Phenolics, fatty acids composition and biological activities of various extracts and fractions of Malaysian Aaptos aaptos
Zalilawati Mat Rashid, Abdul M. Ali, Philippe Douzenel, Nathalie Bourgougnon, Khozirah Shaari, Yosie Andriani, Tengku Sifzizul Tengku Muhammad, Habsah Mohamad✉
1Faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin, Besut Campus, 22200 Besut, Terengganu, Malaysia
2School of Agriculture Science & Biotechnology, Universiti Sultan Zainal Abidin, Besut Campus, 22200 Besut, Terengganu, Malaysia
3Faculté des Sciences et Sciences de l'Ingénieur (UFR SSI), Centre Yves Coppens, Université Européenne de Bretagne, Campus de Tohannic - BP 573,56 017 Vannes Cedex, France
4LBCM, Université Européenne de Bretagne, Campus de Tohannic, BP573, 56017 Vannes Cedex, France
5Laboratory of Natural Products, Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM, Serdang, Selangor, Malaysia
6Institute of Marine Biotechnology, University Malaysia Terengganu, Mengabang Telipot, 21030 Kuala Terengganu, Terengganu, Malaysia
Keywords:Aaptos aaptos Fatty acid p-Hydroxybenzamide Antioxidant Cytotoxicity Antivirus
ABSTRACT Objective: To investigate phenolics, fatty acids composition and biological activities of various extracts and fractions of Malaysian Aaptos aaptos. Methods: Fatty acid methyl ester was analyzed by gas chromatography-flame ionization detector. Antioxidant activity was determined using 2,2-diphenyl-picrylhydrazyl radical scavenging assay and total phenolics content by Folin-Ciocalteu procedure. Vero cells viability was evaluated using methyl thiazole tetrazolium and the inactivation of herpes simplex virus type 1 by neutral red uptake assay.p-Hydroxybenzamide isolated by column chromatography was characterized by utilizing nuclear magnetic resonance spectroscopy and electron impact mass spectrometry. Results:The chloroform, ethyl acetate and methanol extracts of Aaptos aaptos produced higher portions of straight-chain saturated fatty acid, while hexane extract mainly consisted of unsaturated fatty acid. The five majors of fatty acid methyl ester were identified as behenic acid, cis-10-heptadecenoic acid and cis-10-pentadecenoic acids, palmitic acid and tricosanoic acid. In addition, among all organic extracts, chloroform extract inactivated herpes simplex virus type 1 while exhibited weak cytotoxic activity against normal Vero cells and also exhibited strong cytotoxic activity on HL-60, MCF-7, K562, CEM-SS and WEHI-3B cells. A phenolic compound, p-hydroxybenzamide was also isolated from the sponge. Conclusions: Aaptos aaptos could be a source to derive the potential antiviral and anticancer agents. However,further studies are needed to determine the mechanism involved in the process.
1. Introduction
Sponges are parts of the phylum Poriferae with approximately 15 000 living species available worldwide. Marine sponges have been found to contain the highest number of completely new molecules which are biologically active against human pathogens and other ailments. For examples, these include bioactive marine alkaloids, purines,pyrimidines and their nucleosides, amino acids, peptides,guanidine, nitrogenous marine toxins etc[1]. In addition, these species are rich of lipid-containing metabolites such as unusual fatty acids (FA) with high percentage of long chain FA (C24-C30),sterols and phospholipid[2-4]. Some novel marine FA has displayed antimycobacterial, antimalarial and antifungal properties[5].Yakushinamides A and B, prolyl amides of polyoxygenated fatty acids that are isolated from the marine sponge Theonella swinhoei are reported as inhibitors of histone deacetylases and sirtuins[6].Pyrrole alkaloids that are conjugated with various FA obtained from marine sponges from the genera of Mycale are reported to have antileshmanial activity against Leishmania mexicana promastigotes.The cytotoxicity exhibited by these compounds was affected by the length, number and position of the unsaturations of the fatty acid chains[7]. Besides, hexadecanoic, pentadecanoic, docosanoic,tetracosanoic, octadecanoic, eicosanoic, tetradecanoic and 2-hydroxyhexadecanoic acids isolated are from seaweed Sargassum granuliferum and Dictyota dichotoma which are shown to have a promising antifouling property[8].
