Design and selection of an artificial diet for the coconut black-headed caterpillar, Opisina arenosella, based on orthogonal array analysis
JlN Tao, LlN Yu-ying, JlN Qi-an, WEN Hai-bo, PENG Zheng-qiang
Key Laboratory of Monitoring and Control of Tropical Agricultural and Forest Invasive Alien Pests, Ministry of Agriculture/Environment and Plant Protection Institute, Chinese Academy of Tropical Agricultural Sciences, Haikou 571101, P.R.China
Abstract Opisina arenosella has been an outbreak pest of coconut trees in southern China since 2013. To develop efflcient control methods for this invasive pest, adequate rearing protocols are desirable. In this study, an orthogonal array of artiflcial diets with 11 factors at 3 levels was deployed for both 2nd–4th and 5th–6th instar larvae of O. arenosella. Biological parameters including survival time of larvae, development time from larva to pupa, pupation rate, emergence rate, and pupal weight were monitored to reveal the most important components in the diet formulas. Biological parameters in O. arenosella were most affected by brewer’s yeast, sucrose, ascorbic acid, and wheat germ. Statistical analysis indicated that different diet combinations supported optimum performance of biological parameters for 2nd–4th and 5th–6th instar larvae. The validity of the optimization predicted by the orthogonal array analysis was conflrmed in a follow-up bioassay with similar optimized diets for both 2nd–4th and 5th–6th instar larvae. The optimal artiflcial diet has great potential for the mass rearing technique,and can provide valuable results for using parasitoids in biological control of O. arenosella.
Keywords: Opisina arenosella, orthogonal analysis, diet optimization, mass rearing
1. lntroduction
Coconut is a major tropical crop and is one of the most commercially important palms worldwide. Coconut palms are used in landscapes for aesthetic value, as windbreaks in forests, and are cultivated in urban areas along streets,walkways, and seaside coasts, forming an indispensable component of the tropical and subtropical landscape in southern China. However, coconut trees are vulnerable to, and easily infested by, pests (Borowiecet al.2010;Jinet al.2014; Dembilioet al.2015). The coconut blackheaded caterpillar,Opisina arenosellaWalker (Lepidoptera:Xyloryctidae), is the most destructive leaf-eating pest on coconut palm in South Asia, including India, Sri Lanka,Bangladesh, and Myanmar (Nasserand Abdurahiman 2001;Mohanet al.2010). This species has gradually become one of the most harmful coconut pests in China since it spread to Hainan Island in 2013 (Lüet al.2013; Lu 2013).
O. arenosellaattacks coconut palms during all stages,from seedlings to maturity. Larvae remain concealed inside a gallery woven with silken thread and excreta and bitten or chewed leaf bits (Mohanet al.2010). The larvae feed on the upper side of leaf tissues and disregard the lower surface of leaves. Severe damage results in leaf dryness and defoliation, a reduction in the rate of production of flower spikes, retarded growth and even major declines in yield (Mohanet al.2010). Most chemical sprays are inefflcient and waste labor because the palm trees have high and straight trunks andO.arenosellaremains hidden under the leaves (Lüet al.2013). Therefore, attempts to control this pest rely primarily on cultural or biological control.Over 40 parasitoids and 20 predators attackO.arenosellaat nearly all growth stages (Pillaiand Nair 1993), and if a successful mass rearing technique could be developed and implemented for this pest, mass production of these potential biocontrol agents could provide strategies for managing the coconut black-headed caterpillar.
Generally, colonies ofO.arenosellaare maintained on coconut leaflets in the laboratory, and it is necessary to constantly provide a large quantity of fresh coconut fronds for mass rearing (Nasserand Abdurahiman 2001; Mohanet al.2010). The fronds required for healthy growth of coconut trees are quite expensive, and landholders limit the number that can be obtained. The development of artiflcial diets facilitates the mass rearing of this herbivorous pest. These diets save labor, time, space, and the costs associated with growing host plants in the laboratory. For decades,considerable and intensive research has been conducted on formulating main components and supplementary components, and on rearing substrate material for artiflcial diets of lepidopteran pests (Grisdale 1973; Caoet al.2014;Hervetet al.2016). Most diets combine purifled natural products such as wheat, corn meal, and sugar with pure nutritional chemicals. The diet formulas are simplifled to rear most primarily pestiferous species based on measures of insect fltness at different life stages. However, the nutritional quality and variety of formulations can be manipulated for speciflc insect species, according to the research aims,including for insect development, bioassay use, or mass production, among others. The principal purpose of the present study was to rearO.arenosellain the laboratory on available diet components to avoid the often-costly effort necessary to maintain fresh coconut leaves.
