Effects of Salacia lehmbachii ethanol root bark extract on estrous cycle and sex hormones of female albino rats
Grace A. Essiet, Godwin C. Akuodor, Daniel OJ Aja, Mathew O. Nwokike, Desmond O. Eke, Anuli N.Chukwumobi
1Department of Pharmacology, Faculty of Basic Medical Sciences, College of Medical Sciences, University of Calabar, Calabar, Nigeria
2Department of Pharmacology and Therapeutics, Faculty of Medicine, Ebonyi State University, Abakaliki, Nigeria
3Department of Internal Medicine, Chukwuemeka Odumegwu Ojukwu University Teaching Hospital, Awka, Nigeria
Keywords:Salacia lehmbachii Ovulation Estrous cycle Root bark Female sex hormones
ABSTRACT Objective: To evaluate the effect of Salacia lehmbachii (S. lehmbachii) ethanol root bark extract on estrous cycle and sex hormones in female rats. Methods: Forty-eight virgin rats with regular 4-day cycle were grouped into four and each group was further subdivided into ‘a’ and ‘b’ (n=6).Each group was orally treated for 28 days with 2 mL of distilled water (control), ethanol root bark extract of S. lehmbachii in doses of 250, 500 and 750 mg/kg/body weight (groups 2, 3,4, respectively). Estrous cycle was determined daily using the vaginal smear method. Rats in‘a’ subgroups were weighed and sacrificed on the 29th day, and blood was collected for serum generation which was used for hormonal assay and sex organs were removed and weighed.Rats in ‘b’ subgroups were discontinued from treatment for 2 weeks and the parameters above were reassessed. Results: The mean length of estrous cycle and duration of diestrous of treated rats were prolonged dose dependently compared to control. The increase was significant(P<0.05) at 500 and 750 mg/kg. The other estrous phases were shortened in the same pattern.Relative weights of sex organs were reduced significantly (P<0.05) at the highest dose. Sex hormones levels were significantly (P<0.05) reduced compared to control. The above changes reverted towards the control values two weeks post treatments. Conclusions: Ethanol root bark extract of S. lehmbachii (high doses) has antifertility effect in female rats as it prolongs the estrous and diestrous cycle, and reduces serum sex hormones levels. The observed alterations were reversible.
1. Introduction
From the earliest days of mankind, medicinal plants have been used for diverse health issues including controlling pathological conditions and physiological activities. In the course of preclinical evaluation of these plants for safety, some of them have been reported to interfere with female reproductive functions especially the estrous cycle[1,2]. Commonly, such interference is either expressed as a change in normal components of vaginal smear and disruption in the frequency of particular stages of the estrous cycle or both[3]. Xenobiotics may affect estrous cycle by acting at different levels of hypothalamic-pituitary axis resulting in alterations in the levels of gonadotrophins, namely leutinizing hormones (LH) and follicle stimulating hormones (FSH) or at ovarian level to inhibit ovulation[4] .
Ovulation is fundamental to successful establishment of pregnancy,but may also impact the developmental potential of resultant embryos. The ovulatory cascade of events is complex and triggered by a surge of LH which stimulates the activity of multiple intracellular signaling pathways in granulosa cells culminating in altered transcriptional complexes mediating expression of ovulatory genes[5,6]. The observed effects of LH are mainly mediated by adenylate cyclase and increased cyclic adenosine monophosphate(cAMP). The cAMP in turn via cAMP-dependent protein kinase affects three distinct steps which are crucial in the ovulatory process. They include stimulation of steroidogenesis, induction of cyclooxygenase (COX)/ lipooxygenase leading to increased synthesis of prostaglandins/leukotrienes and stimulation of plasminogen activator which catalyzes the conversion of plasminogen to plasmin.The involvement of cyclooxygenase enzymes signifies that ovulation is an inflammatory process. Thus, it may be blocked by chemicals with anti-inflammatory properties especially when they are administered at high doses before the LH surge[7]. Such chemicals are present in some medicinal plants that are used locally for the treatment of diverse ailments. An example of such plants is Salacia lehmbachii (S. lehmbachii).