In Malaysia, the studies on marine sponges only have been initiated in the late 1980s, with most of the studies focusing on the bioactivies screening, instead of taxonomic studies[9-11]. Only a few studies emphasized on the isolation of chemical constituents of Malaysian marine sponges which involved Leucoploeus fenestrata[12]and Pseudaxinyssa sp.[13], Aaptos sp.[14-18] and Xestospongia[19].In addition, other previous researchers also have explored the characterization of chemical compounds from marine-sponge derived fungi [20,21], the isolation of marine bacterium associated with Theonela sp.[22,23] and Haliclona amboinensis[24]. Other than that, studies on the cultivation of sponge Aaptos sp. and Theonella sp. in open-sea system also has been successfully carried out[25].Recently, identification of three poly-hydroxyalkanoatesynthase genes (phaC) isolated from the marine bacteria metagenome of Aaptos aaptos (A. aaptos), a marine sponge in the waters of Bidong Island, Terengganu, Malaysia has been reported[26]. In addition to the list, three methanol extracts of Malaysian marine sponges species,namely Aaptos sp., Stryphuous ponderosus and Theonella sp. were reported to exert cytotoxic effects against human breast cancer cell line, MCF-7. Methanol extract of Stryphuous ponderosus has revealed apoptotic-induced cytototoxicity against MCF-7 cell line[27].
Previous researches on genus Aaptos have gained great attention worldwide due to the interesting biological activities of its aaptaminoids compounds. These biological activities include the prevention of neoplasm that acts as an α-adrenoceptor blocker[28],antiamoebic activity[29], the prevention of herpes simplex virus replication[30], an antioxidant activity[31], an activator of p21 promoter stably transfected in MG63 cells[32] and antidepressant-like activity[33], as well as exhibited promising activity against cancer cell lines including A549 (human lung adenocarcinoma), KB16(human mouth epidermoid carcinoma), P-338 (murine lymphocytic leukemia) and HT-29 (human colon adenocarcinoma)[34]. Recently,a study on Aaptos from Pramuka Island, Jakarta has revealed the identification of sponge-associated bacteria based on 16S-rRNA,that was proven to have ability to inhibit Vibrio sp. in vitro and in vivo. Other than that, the study also focused on encoding the genes’bioactive compounds (NRPS and PKS genes) on Aaptos sp. and Hyrtios[35].
Aaptos sp. is one of the abundance marine sponges found in the east coast of Peninsular Malaysia, particularly along the Terengganu coast. Previously, we have reported the isolation of cholestanyl myristate, 5α-cholestan-3β-ol, aaptamine, two new derivatives of the aaptamine which are 3-(isopentylamino)demethyl(oxy)aaptamine and 3-(phenethylamino)-demethyl(oxy)aaptamine[14,15].Aaptaminoids have seemed to be important metabolites for the genera of Aaptos since several derivatives which included aaptamine, demethylaaptamine, isoaaptamine, aaptosamine,aaptosine, demethyloxy-aaptamine[28], aaptosine[36], aaptosamine[37],4-methylaaptamine[30], bisdemethylaaptamine and bisdemethylaaptamine-9-O-sulfate[38] were isolated from Aaptos sp. collected in Indonesia, Philippine and Okinawa. According to summarization of studies from all parts of the world by Larghi et al[39], the derivatives that are collected from Indonesia, Philippine and Okinawa, are isolated from Aaptos sp. and have gone through the process of derivatization of aaptaminoids. Later, an aromatic alkaloid,N-demethylaaptanone has been isolated from Vietnamese marine sponge A. aaptos[40]. However, to the best of our knowledge, there were no studies done on the FC constituent and phenolics content of Aaptos sp. Thus, this study is conducted to determine the FC constituent, phenolics contents and biological activities of hexane,chloroform, ethyl acetate and methanol extract of A. aaptos.