The orthogonal array design (OAD) is a fractional factorial design approach for analysis of optimal levels of combined parameters in multi-parameter and multi-level experiments,which requires fewer treatments than in a conventional parametric design (Sharmaet al.2005). An OAD analysis indicates representative combinations of factors and levels for laboratory experiments and can also be used to aid in the design of an optimized artiflcial diet for an insect based on the combination of ingredients (Bianet al.2014; Lüet al.2014). In the present study, initial successes using primary nutrition sources such as wheat germ, corn meal, coconut frond powder and brewer’s yeast to feedO.arenosellaled to development of an experimental OAD with eleven factors at three levels. Different combinations of ingredients were fed toO.arenosella, and parameters including survival time of larvae, development time from larva to pupa, pupation rate, emergence rate, and pupal weight were monitored to reveal the most important ingredients and optimize diet composition. A follow-up bioassay was used to assess the optimized formula. The flnal artiflcial diet formula can be used to optimize artiflcial rearing ofO.arenosellain order to study effective control measures of this invasive pest.
2. Materials and methods
2.1. Insects
The population ofO.arenosellatested in this experiment was originally collected in August 2015 from coconut palms in Danzhou, Hainan Province, China (109°17´E, 19°39´N).Approximately 1 000 adults were transported with their host leaves to the quarantine facilities of the Chinese Academy of Tropical Agriculture Sciences in Danzhou. The colony was reared at (25±2)°C under a 16 h L:8 h D photoperiod with (70–80)% RH on coconut palm leaves in the laboratory.The adults were supplied with 20% honey as a nutrition supplement and fresh coconut leaves for laying eggs.Newly hatched larvae were separated daily and provided with fresh new coconut leaves as food in a plastic container for the experiment.
2.2. Optimization of artificial diet using an OAD
Several ingredients prepared for the diet test were based on the McMorran formula for Lepidoptera larvae(McMorran 1965). Twelve ingredients were included in the artiflcial diet forO.arenosella: wheat germ, sucrose, corn meal, brewer’s yeast, coconut frond powder, Wesson’s salt, cholesterol, choline chloride, inositol, sorbic acid,ascorbic acid, and agar. Agar is a thickener, and different concentrations might affect the physical condition and shape of the diet, which could create side effects in the biological parameters of the test insect; therefore, the agar concentration was set at 1% in all diet treatments. An L27(311) OAD was employed to assign the other 11 ingredients(factors from A to K) at 3 concentrations (levels from 1 to 3)(Table 1), with 27 tests conducted according to the matrix given in Table 2. Each row of the orthogonal array was a speciflc set of factor levels to be tested.
The ingredients of the basic artiflcial diets used in this study are given in Table 1. Sterile water was added as a supplement to reach a total of 100 g for all formulas. To prepare artiflcial diets, each treatment used 1 g of agar that was completely dissolved in boiling sterile water. Then, the primary nutrients, including wheat germ, sucrose, corn meal,brewer’s yeast, and coconut frond powder, were added, and the fluid solution was heated at 80°C for approximately 2 min.Complementary nutrients, including Wesson’s salt,cholesterol, choline chloride, inositol, sorbic acid, and ascorbic acid, were added to the fluid solution when it cooled to 60°C. The entire fluid solution was stirred continuously until all ingredients were completely dissolved. All solid formula of artiflcial diets was stored at 4°C.

Table 1 Factors and levels in an orthogonal array design (OAD) to optimize an artiflcial diet for Opisina arenosella larvae
2.3. Rearing method and biological parameters assessed
To evaluate the efflcacy of the proposed diet for different stages of larvae, 20 individuals of 2nd–4th or 5th–6th instar larvae were placed in separate plastic containers (15 cm×10 cm×10 cm) with feed according to each formula in Table 1. During the rearing process, the numbers of living larvae, pupae, and adults were counted daily. Pupal weight was obtained on the flrst day after pupation. Survival time of larvae, development time from larva to pupa, pupation rate,and emergence rate were calculated as follows:

High values of survival time of larvae, pupation rate, and emergence rate and a low value of development time from larva to pupa were considered characteristic of an optimized diet for theO.arenosellapopulation.
