Impact of replacing egg yolk with lecithin on quality of pre-freeze and post-thaw buffalo spermatozoa
Asmaa A. Mostafa, Mohamed. S. El-Belely, Sayed. T. Ismail, Reda. I. El-Sheshtawy, Mohamed I. Shahba✉
1Abassia Frozen Semen Center, General Organization for Veterinary Services, Cairo, Egypt
2Department of Theriogenology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
3Animal Reproduction and Artificial Insemination Department, National Research Centre, Dokki, Giza, Egypt
Keywords:Buffalo Semen Preservation Soybean lecithin
ABSTRACT Objective: To estimate the result of egg yolk replacement with alternative cryopreservatives such as plant-derived lecithin from soybean on sperm quality parameters pre and post freezing in buffalo bulls. Methods: The control cryopreservation extender was tris-citric acid-fructose-egg yolk-glycerol (TCFYG) diluent. Semen samples were extended gradually 1:10 with TCFYG control extender and tris-citric acid-fructose-glycerol (TCFG) extender plus variable concentrations of soybean lecithin (0.5%, 1.0%, 1.5%, 2.0%, 2.5% and 3.0%) to ensure 60 million active spermatozoa/mL of the extended semen. The diluted semen samples were refrigerated slowly (roughly for 2 h) up to 5 ℃ and equilibrated for 2 h. Semen was filled into 0.25 mL polyvinyl French straws (IMV, France). After equilibration period, the straws were placed horizontally on a rack and frozen in a vapor 4 cm above liquid nitrogen for 10 min and were then dipped stored in liquid nitrogen at -196 ℃. Results: The respective overall percentages of forward motile spermatozoa, live spermatozoa, morphologically normal spermatozoa, acrosome integrity and hypo-osmotic swelling reactivity observed primarily in fresh semen, after equilibration (pre-freeze stage) and post freezing (post-thaw stage) in TCFYG (control) extended semen declined progressively and statically (P<0.01)during these periods of study. Pre-freezing stage: replacement of egg yolk into TCFG with soybean lecithin at concentrations of 1.0% and 1.5% significantly (P<0.01) ameliorated the maintenance of (motility, viability, acrosome and membrane integrity %), meanwhile it had significantly (P<0.01) reduced the abnormality % of spermatozoa to the lowest value compared to control TCFYG and to some other concentrations in use. Post-thaw stage: the replacement of egg yolk with 1.0% soybean lecithin (SL) showed significantly (P<0.01)higher percentage of sperm progressive motility compared to 1.5% SL and TCFYG control.These values were significantly (P<0.01) higher than 0.5%, 2.0%, 2.5% and 3.0% SL. The post thawing live sperm percentage mean values were significantly (P<0.01) higher in 1.0%SL and 1.5% SL compared to control. These values were significantly (P<0.01) higher than in 0.5%, 2.0%, 2.5% and 3.0% SL. The mean values of post-thaw morphological normal sperm percentage did not differ between 1.0% SL and control groups but significantly(P<0.01) higher than 0.5%, 1.5%, 2.0%, 2.5% and 3.0% SL. The respective percentage mean values of post-thaw sperm with head, mid-piece and tail abnormalities were significantly(P<0.01) lower in 1.0% SL than all other SL concentrations. Concerning the post-thaw percentages of acrosome and sperm membrane integrity, the respective mean values were significantly (P<0.01) higher in 1.0% SL and 1.5% SL as compared to control. Mean values of both parameters in the 0.5% SL were intermediate between 1.0% and 1.5% SL versus control groups. The previously mentioned mean values in acrosome/membrane integrity were significantly (P<0.01) higher than 2.0% SL, 2.5% SL and 3.0% SL. Conclusions:Lecithin-based diluent can be a potent proper alternative extender for preservation of spermatozoa during pre- and post-freezing process. SL 1.5% extenders have supplied an optimal environment and condition for ameliorating the quality of pre-freezing and postthaw buffalo spermatozoa by means of improved motility, viability, functional acrosome,sperm membrane integrity and morphologically normal spermatozoa.