S. lehmbachii is a small flowering tree of 3-5 meters in height. It is one of the 52 species in the genus Salacia belonging to family of Celastraceae[8]. The plant is commonly used by the local dwellers in some parts of Southeastern Nigeria and Cameroun particularly the Bakassi forest reserves, for the treatment of febrile illnesses like malaria. It is known in vernacular as ‘eba-enang-enang’ (peoples of Akwa Ibom and Cross River States) and ‘ora-mmanu’ (the Igbos), all of Nigeria. The median lethal dose of the root bark extract of the plant in albino rats is above 5 000 mg/kg while its chemical constituents include alkaloids, glycosides, flavonoids, tannins, saponins and polyphenols[9]. The plant has a wide range of pharmacological actions including analgesia and antiinflammation[9], inhibition of male sex hormones[10] and antifertility in male rats[11]. The hepatotoxic, hematotoxic, embryotoxic and teratogenic potentials of the plant have also been reported[12-14]. Though, the root bark extract of S. lehmbachii is reported to have antifertility effect in male rats, till date there has not been study to evaluate similar effect in female rats. The present study was undertaken to evaluate the effect of the ethanol extract of the plant’s root bark on the estrous cycle and sex hormones in female albino rats.
2. Materials and methods
2.1. Collection and identification of plant material
The roots of S. lehmbachii were purchased from Watt market,a local market in Calabar, capital of Cross River State, Nigeria.The plant was authenticated by the Department of Botany of the University of Calabar where a voucher specimen with herbarium number 688 was deposited.
2.2. Preparation of the extract
The roots were washed with clean water to remove dirt and dried in their lengths in an electric oven, and thermostatically controlled at 40 ℃ for 12 h. The root bark powder of the plant was obtained following an earlier described method[10]. The dry ethanol extract was derived from a two-staged Soxhlet extraction of the root bark powder using ethanol (99.8%, BDH Chemical Limited, England)as solvent and the resultant extract solution dried into dry powder following the earlier described method[10]. The solid extract which formed the yield was weighed and preserved in a clean and dry container until required for the experiments. The extract was constituted in distilled water to give the doses required for the study.
2.3. Experimental animals
Twelve weeks old virgin rats, 48 in number and weighing between 170-190 g were purchased from the animal house of the Department of Pharmacology, University of Calabar and housed in plastic cages with wire gauzed top. Each cage contained six rats which were properly identified with dilute picric acid. The animals were acclimatized for seven days to normal laboratory conditions [relative humidity: (50±5)%; temperature: (28±2 ) ℃ and 12 h of light-dark cycle] before the start of the experiment and maintained at the same conditions throughout the duration of the study. They were fed with standard rat chow (Agro-Feeds, Calabar) and water (Water board,Calabar) ad libitum. The guidelines on Care and Use of Laboratory Animals were followed (NIH Publication, No. 85-23, revised 1985).The study protocol (No: 010PA21016, dated 24/10/2016) was approved by the Research and Ethical Committee of the Faculty of Basic Medical Sciences, University of Calabar, Nigeria.
2.4. Determination of the estrous cycle
Estrous cycle of rats was determined between 7 a.m. and 9 a.m.each day using the vaginal smear method[15]. Vaginal lavage of rats carried out with 10 μL of normal saline (NaCl 0.9%) using a plastic pipette resulted in vaginal fluid, a drop of which was placed on a glass slide and examined under a light microscope unstained,without a cover slide and condenser lens and using 40 × objective lens. The proportion of characteristic cell types (leucocytes, cornified and epithelial cells) was used to determine the phases of estrous cycle and a different slide was used per animal[16]. After confirming regular 4-day cyclicity for sixteen days (4 cycles), the animals were selected for this study.