2.4. Data analyses
The orthogonal array (L27) was designed and analyzed using SPSS 17.0 software (Statistical Package for the Social Sciences, Chicago, IL, USA) (Bianet al.2014; Lüet al.2014). All biological parameters were analyzed in each artiflcial diet trial with different combinations of the eleven ingredients (Table 2). Range analysis was used to demonstrate the effect of each factor and to determine optimal levels of the various factors. The range (Rj) was deflned according to the following equation (Denget al.2012; Lüet al.2014):

Where,jis the factor letter (j=A, B, C, D, E, F, G, H, I,J, K),iis the factor level (i=1, 2, 3), andindicates the mean value of the sum of the evaluation indices of all levels for each factor; this equation was used to determine the theoretically ideal level and the optimal combination of factors (Wuet al.2011). A relatively largeRjvalue indicates that the ingredient is a more suitable factor, and the optimal level for each factor is indicated by the largest value ofkji(Cuiet al.2010). The range analysis cannot distinguish whether the difference between the data at each factor level was caused by experimental variables or by experimental errors, so analysis of variance (ANOVA) was employed to analyze the OAD results to assess the effect of the factors(ingredients) on biological parameters (Lüet al.2014);differences among mean values were considered signiflcant atP<0.05.
The most similar optimized formulas between the 2nd–4th and 5th–6th instar larvae were selected for the second bioassay. Each diet had 3 replicates, and each replicate had a total of 20 individuals for the test. The data were analyzed with a one-way ANOVA to test for signiflcant differences. Mean values were separated using Tukey’s test. In all experiments, differences among mean values were considered signiflcant atP<0.05.
3. Results
3.1. Optimization of artificial diet using OAD
According to the L27orthogonal array, 27 separated treatments were analyzed for the 2nd–4th and 5th–6th instar larvae, with the biological parameters shown in Table 2. Variations in the biological parameters indicated thatO.arenosellalarvae could complete development on all 27 formulas. Range analyses of the artiflcial diets for the 2nd–4th and 5th–6th instar larvae are shown in Tables 3 and 4, respectively.A largeRjvalue indicates that a factor was considered more suitable at the different levels, and the signiflcance of each ingredient for the different biological parameters was shown as follows.First, brewer’s yeast(D) was the most important ingredient and coconut frond powder (E) was the least important ingredient for survival time of both 2nd–4th instar and 5th–6th instar larvae.Sucrose (B) had the strongest effects on development time from larva to pupa and emergence rate of both 2nd–4th instar and 5th–6th instar larvae. Pupation rate of 2nd–4th instar and 5th–6th instar larvae was most affected by ascorbic acid(K) and sucrose,respectively. Wheat germ (A) was the most important factor on pupal weight of both 2nd–4th instar and 5th–6th instar larvae.
Based on thevalues of each factor,the artiflcial diets theoretically yielding optimum biological parameters are shown in Table 3 and were the following: the highest survival time, A2B1C3D3E3F2G1H3I1J1K2 (2nd–4th instar) and A2B2C3D3E3F1G2H1I2J3K2 (5th–6th instar);the shortest development time from larva to pupa,A1B1C2D1E3F1G3H3I1J2K1 (2nd–4th instar) and A2B1C1D1E1F3G2H1I2J2K1 (5th–6th instar); the highest pupation rate, A3B3C3D3E3F3G1H3I1J3K3 (2nd–4th instar) and A1B2C1D2E2F2G2H1I3J3K2 (5th–6th instar);the highest emergence rate, A3B3C3D1E2F2G2H1I2J2K2(2nd–4th instar) and A1B2C2D2E2F1G2H1I2J2K2(5th–6th instar); and the heaviest pupal weight,A3B1C3D1E1F1G3H2I3J3K3 (2nd–4th instar) and A1B1C2D1E3F1G1H2I1J1K2 (5th–6th instar).