1. Introduction
Artificial insemination is one of the assisted fertility tools which is used to improve the genetic potential of livestock breeds and to exploit the spermatozoa from superior ones. Semen from farm animals used for these purposes could be preserved either for shortterm at 4 ℃ in the liquid form or for long-term in cryopreserved state with liquid nitrogen[1]. However, declines of about 50% in spermatozoal motility, livability and sperm membrane status are major problems occurring during the freezing process, caused by severe deterioration in sperm membrane during the freezing process.Recent studies have been performed to constitute semen diluents for protecting bull spermatozoa in post-freezing and post-thawing process[2-7].
Commonly, buffalo semen is preserved in milk[8-11], tris-egg yolk[12-14] and egg yolk-citrate[3,15] diluents. These diluents contain additives of animal source (egg yolk and/or milk) which may pose an extreme hazard of microbial contaminants[16,17]. This sanitary risk may reduce fertility of frozen semen directly through producing hazardous metabolites and toxins which deteriorates the semen characteristics, or indirectly through local infection leading to abortion[6,18]. Also, egg yolk in semen diluent can lower the activity and motility of ram spermatozoa[19]. Furthermore, high density lipoproteins in egg yolk lowers the quality of semen by causing cholesterol efflux out of the spermatozoal membrane, which leads to change in flexibility and increases the liability to cold shock[20].The main functional portion of egg yolk is low density lipoproteins fraction like lecithin, which protects the membrane integrity all through preservation[21,22]. A high quality alternative instead of the ingredients of animal origin in extenders for freezing of semen is soybean lecithin (SL), a phospholipid that is the principal component of soybean[23]. SL may decrease the hygienic risks and improve freezability and fertilizing capacity of bovine spermatozoa[6,24].
Therefore, the current investigation was designed to estimate the result of egg yolk replacement with alternative cryopreservatives such as plant-derived lecithin from soybean on sperm quality parameters pre and post freezing in buffalo bulls.
2. Materials and methods
2.1. Semen collection and initial evaluation
Four buffalo bulls (aged 3.5-5 years) kept at the Abassia Buffalo Semen Freezing Center, Central Organization for Veterinary Services, Ministry of Agriculture, Egypt, were chosen to be the source of semen. The buffalo bulls were kept under uniform standard nutrition and managerial practices. They were in healthy conditions(600-800 kg body weight), free from general and genital diseases.The bulls were under constant weekly intervals semen collection program with an artificial vagina. Semen collections were carried out early in the morning. Two successive ejaculated semen samples were collected using an artificial vagina at each collection process with 10-15 min interval. The ejaculates were pooled to enlarge the semen volume for different aliquotes and to avoid the evaluated samples variability. The semen ejaculates (10 ejaculates per bull, total 40 ejaculates in each experiment) after collection were immediately transferred into a water bath at 35 ℃ for 10 min and evaluated for visual motility using a high power ordinary microscope (at 400 ×)with closed circuit television, sperm concentration using Neubauer haemocytometer and abnormality % using eosin-nigrosin stain.The semen samples with 70% motility, total sperm defects lower than 20% and with concentration of 600 ×106spermatozoa/mL of the ejaculates were selected for processing. Additionally, the hypoosmotic swelling test was performed to assess the sperm membrane functional integrity of % as recorded by Jeyendran et al[25]. The sperm with swollen twisting tail was considered intact. Sperm acrosomal integrity % was undertaken as mentioned by Watson[26].Normal acrosome was identified by normal apical ridge.