2. Materials and methods
2.1. Extraction
The A. aaptos were collected from the coastal waters of Terengganu,on the eastern part of Peninsular Malaysia (Kapas, Perhentian, and Bidong Islands) via scuba diving at a depth of 8 to 15 m. Some of the collected specimens were deposited at the Biodiversity Museum,Institute of Oceanography, Universiti Malaysia Terengganu. Five samples from different locations were frozen immediately after the collection. Next, the samples were cleaned and cut into small cubes (1 cm × 1 cm) and they were dried in air-crafted oven at 40 ℃After the dried samples were extracted with methanol, the extracts were filtered and dried under reduced pressure yielding sample of methanolic extracts (D, G, H, J, K). Furthermore, due to the abundant supply of extracts, sample from Bidong Island (G) was selected for successive extraction with hexane, chloroform, ethyl acetate and methanol for three times, with each yielding different polarity of extracts. Each extract underwent the process of evaporation under reduced pressure to consequently acquire the hexane fraction extract(ABHE), chloroform fraction extract (ABCE), ethyl acetate fraction extract (ABEE) and methanol fraction extract (ABME).
2.2. Lipid extraction and fatty acid methyl ester (FAME)content analysis
ABHE, ABCE, ABEE and ABME samples were used in the preparation of FAME content analysis. The derivatization of these fatty acids and its FAME analysis were done precisely as the method described by Bazes et al[41].
2.3. Total phenolics content
The amount of total phenolics in the extracts was determined according to the Folin-Ciocalteu procedures[42], with some modifications where the concentration of the test samples (ABHE,ABCE, ABEE and ABME) were changed to 1 mg/mL and the absorption at 765 nm was recorded (Bio-rad spectrophotometer).The total phenolic content in methanolic crude extracts in GAE was determined by calculating it using the following formula:

Where
C=total phenolic content of methanolic crude extracts (mg/g)
c=concentration of gallic acid established from the calibration curve(mg/mL)
V=volume of extract (mL) and m is the weight of pure plant methanol extract (g).
2.4. Anti-oxidant activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical method was adopted to determine the DPPH free-radical scavenging activity in order to assess the antioxidant activity. The method used was based on Habsah et al[15] and Von Gadov et al[43], with a modification where the absorbance (Bio-rad spectrophotometer) was read at 517 nm against a blank. Buthylated hydroxyanisole (BHA) and quercetin were used as positive control.
2.5. Culture of cells and cytotoxic activity against cancer cell lines
The samples of the six cell lines used were human acute promyelocytic leukemia (HL-60), humanbreast adenocarcinoma(MCF-7), human chronic myelogenous leukemia (K-562), human cervix adenocarcinoma (HeLa), acute lymphoblastic leukemia(CEM/C2) and murine myelomonocytic leukemia (WEHI-3B).These samples have been supplied by American Type Culture Collection (ATCC). The cell lines were cultured and maintained as described by Ali et al[44]. For cytotoxic assay, the microculture cytotoxicity was screened using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, a procerdure adopted from Shaari et al[14] and Mosmann[45] with some modification where the concentrations of where final concentration of sample ranging from 30 g/mL to 0.46 g/mL. The determination of 50% cytotoxic concentration (CD50) was done in three replicates.
2.6. Antiviral activity
2.6.1. Cell and virus culture
Vero (ATCC®CCL-81TM) originated from Cercophithecus aethiops African green monkey kidney and herpes simplex type 1 virus (HSV-1) stock was maintained and cultured according to Rashid et al[16] and Muench[46].
2.6.2. Neutral red uptake assay
Neutral red uptake assay was done following a procedure as described by Rashid et al[16] and McLaren et al[47]. Acyclovir was used as positive control.
2.6.3. Cytotoxicity assay by cell viability
Method of Rashid et al[16] was adopted with a modification by changing the concentration of samples ranging from 0.5 to 100 μg/mL.
2.6.4. Antiviral assay by cell viability
The same experiment protocol as the cytotoxicity test described by Rashid et al[16] and Langlois et al[48] was used except that the MEM was replaced by 50 μL HSV-1 virus-infected cell suspensions at multiplicity of infection (MOI) of 0.001 ID50/cells (2 × 108.5ID50/mL)HSV-1.
2.7. Statistical analysis
All of the data extracted were determined statistically by using analysis of variance (ANOVA) in SPSS version 11.5 for Windows at 95% confident interval (CI) to compare the significant different between doses of sample’s treatment, while the independent sample Student t-test was used (at 95% CI) to compare the significant different between control (untreated) and doses of sample’s treatment.