Table 2 Orthogonal array design (OAD) for three levels of eleven factors used for diet optimizationwith the corresponding biological parameters of Opisina arenosella larvae
The ANOVA results for the primary factors affecting different biological parameters are shown in the supplementary material. ANOVA indicated that wheat germ signiflcantly affected development time from larva to pupa (P=0.04) and pupal weight (P=0.021) of 5th–6th instar larvae; sucrose signiflcantly affected development time from larva to pupa of both 2nd–4th and 5th–6th instar larvae (P=0.008 andP=0.048, respectively); brewer’s yeast signiflcantly affected survival time of larva at the 5th–6th instar (P=0.039); and cholesterol and sorbic acid signiflcantly affected development time from larva to pupa of 2nd–4th instar larvae (P=0.03 andP=0.028, respectively).ANOVA conflrmed the results of the range analysis: wheat germ, sucrose, and brewer’s yeast influenced survival time,development time, and pupal weight, respectively.
3.2. Verification of the optimized artificial diet for both 2nd–4th and 5th–6th instar larvae
The ideal diet formula for both 2nd–4th and 5th–6th instar larvae ofO.arenosellawas identifled as follows. Diet 1 (A2B1C3D3E3F2G1H3I1J1K2) and diet 2(A2B2C3D3E3F1G2H1I2J3K2) had identical levels of 5 ingredients, with the composition A2C3D3E3K2,and diet 3 (A3B3C3D1E2F2G2H1I2J2K2) and diet 4(A1B2C2D2E2F1G2H1I2J2K2) had identical levels of 6 ingredients, with the composition E2G2H1I2J2K2.Therefore, these four formulas were selected for the second bioassay test, and the results are shown in Fig. 1. Among the four diets, for the 2nd–4th instar larvae, no signiflcant differences were detected in average development time from larva to pupa (30.33–36.2 days), average pupation rate (28.3–63.3%), or average emergence rate (68.3–84%).For the 5th–6th instar larvae, no signiflcant differences were detected in survival time of larva (9.03–10.85 days),average emergence rate (62.7–95%) or pupal weight(31.7–65.3 mg). However, a signiflcantly lower survival time of larva (15.53 days) with diet 3 and pupal weight(26.3–36.7 mg) with diets 2–4 were detected for 2nd–4th instar larvae, and the lowest development time from larva to pupa (11.1 days) was found with diet 1 for 5th–6th instar larvae. Thus, diet 1 was superior to diets 2–4 for rearing both 2nd–4th and 5th–6th instar larvae. The following ingredients were selected for the optimal artiflcial diet because this combination had the best performance for larvae in all stages: 7.5 g wheat germ, 2 g sucrose, 6 g corn meal, 4 g brewer’s yeast, 6 g coconut frond powder,0.75 g Wesson’s salt, 0.05 g cholesterol, 0.05 g choline chloride, 0.01 g inositol, 0.2 g sorbic acid, 0.4 g ascorbic acid, 1 g agar, and 72.04 mL sterile water.
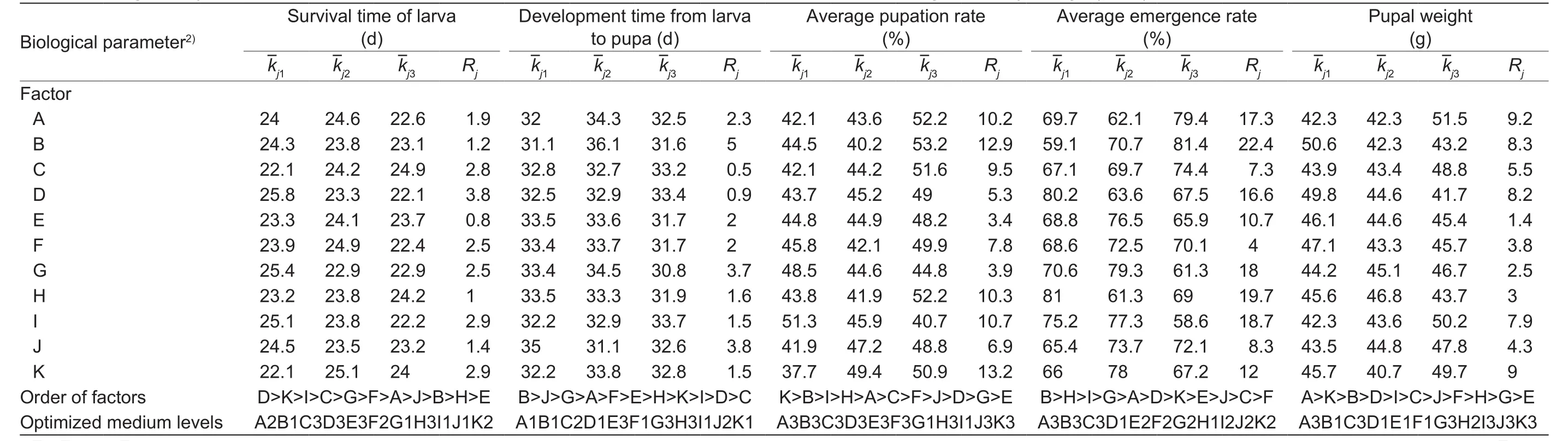
Table 3 Range analysis of artiflcial diets for 2nd–4th instar larvae of Opisina arenosella tested in the orthogonal array design (OAD) experiment1)
4. Discussion