2.2. Experimental design
This experiment was designed to investigate the effect of variable levels of SL, as an alternative for egg yolk in buffalo semen diluent on post-cooling and post-thawing functional sperm quality. The control cryopreservation extender was tris-citric acid-fructose-egg yolk-glycerol (TCFYG) diluent prepared by dissolving 3.028 g tris, 1.678 g citric acid and 2.000 g fructose in 100 mL bi-distilled water, then by adding 20% egg yolk and 7% glycerol combined with antibiotics according to Ijaz et al[27]. Semen samples were extended gradually 1:10 with TCFYG control extender and tris-citric acidfructose-glycerol (TCFG) extender plus variable concentrations of SL (0.5%, 1.0%, 1.5%, 2.0%, 2.5% and 3.0%) to ensure 60 million active spermatozoa/mL of the extended semen. The diluted semen samples were refrigerated slowly (roughly for 2 h) up to 5 ℃ and equilibrated for 2 h. Semen was filled into 0.25 mL polyvinyl French straws (IMV, France). After equilibration period, the straws were placed horizontally on a rack and frozen in a vapor 4 cm above liquid nitrogen for 10 min and were then dipped in liquid nitrogen at -196 ℃.
2.3. Statistical analysis
The data are tabulated as mean±standard deviation (mean±SD).All data were submitted to one way analysis of variance by using computerized statistical analysis.
The analytical design was factorial design (general linear model procedure) to clarify the result of cryoprotectant (type and concentration) in extenders including egg yolk (control) and SL on sperm motility, viability, abnormalities, sperm membrane and acrosome integrities pre and post freezing. Treated means were compared by the least significant difference test at 5% and 1% levels of probability. Each treatment was repeated five times, for each replicate; four straws were thawed and pooled for assessing of sperm characteristics. All statistical methods were performed as recorded by Snedecor and Cochran[28].
3. Results
3.1. Effects of freezing stage on semen quality traits
As shown in Table 1, the respective overall percentages of forward motile spermatozoa, live spermatozoa, morphologically normal spermatozoa, acrosome integrity and hypo-osmotic swelling reactivity observed primarily in fresh semen (initial stage), after equilibration (pre-freeze stage) and post freezing (post-thaw stage)in TCFYG (control) extended semen declined progressively and statically (P<0.01) during these periods of study.
3.2. Effect of egg yolk- and lecithin-based extender on semen quality traits
3.2.1. Pre-freezing stage
Concerning the results in Table 1, replacement of egg yolk into TCFG with SL at concentrations of 1.0% and 1.5% significantly (P<0.01)ameliorated the maintenance of motility%, viability %, acrosome %and membrane integrity %, meanwhile it had significantly (P<0.01)reduced the abnormality % of spermatozoa to the lowest value compared to control TCFYG and to some other concentrations in use.The highest mean values of motility percentage were observed in 1.0% SL and 1.5% SL as compared to control. These values were significantly (P<0.01) higher than 0.5%, 2.0%, 2.5% and 3.0% SL pre-freezing.
Similarly, the highest mean values of live sperm percentage were detected in 1.0% and 1.5% SL as compared to control. These values were significantly (P<0.01) higher than 0.5%, 2.0%, 2.5% and 3.0%SL pre-freezing.
The values of morphologically normal spermatozoa percentage were apparently higher (P<0.05) in 0.5%, 1.0% and 1.5% SL as compared to control. These values were significantly (P<0.01)higher than 2.0%, 2.5%, 3.0 % SL pre-freezing.
Table 2 revealed a reverse trend in morphologically abnormal sperm percentage values i.e. higher total sperm abnormality percentages in control extender as compared to 0.5%, 1.0% and 1.5% SL extenders. Pre-freezing sperm head, mid-piece and tail abnormalities were significantly lower in 0.5%, 1.0% and 1.5% SL, respectively,compared to control.
The respective mean percentages of spermatozoa with intact acrosome and membrane integrity were significantly (P<0.01)higher in 1.0% SL and 1.5% SL as compared to the control. These respective values were significantly (P<0.01 and P<0.05 for acrosome/membrane integrity, respectively) higher than in 0.5% SL,2.0% SL, 2.5% SL and 3.0% SL pre-freezing.

Table 1Semen characteristics in buffalo bulls at different stages of cryopreservation in TCFYG extender and egg yolk free extender (TCFG) containing different concentration of SL (n=4) (Mean±SD).
