ratio, etc., needs further study.

Table 3 Effects of K/Mg supply ratios on Mg concentration of three tomato cultivars

Table 4 Effects of different K/Mg supply ratios on K concentration of three tomato cultivars

Table 5 Effects of different K/Mg ratios on Mg uptake and allocation of three tomato cultivars
When the K/Mg ratio was increased from 4:1 to 8:1, the total biomass of tomato was decreased by 21.6% (Table 2),indicating high K inhibited the tomato growth. It is consistent with the other study (Farhatet al.2013). The antagonism between K+and Mg2+is related to their similar chemical properties (Viadéet al.2011). The K/Mg8:1treatment did not signiflcantly change the Mg concentration in shoot;however, it signiflcantly reduced the Mg concentration in roots of tomato during the late growth stages (Table 3). The total uptake of Mg in shoots and roots were also signiflcantly decreased (Table 5). Decreased Mg content and uptake at high K concentrations were also found inCitrus(Lavon 1999) and cowpea (Narwalet al.1985).
The antagonism between K+and Mg2+may occur in the processes involved in the nutrient uptake and translocation within the plant. Competition for the similar transporters on the cell membrane is the main reason for antagonism between K+and Mg2+(Dinget al.2008; Shewmakeret al.2008; Kamiyaet al.2012). Therefore, the presence of abundant K+in solution prevents Mg uptake (Maathuis 2009). The Mg transport process from roots to the shoots is another reason for antagonism between K+and Mg2+. And Mg translocation is inhibited by high K+solution (Ohno and Grunes 1985; Farhatet al.2013). In present study, there were no signiflcant differences in the ratio of Mg in shoot to total biomass between the K/Mg4:1and K/Mg8:1treatment.It further indicates that inhibiting Mg uptake by higher K/Mg ratio may be the key factor for the antagonism of K+and Mg2+. The very high level of available K in the solar greenhouse in the calcareous soil in North China because over-application of K fertilizer was considered as the main reason of Mg deflciency (Yanet al.2016). Therefore, to alleviate Mg deflciency of tomato in the greenhouse, overapplication of K fertilizer should be avoided.
4.2. Response of different tomato cultivars to K and Mg supply
In this study, three tomato cultivars showed signiflcantly different responses towards plant growth, Mg deflciency symptoms, and Mg uptake under different K/Mg ratios.When Mg was deflcient (K/Mg4:0treatment), MF showed the lowest growth rate and plant biomass. MF seedlings showed typical Mg deflcient symptoms at flrst, followed by JP, and ZZ seedlings. When K/Mg ratio was high, plant biomass and Mg uptake of MF was also lowerthan that of JP and ZZ. Under different stressed conditions (K/Mg4:0and K/Mg8:1treatments), MF showed the lowest shoot Mg concentration and the highest root Mg concentration among the three cultivars, indicating that the MF had the lowest transport ability for Mg from roots to shoots. Similar results are reported for the different cultivars of Jerusalem artichoke(Helianthus tuberosusL.), sweet potato (Ipomoea batatasLam.) and grape (Vitis vinifera) (Shang 2007; Sunet al.2012; Maet al.2015). It indicates there are large variations in Mg uptake among different crop cultivars. Therefore, it needs further study to select tomato cultivars with high Mg uptake efflciency.
5. Conclusion
The Mg deflcient symptom was observed when the Mg content in shoot of tomato was lower than 4 mg g–1DW,indicated that Mg played a key role in chlorophyll and biomss formation of plant leaves. When the K+/Mg2+ratio was increased from 4:1 to 8:1, the total biomass and Mg uptake of tomato were decreased signiflcantly, conflrming that high K level inhibited Mg uptake and plant growth. The differences of total biomass and Mg uptake of the three cultivars were signiflcant; and the Mg uptake of the three different cultivars ranked as ZZ>JP>MF under Mg deflciency and high K condition. Adequate application K fertilizer and selection of appropriate tomato cultivars are two reasonable ways to alleviate Mg deflciency in tomato production.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (41671295) and the Agricultural Scientiflc and Technological Project in Shaanxi Province,China (2014K01-14-03).
 Journal of Integrative Agriculture2018年12期
Journal of Integrative Agriculture2018年12期
- Journal of Integrative Agriculture的其它文章
- Analysis of three types of resistance gene analogs in PmU region from Triticum urartu
- Overexpression of the Suaeda salsa SsNHX1 gene confers enhanced salt and drought tolerance to transgenic Zea mays
- Effects of planting methods on yield and quality of different types of japonica rice in northern Jiangsu plain, China
- The role of rhizobacteria in rice plants: Growth and mitigation of toxicity
- Postponed and reduced basal nitrogen application improves nitrogen use efficiency and plant growth of winter wheat
- Effects of variety and chemical regulators on cold tolerance during maize germination