2.5. Animal treatment
The effects of the extract on estrous cycle of female rats were assessed following the earlier described protocol[17] but with slight modifications. Rats with regular 4-day cycle were unbiasedly shared into 4 groups of twelve rats per group. Each group was further subdivided into ‘a’ and ‘b’ (n=6). Group 1 (Control) received 2 mL of distilled water; groups 2, 3 and 4 had 250, 500 and 750 mg/kg body weight of the ethanol root bark extract of S. lehmbachii. All treatments were through a gavage starting from proestrous phase and lasted 28 days (7 estrous cycles). The length of estrous cycles and duration of each phase of the cycle were recorded as described previously[18]. Twelve hours after the last treatment, rats in all the ‘a’subgroups were weighed before being sacrificed under chloroform anaesthesia and blood was collected via cardiac puncture into plain bottles for generation of serum which was used for hormonal assay.The uterus and ovaries were dissected out, cleaned of blood in between filter papers, and weighed (absolute weight). For the rats in the ‘b’ subgroups, all administrations were withdrawn for two weeks and their estrous cycles were studied for another 28 days (postextract period), at the end of which, the sex hormones, weight of animals and that of uterus and ovary were reassessed. The aim of this later exercise was to evaluate the reversibility of observed changes in the first part of the study.
2.6. Relative organ weights
The relative weights of the harvested organs of each rat in 2.5 g above were calculated as earlier documented using the formular below[19,20]:
Relative organ weight (mg/100 g body weight) = Absolute organ weight (g) × 100 / Body weight of rat on sacrifice day (g)
2.7. Hormonal assay
Sera generated from the collected blood samples by allowing them stand for 3 h to ensure complete clotting, centrifuging the clotted samples at 3 000 rpm for 10 min and aspirating off the serum with sterile needles and syringes were used for hormonal analysis. The enzyme-linked immunoassay technique was used for the assay. The procedures in the hormone assay kits (Life Science Inc, USA) were followed according for LH and FSH, estradiol and progesterone[21].
2.8. Statistical analysis
Results were expressed as mean ± stand error (mean ± SE) of six observations. Means were analyzed using a one-way ANOVA followed by Student’s test to compare the difference between the control and treated values and P<0.05 was considered significant.
3. Results
Estrous cycle length was dose dependently prolonged in treated rats compared to control (Table 1). Treated rats changed estrous cycle length from 4 days (Control) to (4.31±0.35) days, (5.41±0.94)days and (6.63±1.01) days at doses of 250, 500 and 750 mg/kg respectively. The extract-induced elongation of estrous cycle was significant (P<0.05) only at high doses (500 mg/kg and 750 mg/kg).

Table 1Effect of ethanol root bark extract of S. lehmbachii on rat estrous cycle.
The duration of dietrous phase was prolonged while that of other phases (proestrous, estrous, metestrous) were shortened also dose dependently (Table 2). S. lehmbachii ethanol root bark extract increased the duration of diestrous from 25.00 (% no. of days) in control rats to 41.06 and 47.72 at doses of 500 and 750 mg/kg body weight respectively, showing significance (P<0.05) only at high doses.

Table 2Effect of ethanol root bark extract of S. lehmbachii on phases of rat estrous cycle (% number of days).
The relative weights of rats uteri and ovaries were significantly(P<0.05) different from control only in rats treated with 750 mg/kg body weight (Table 3).

Table 3Effect of ethanol root bark extract of S. lehmbachii on relative weights of rat uterus and ovary (mg/100g body weight).
Serum concentrations of FSH, LH, progesterone and estradiol were dose dependently reduced by the extract and at high doses(500 and 750mg/kg body weight), and the percentage reduction was significantly (P<0.05) different of the control value (Table 4).
Reassessment of each of the above parameters after two weeks of withdrawal of treatments gave values showed a reversal toward the control values (Tables 1, 3, 5).
4. Discussion
Estrous cycle in rats occurs rapidly between 4 to 5 days and the events within the cycle are regulated by gonadotropin releasing hormone from the hypothalamus, gonadotropins from the anterior pituitary gland and sex hormones from the gonads[22]. The cyclic vaginal change which characterizes estrous cycle is an index of good functioning of the neuroendocrine- reproductive system and ovarian activity, while a distortion of normal estrous cycle indicates disruption of ovarian progesterone and estrogen balance[1,2]. In our study, the length of the estrous cycle was dose dependently and significantly (P<0.05) increased in the extract-treated groups compared to control. The mean cycle lengths of 4.31 and 5.41 days from rats treated with 250 and 500 mg/kg body weight of the extract aggree with the results of other investigators who recorded a mean cycle length of 5.4, 4.5 and 4.4 days[23-25]. The cycle length of 6.63 days from rats treated with high dose (750 mg/kg) of the extract clearly show a prolonged cycle length and this value was higher but statistically insignificant (P>0.05) to that from 500 mg/kg-treated groups.