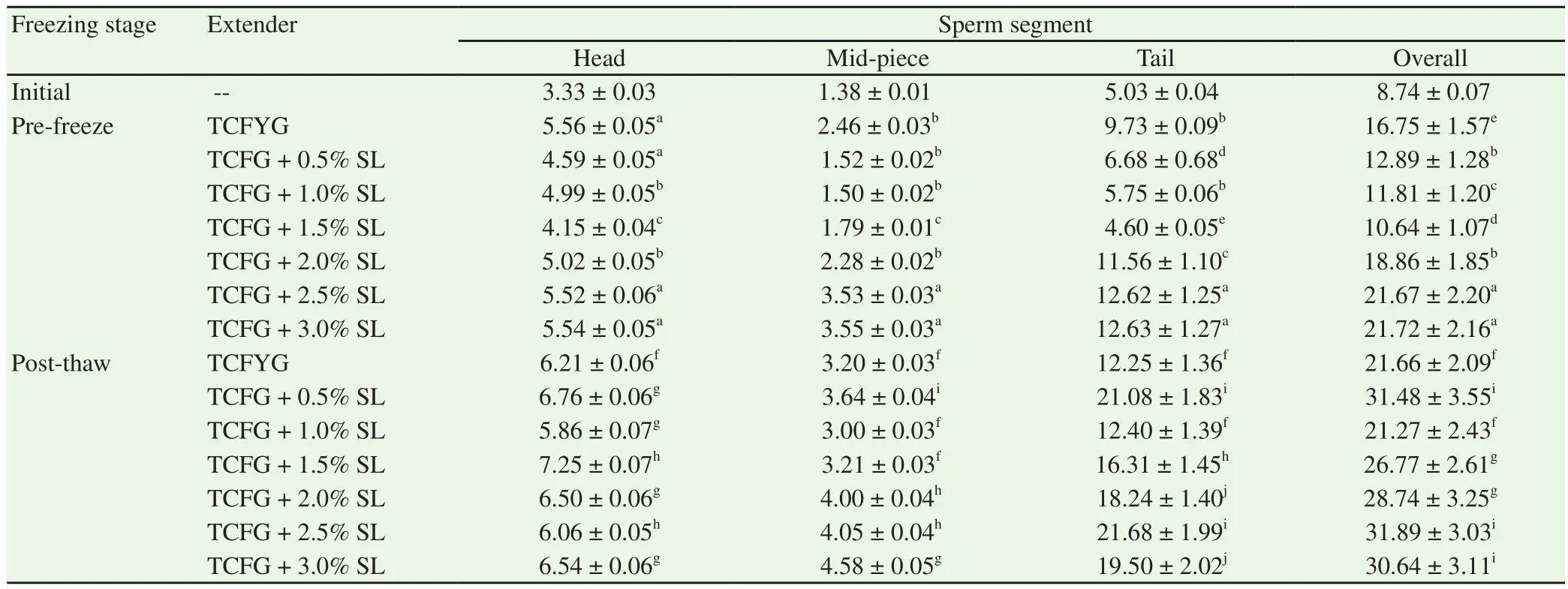
Table 2Segment wise sperm abnormalities in buffalo bull semen at different stages of cryopreservation in TCFYG extender and egg yolk free extender (TCFG)containing different concentration of SL (Mean±SD).
3.2.2. Post-thaw stage
Table 1 revealed that the replacement of egg yolk with 1.0%SL showed significantly (P<0.01) higher percentage of sperm progressive motility compared to 1.5% SL and TCFYG control.These values were significantly (P<0.01) higher than 0.5%, 2.0%,2.5% and 3.0% SL.
The post thawing live sperm percentage mean values were significantly (P<0.01) higher in 1.0% SL and 1.5% SL compared to control. These values were significantly (P<0.01) higher than in 0.5%, 2.0%, 2.5% and 3.0% SL.