2.8. Purification and characterization of p-hydroxybenzamide
Methanol extract of A. aaptos underwent solvent partitioning to give diethyl ether, butanol and aqueous extract. Approximately 15.2 g of this butanol extract was fractionated using dry vacuum column chromatography on silica gel and gradually eluted with n-hexane,n-hexane/DCM, DCM, DCM/MeOH, and MeOH. A total of 17 fractions including fractions IK and IL were collected. The combined fraction IK and fraction IL (11.0 g) were chromatographed on silica gel by eluting them with CHCl3and MeOH with the ratio of 9.5:0.5; 9:1 and 8:2 to give 21 fractions. After that, fractions 8-12 (3.0 g) were further separated on silica gel column eluted with CHCl3/MeOH, 8:2 to yield 12 subfractions including fraction CAMI8-12C. Fraction CAMI8-12C was rechromatographed over LH-20 column chromatography eluted with CHCl3/MeOH, 1:1 to give 5 fractions including fraction CAMI8-12CV.Next, fraction CAMI8-12CV was further purified by washing with MeOH. Separation of soluble and unsoluble compounds in methanol by filtering yielded two compounds. p-Hydroxybenzamide which was soluble in MeOH was isolated as white powdered compound.Infrared (IR) spectrum was recorded with Perkin Elmer FTIR (model 1725X) spectrophotometer using KBr discs. Proton Nuclear Magnetic Resonance (1H-NMR) spectra were recorded on Bruker ARX 400 NMR spectrometer with tetramethylsilane as internal standard. Mass spectra were recorded by Direct Induction Probe using a Shimadzu GCMS-QP5050 spectrometer with ionization induced by electron impact at 70 eV.
3. Results
3.1. FAME content analysis
FAME profiles of ABHE, ABCE, ABEE and ABME (Table 1)showed notable differences with predominance of behenic acid(C22:0), cis-10-heptadecenoic acid (C17:1), palmitic acid (C16:0),and cis-10-pentadecenoic acid (C15:1). Generally, the FAME pro files of all samples were quite similar. Hexane extract was rich in cis-10-heptadecenoic acid (C17:1, 19.5%), followed by cis-10-pentadecenoic acid (C15:1, 16.4%), palmitic acid (C16:0, 9.2%),palmitoleic acid (C16:1, 7.6%), pentadecanoic acid (C15:0, 7.6%)and behenic acid (C22:0, 6.7%). Meanwhile, the concentration of palmitic acid and behenic acid