In this study, we developed an artiflcial diet forO.arenosellalarvae. The results identifled the roles of various ingredients and the optimal ingredients based on an orthogonal experimental analysis. The order of factors revealed that: (1) brewer’s yeast was the primary ingredient affecting the survival time of larvae; (2) sucrose was the key ingredient affecting the development time of larvae and the emergence rates; (3) sucrose and ascorbic acid were the primary ingredients affecting pupation rate; and(4) wheat germ was the key ingredient affecting pupal weight. However, the optimal formula for each biological parameter was different between the 2nd–4th and 5th–6th instar larvae. Diet 1 was verifled in the follow-up bioassay to have the most optimal biological parameters in common and therefore was suitable to rear both 2nd–4th and 5th–6th instar larvae ofO.arenosella. This mixed diet led to a high survival rate that was similar to rearing on natural leaves. This diet has been used for successful mass rearing this insect in our laboratory for over three generations. However, it is worth noting that the physical conditions of the diet are slightly sticky and caused some mortality of smaller individuals observed in our laboratory.Therefore, we suggest that the flrst instar ofO.arenosellashould be reared on fresh coconut leaves. Further, keeping rearing conditions in a dry or air-circulating environment is one of the most important factors of the system. These conditions are necessary because larvae ofO.arenosellain nature are found in the space covered with their chewed leaf bits and excreta, which are easily dried by atmospheric circulation on the palm trees. Air-circulating conditions in the laboratory similarly dried excreta and provided a comfortable niche for this insect.
The McMorran diet has been widely used to rear Lepidoptera and many other insect species for decades(McMorran 1965; Hervetet al.2016). This formulation was subsequently modifled or more ingredients were added to reduce the incidence of deformities or to improve the biological fltness of different species (Hervetet al.2016). Our original ingredients for theO.arenoselladiet were based on the McMorran diet but were modifled according to published literatures (McMorran 1965; Caoet al.2014; Hervetet al.2016) and our previous rearing experience. Because coconut leaf is the primary natural food for this insect, including coconut frond powder as a factor was an important consideration in the design of an optimal formulation. However, no signiflcant correlation was found between coconut frond powder and the biological parameters we monitored. Thus, coconut frond powder was not a key factor affecting the biological fltness ofO.arenosella. In some formulations, linseed oil is added to the diet to reduce wing deformities in Lepidoptera (Grisdale 1973). However, linseed oil makes the diet more viscous and sticky, and because the larvae ofO.arenosellahidden in palm leaves likely prefer a drier environment, linseed oil or other oils were not considered for the design of this artiflcial diet.

Table 4 Range analysis of artiflcial diets for 5th–6th instar larvae of Opisina arenosella tested in the orthogonal array design(OAD) experiment1)

Fig. 1 Biological parameters of Opisina arenosella reared on artiflcial diets 1–4. Mean values with different letters are signiflcantly different (Tukey’s test: P<0.05). Error bars indicate SE.