The mean values of post-thaw morphological normal sperm percentage did not differ between 1.0% SL and control groups but significantly (P<0.01) higher than 0.5%, 1.5%, 2.0%, 2.5% and 3.0%SL. The respective percentage mean values of post-thaw sperm with head, mid-piece and tail abnormalities (Table 2) were significantly(P<0.01) lower in 1.0% SL than all other SL concentrations.
Concerning the post-thaw percentages of acrosome and sperm membrane integrity, the respective mean values were significantly(P<0.01) higher in 1.0% SL and 1.5% SL as compared to control.Mean values of both parameters in the 0.5% SL were intermediate between 1.0% and 1.5% SL versus control groups. The previously mentioned mean values in acrosome/membrane integrity were significantly (P<0.01) higher than 2.0% SL, 2.5% SL and 3.0% SL.
4. Discussion
The present overall mean percentages of semen quality characteristics recorded primarily in fresh buffalo semen had declined progressively and statistical significantly (P<0.01) after extension with control TCFYG extender during pre-freezing and post-thaw stages. This finding is in accordance with Fukui et al[29], Khalifa and Abdel-Hafez[30,31], Chaudhari et al[7] and El-Sisy et al[32], who reported deterioration in sperm characteristics during refrigeration and freezing. This marked reduction was related to changes in the pH of extension and osmolarity in addition to bacterial and fungal contamination present in egg yolk-based extender[33]. The microbial contamination results in endotoxins that decrease the liveability of sperm[34].
The replacement of egg yolk into TCFG with SL ameliorated to a great extent the maintenance of semen characteristics (motility,livability, acrosome and membrane integrity %), it had significantly(P<0.01) reduced the spermatozoa with total, head, mid-piece and tail abnormalities during pre-freezing and post-thaw stages of cryopreservation Increasing vitality of spermatozoa obtained with SL-based extender is caused by phosphotidyl choline from SL that restores the membrane phospholipids to preserve the membrane integrity and maintains sperm motility at low temperature.Furthermore, SL plays a vital physiological function in reducing the cooling point and lowering the replacement of plasmalogens to reduce the possible mechanical injuries of the sperm membrane[4].
Results of the present investigation showed that among the wide range (0.5% to 3.0% SL) tested, concentrations of SL for cooling and freezing of buffalo semen were 1.0% and 1.5%. In particular 1.0% SL, these concentrations showed best semen parameters after both stages of cryopreservation. The reported optimal concentrations of SL in extender used for semen freezing in the literatures were ranged from 0.8% in dogs[35], 1.0% in rams[31,36-38], human[39] and cat[40] and 1.5% in bovine bulls[41-46] and goats[47-52].
The current results regarding greater effectiveness of 1.0% and 1.5% lecithin-based extender than egg yolk-based extender in preserving forward motility, live, morphologically normal and acrosomal status and membrane integrity of buffalo bull spermatozoa are in accordance with those reported by Amirate et al[20], Bard[53],El-Sherbieny[6] and El-Sisy et al[32] in buffaloes but are in confliction with those recorded in buffalo bulls[3,7,54,55] and bovine bulls[56,57].These authors observed no differences in the effect of SL extender and egg yolk-based extender concerning percentage values of the aforementioned semen traits of buffalo. Apparent differences between these findings are attributed to the variations in cooling and freezing rates and type of commercially available SL-based extenders, but is also likely attributed to breed and species variations with subsequent changes in sperm membrane and seminal plasma composition[58,59].
According to our findings, optimal SL concentration (1.0%and 1.5% SL) is the best for protection of spermatozoa during temperature variation. Concentration of SL below or above the optimal may have deleterious effect and this may be the effect with 0.5%, 2.5% and 3.0% SL extenders. The reduction in most of semen characteristics in extenders containing 0.5% SL may be related to insufficient support to offer great cryoprotection of sperm membrane integrity[6]. SL at concentrations higher than 1.5%were toxic for sperm motility and viability and this was likely that higher concentration of SL amplified thickness of extenders with much debris observed in the extender with 2% and more lecithin.Also, spermatozoa are able to move more easily in semen diluents containing optimum levels of SL than in other extenders which would lead to better sperm motility.