were much higher in the chloroform extract, which were 18.3% and 10.2% respectively. However,the highest content of behenic acid was detected in ethyl acetate(35.7%) while other FAMEs contained much lower values with palmitic acid, 5.7% and nervonic acid (C24:1), 4.2%. On the other hand, the different major FAMEs were found in methanol extract which included tricosanoic acid (C23:0, 20.0%), heneicosanoic acid (C21:0, 11.1%), behenic acid (19.8%) while higher content of nervonic acid (8.6%) were also detected.
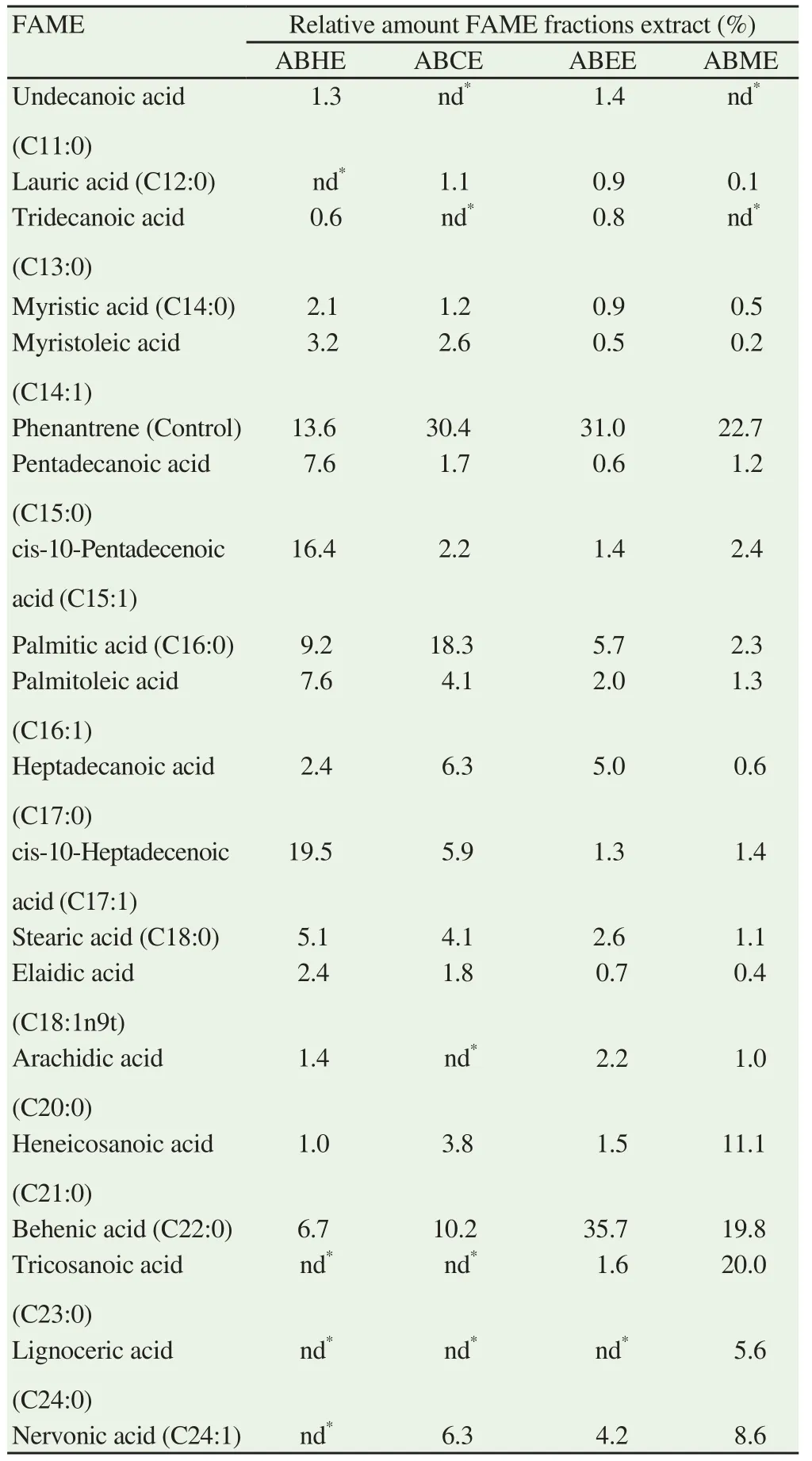
Table 1 Fatty acid compositions of hexane, chloroform, ethyl acetate and methanol extracts of A. aaptos quantitatively analysed by GC-FID.
3.2. Total phenolics content and anti-oxidant activity
The content of phenolic compounds in the samples were determined from regression equation of calibration curve (y=0.007 4x-0.153 7,R2=0.844 8) and expressed in gallic acid equivalent (GAE; mg/g).The total phenolic contents of methanol crude extracts from five different locations and four solvent fractions varied widely with values ranging from 21.8 to 68.5 mg/g GAE and 6.5 to 14.3 mg/g GAE, respectively (Table 2). In addition, the chloroform (ABCE)and methanol (ABME) fraction extracts showed potential DPPH free radical scavenging activity, with the respective percentage of inhibition being 67.6% and 78.2% respectively. Their IC50values were 1.71 and 4.62 mg/mL respectively (Table 2).

Table 2 DPPH free-radical scavenging activity and total phenolic content of methanolic extracts of Aaptos sp. from different locations.
3.3. Cytotoxic activity against cancer cell lines
The preliminary cytotoxic screening of methanolic crude extracts(CE) of A. aaptos that were collected from various locations of Terengganu islands against HL-60 (human leukemia cell line) and MCF-7 (breast cancer cell line) has revealed that CE from Perhentian Island coast, B and D exhibited the strongest activity against HL-60 cell line (CD50; 7.9 and 10.4 g/mL respectively), moderate activities by J and L (CD50; 12.0 and 13.2 g/mL respectively) from Kapas Island coast, while weak activity was shown by sample A (CD50;27.6 g/mL) from Perhentian Island coast (Table 3).