The primary nutrition sources were not easily determined in our study because different dietary components may satisfy the same nutritional components. For example,both brewer’s yeast and casein have an enriched protein component and provide a highly nutritional substance for artiflcial diets of insects, and nearly all formulations have one or both of these ingredients (Vanderzant 1974;Hervetet al.2016). Our data showed that the survival time ofO.arenosellalarvae was signiflcantly affected by the concentration of brewer’s yeast and indicated that 4%brewer’s yeast should be adopted for the artiflcial diet.Sucrose, or another sugar, is an indispensable ingredient in artiflcial diets for Lepidoptera (Hervetet al.2016), Diptera(Vanderzant 1974; Tachibanaand Numata 2001; Chenet al.2014), and Coleoptera (Vanderzant 1974; Ichikiet al.2009; Tanet al.2015). In the present study, sucrose was the primary ingredient affecting the development time of larvae, pupation rate, and emergence rate ofO.arenosella,similar to the results found forCeratitis capitata(Nesteland Nemny-Lavy 2008),Drosophila melanogaster(Rovenkoet al.2015), andChilo suppressalis(Hanet al.2012).Wheat germ provides carbohydrates, proteins, fatty acids,and sterols that are required by insects. As a primary constituent, wheat germ has been used in most artiflcial diets for over 200 species of insects for many years (Vanderzant 1974; Hervetet al.2016). Our results showed that wheat germ affected pupal weight inO.arenosella, similar to the results found forPectinophora gossypiella(Adkissonet al.1960),Ceratitis capitata(Vargaset al.1994), andDiatraea saccharalis(Roeet al.1982).
A nutritionally complete diet must contain all or most of the substances required by an insect. Therefore, some trace complementary ingredients are indispensable to balance the nutritional requirements of insects. Based on the current state of knowledge, plant-feeding insects likely require complementary nutrients such as cholesterol, inositol, sorbic acid, ascorbic acid, and trace amount of other ingredients in artiflcial diets (Vanderzant 1974; Chippendale 1975; Hervetet al.2016). Our results showed that the biological fltness ofO.arenosellawas not signiflcantly influenced by the complementary supplements, with the exception of ascorbic acid (Vitamin C), which was the primary ingredient that affected pupal weight. Ascorbic acid is a feeding stimulant and is an essential nutrient for the growth forDiatraea grandiosella(Chippendale 1975),Lymantria dispar(Rothet al.1994), andNilaparvata lugens(Panet al.2014),among other insect species. Therefore, the complementary nutrient ascorbic acid and other additional trace elements should be included in formulations and modifled to improve the biological fltness ofO.arenosellawhen reared in the future.
OAD is often used to design experiments with multiple factor levels to minimize assay numbers, time and experimental cost, and the optimum parameters determined in the laboratory can be utilized at larger scales of production(Oles 1993). OAD has been used previously to design and analyze combinations of factors and levels for the optimization of artiflcial diets for other insect species (Assemiet al.2012; Lüet al.2014). The present study demonstrated that orthogonal array analysis is a useful method to obtain optimal combinations of ingredients for artiflcial diets to rear larvae ofO.arenosella. The most reasonable formula was selected for both early and late instars after veriflcation from a follow-up bioassay. This new formulation allows us to have successive indoor populations, which may be useful for mass production ofO.arenosella.
5. Conclusion
The following artiflcial diet with the best performance was to rear larvae ofO.arenosella: 7.5 g wheat germ, 2 g sucrose, 6 g corn meal, 4 g brewer’s yeast, 6 g coconut frond powder, 0.75 g Wesson’s salt, 0.05 g cholesterol, 0.05 g choline chloride, 0.01 g inositol, 0.2 g sorbic acid, 0.4 g ascorbic acid, 1 g agar and 72.04 mL sterile water. Our study indicates that orthogonal array analysis is a useful method to obtain the best composition of an artiflcial diet for insects.
Acknowledgements
This study is supported by the National Key Research and Development Program of China (2016YFC1201200), the National Key Technologies R&D Program of China during the 12th Five-Year Plan period (2015BAD08B03), and the Central Public-interest Scientiflc Institution Basic Research Fund for Chinese Academy of Tropical Agricultural Sciences(1630042017013 and 1630042017012).
 Journal of Integrative Agriculture2018年12期
Journal of Integrative Agriculture2018年12期
- Journal of Integrative Agriculture的其它文章
- Analysis of three types of resistance gene analogs in PmU region from Triticum urartu
- Overexpression of the Suaeda salsa SsNHX1 gene confers enhanced salt and drought tolerance to transgenic Zea mays
- Effects of planting methods on yield and quality of different types of japonica rice in northern Jiangsu plain, China
- The role of rhizobacteria in rice plants: Growth and mitigation of toxicity
- Postponed and reduced basal nitrogen application improves nitrogen use efficiency and plant growth of winter wheat
- Effects of variety and chemical regulators on cold tolerance during maize germination