The sperm membrane/acrosome integrity of spermatozoa is important to keep sperm functionality during storage in the female genital tract[60]. The deterioration of sperm membrane fluidity due to disarrangement of lipids within the membrane during cooling and freezing may induce further cellular and subsequent sperm damage[61]. In the current study, 1.0% and 1.5% SL resulted in higher membrane and acrosome integrity compared to other SL-based and control egg yolk-based extenders. Amirate et al[20]reported higher sperm percentage with normal acrosome frozen in SL-based diluent as compared to an egg yolk-based diluent and suggested that presence of higher calcium ions of egg yolk might be concerned with the acrosomal damage. It was declared that the acrosome status and normal membrane of spermatozoa has direct correlation with sperm motility[62,63]. Then it seems that improving impact of 1.0% and 1.5% SL on progressive motility of sperm may be partly related to plasma membrane/acrosome integrities. Although the perfect mechanism by which lecithin induces its effect on plasma membrane of spermatozoa during pre- and post-freezing process is not clear, it has been explained that lecithin in soybean protects sperm membrane phospholipids by occupying sites on the sperm membrane and ameliorates its tolerance to the freezing process[21,60].It has been identified that sperm motility is done via propelling forces of its tail in conjugation with lateral displacement of its head based on the energy supplied by mitochondria in the mid-piece[64,65].The findings of this study show a considerable correlation between the morphological structures of the three segments of live spermatozoa with motility percentage. The present findings documented a strong positive correlation between morphologically normal spermatozoa with its three segments and progressive motility in 1.0% SL-based extender pre and post freezing. Evaluation of frozen-thawed spermatozoa with Rhodamine 123 fluorescent dye showed that the percentage of active mitochondria was superior in 1.0% SL with respect to 2.0% SL diluent[66].
Our findings postulated that the percentages of live spermatozoa pre and post freezing were considerably higher in 1.0% and 1.5% SL extenders compared to control egg yolk-based extender. Emamverdi et al[60] confirmed that 1.0% and 1.5% SL extenders contain low percentages of early apoptotic spermatozoa compared to egg yolkbased extender. Del Valle et al[67] reported that lecithin is able to perfectly preserve sperm against cooling and cryoinjury, as its addition resulted in increased percentage of viable and non-apoptotic spermatozoa. In the existing study, we have not evaluated the lipid peroxidation in spermatozoa, but it seems that SL-based extenders can decrease damage in spermatozoa via decreasing fatty acid peroxidation in plasma membrane[68,69] which may cause apoptosis in these cells.
In conclusion, our findings documented that lecithin-based diluent can be a potent proper alternative extender for preservation of spermatozoa during pre- and post-freezing process. Between lecithin-based extenders tested in this study and, to a lesser extent,1.5% SL extenders have supplied an optimal environment and condition for ameliorating the quality of pre-freezing and post-thaw buffalo spermatozoa by improving motility, viability, functional acrosome, sperm membrane integrity and morphologically normal spermatozoa.
Conflict of interest statement
All authors declare that there is no conflict of interest.
 Asian Pacific Journal of Reproduction2018年6期
Asian Pacific Journal of Reproduction2018年6期
- Asian Pacific Journal of Reproduction的其它文章
- Comparison of Bishop score and cervical length measurement through transvaginal ultrasound as prediction against labor induction
- Effects of Salacia lehmbachii ethanol root bark extract on estrous cycle and sex hormones of female albino rats
- Effect of buffalo bull breeds on developmental competence and vitrification of invitro produced embryos
- Effect of paclitaxel and resveratrol on New Zealand rabbit semen
- Inhibitory effect of genistein on MMP-2 and MMP-9 expression through suppressing NF-κB activity in peritoneum of murine model of endometriosis
- Role of follicle-stimulating hormone and estradiol benzoate in recovering spermatogenesis in tamoxifen-injured rats