The observed increase in estrous cycle length in this study implies impaired fertility and could be ascribed to the prolonged diestrous and shortening of the other phases also noted in this study. Other workers had also recorded the increased estrous cycle length and prolonged die strus with shortening of the other estrous phases following the administration of other plant extracts and xenobiotics.This includes studies on Garcinia kola seeds[17], Trichosanthes cucumerina[26], Ixora coccinea[27], Mimosa pudica[28], artemether[29]and amodiaquine[30]. A perculiarity of the plants and drugs mentioned above as well as S. lehmbacii being investigated is antiinflammatory activity[31-35,11].
The prolongation of estrous cycle and diestrous phase observed in this study is an indication of impairment of ovulation, and also is regarded as an inflammatory process[36]. Some researchers had earlier used anti-inflammatory drugs to block ovulation[37]. Antiinflammatory drugs inhibit the COX enzymes thereby preventing prostaglandins synthesis. There are two important isozymes of COX enzyme (COX-1 and COX-2) and studies carried out on COX-2 deficient mice showed defective ovulation, implying that COX-2 is involved in ovulation[38]. Since the extract has anti-inflammatory action as earlier mentioned, it may have blocked ovulation in this study by inhibiting COX-2 enzyme thereby inhibiting prostaglandin synthesis.The hypothalamic-pituitary-gonadal axis as already mentioned plays an important role in reproduction. Gonadotrophins (LH,FSH) in females cause follicles to mature, develop into preovulatory follicles and secrete estrogen[7]. In this study, the levels of gonadotrophins were significantly reduced indicating distortion of pituitary-ovarian axis. This finding aggrees with the earlier report that the same extract disrupts the pituitary-testicular axis in male rats resulting in reduced levels of gonadotrophins[16]. FSH stimulates the growth and maturation of ovarian follicles by acting directly on the receptors located on the granulosa cells. The observed reduction in its levels may impair the process of folliculogenesis and delay maturation of the follicle. LH stimulates secretion of sex steroids from the gonads and its surge in females stimulates ovulation. Reduction of its serum level could disrupt ovulation either by decreasing the number of mature follicles or altering the pattern of estrous cycle[39]. Therefore, the observed reduction in the serum LH levels may be due to inhibitory effect of the extract on LH release which then disrupts ovulation process in treated rats. Also, there is a possibility of the extract causing dysregulation in the hormonal secretion via its effect on the anterior pituitary or hypothalamus. Our findings aggree with the results of other workers who also reported decreased release of LH and FSH in rats treated with various extracts[2,10]. The proestrus surge of LH which is responsible for ovulation requires high estrogen levels, and the subsequently formed corpus luteum secretes progesterone whichregulates menstruation and prepares the female for conception amongst other things[40]. Estrogen and progesterone have a feedback inhibitory effect on gonadotropin releasing hormone secretion in the hypothalamus, and it is this inhibition that prevents the mid cycle LH surge and ovulation. In this study, the serum levels of estrogen and progesterone were significantly reduced in the extract-treated rats. The reduction in estrogen levels may be due to inhibitory effect of the extract on gonadotropins as FSH stimulates maturation of the Graafian follicle while LH causes the follicles to synthesize testosterone which is then converted to estrogen by aromatase[41].It may also be attributed to a decreased aromatase activity or substrate supplementation during its synthesis as earlier reported[42].Estrogen level reduction may be a direct toxic effect on follicular cells as the extract was reported to cause such toxicity in the thecca cells of the rats testes[11]. Similar results were obtained following administration of Cnidoscolous aconitifolius leaf extract[43]. The decreased progesterone level is probally caused by poor synthesis because of deficient corpus luteum resulting from lack of ovulation,or a direct toxic effect on the corpus luteum. Administration of water based extract of combined Lepidagathis longifolia and Phyllagathus rotundifolia also reduced both progesterone and estrogen levels in rats[44]. The insignificant difference in the relative weights of uteri and ovaries in rats treated with low doses of the extract implies that the changes produced by the extract did not affect the organs’ relative weights. However, the significant reduction of the organs weight at high dose of the extract (750 mg/kg) may be caused by the observed reduction in estradiol levels since this hormone is directly responsible for the growth and development of reproductive organs[45].
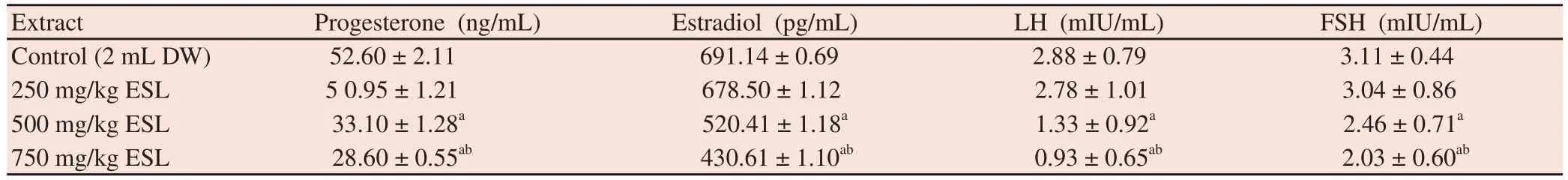
Table 4Effect of ethanol root bark extract of S. lehmbachii on serum levels of sex hormones in female rats.

Table 5Effect of ethanol root bark extract of S. lehmbachii on serum levels of sex hormones in female rats after two weeks of withdrawal of treatments.
Certain biocative compounds found in plant extracts can affect estrous cycle, conception and reproduction. Typical examples are alkaloids and flavonoids which have been shown to reduce plasma concentrations of LH, FSH, estradiol and progesterone[43-45].Therefore, the presence of these phytochemicals in the extract as previously reported[9] may account for the observed alterations in female reproductive hormones levels.
The toxicity of a medication may be reversible or irreversible depending on the type of interactions between the drug and the organism. When interaction brings about reversible toxic effects, the only determining factor is the toxicant’s concentration at the site of action whereas irreversible toxicity occurs when biomacromolecules bind covalently with the toxicant[46]. The reversible effect of the extract on estrous cycle observed in this study is an indication that the cell injury that led to the changes was not permanent but reversible. It is likely that the absorbed extracts were adequately cleared such that plasma level fell below the minimum effective concentration. Other researchers also reported reversed antifertility action from the seeds, roots and pulp extracts of Momordica charantia after withdrawal of therapy in rats[1,2].
From our findings, the root bark of S. lehmbachii prolongs estrous cycle length, increases diestrous duration and reduces sex hormones levels in female albino rats, and thus could impair fertility. We advice cautious usage of the plant on women of reproductive age especially those with reduced fertility.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
The authors are grateful to Mr. Marcus Inyang, Mr. Etim Ifang,Mrs. Mary Useh and Miss Emem Uko for their technical assistance.
 Asian Pacific Journal of Reproduction2018年6期
Asian Pacific Journal of Reproduction2018年6期
- Asian Pacific Journal of Reproduction的其它文章
- Comparison of Bishop score and cervical length measurement through transvaginal ultrasound as prediction against labor induction
- Effect of buffalo bull breeds on developmental competence and vitrification of invitro produced embryos
- Effect of paclitaxel and resveratrol on New Zealand rabbit semen
- Inhibitory effect of genistein on MMP-2 and MMP-9 expression through suppressing NF-κB activity in peritoneum of murine model of endometriosis
- Impact of replacing egg yolk with lecithin on quality of pre-freeze and post-thaw buffalo spermatozoa
- Role of follicle-stimulating hormone and estradiol benzoate in recovering spermatogenesis in tamoxifen-injured rats