On the other hand, the cytotoxicity against MCF-7 resulted in moderate activity shown by extract J (CD50; 12.7 g/mL) and followed by weak activity of sample D (CD50; 25.5 g/mL). According to the result of the experiment other extracts were not active against HL-60 and MCF-7 cell lines with CD50values of more than 30 μg/mL.Thus, the cytotoxic activity of the extracts became dependent on the locality of the sample. Interestingly, the evaluation of cytotoxic activity of ABCE against panels of cancer cell lines has resulted in positive outcomes (Table 4). ABCE showed strong activity against both HL-60 and MCF-7 cells with CD50; (5.6±0.1) g/mL and (3.3±2.1) g/mL,respectively. Subsequently, ABCE also showed moderate activity against K562, CEM-SS and WEHI-3B with CD50values of 12.5, 11.2 and 12.6 g/mL respectively. However, weak activity was obtained against HeLa cell line with CD50value of 20.5 g/mL. There were some significant differences in cytotoxic percentages between the cell treated with higher doses of ABCE (30, 15 and 7.5 μg/mL) and the untreated in all panels of cell lines at P<0.05 (t-test) (Figure 1). Meanwhile, a significant difference could be seen between the higher and lower groups of treatment doses in all treated-cell cultures(P<0.05).

Table 3 IC50 value (g/mL) of 12 crude methanolic extracts of A. aaptos (marine sponges) collected off various locations in Terengganu against HL-60 and MCF-7.
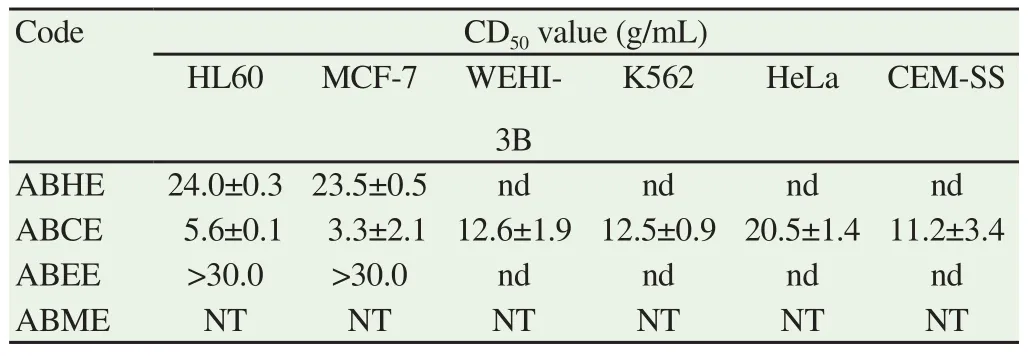
Table 4 CD50 value (g/mL) of hexane, dichloromethane, ethyl acetate and methanol extracts from A. aaptos against HL-60, MCF-7, K562, HeLa, CEM-SS and WEHI-3B.
3.4. Antiviral against HSV-1
Regarding the determination for anti-HSV-1 activity, the ABHE,ABCE, ABEE and ABME were firstly evaluated for their cytotoxic effect alone against Vero cells (mock-treated cells). The samples were exposed to the cells for 72 h under the same culture condition used in anti-HSV-1 assay. The cytotoxic effect of the samples against Vero cells was microscopically visible with all of the cell monolayers detaching from its culture vessels thus resulting the viability of cells are being compromised. Sample ABHE, ABCE and ABEE (at concentration 100 g/mL) showed low cytotoxic activity with the percentage of reduction (destruction) in dehyrogenase enzyme of 10.2% to 3.3% after 72 h of treatment. However, the cytotoxic effect was not observed in the cells that were exposed to ABME. Thus,CC50of all fractions was not determined due to their percentage of cytotoxic effects being too small and not exceeding 50% (Table 5).After studying the cytotoxicity effect of samples against normal Vero cells, this study was followed by an experiment conducted to assess the antiviral effect of samples against HSV-1. 0.001 ID50/cells of MOI at 2 × 108.5ID50/mL. Table 5 showed the potential of antiviral activity of ABCE when it was compared to other fractions in which 100% of protection was achieved at 100 g/mL. The EC50value of ABCE was(60.5±1.2) g/mL. No activity was displayed in HSV-1 treated-ABHE,ABEE and ABME with percentage of virus inactivation being lower than 50% (11.6%, 15.7% and 27.8%, respectively).

Table 5 Inactivation effect of HSV-1 assayed by neutral red uptake method.

Figure 1. Cytotoxicity of ABCE (chloroform extract) against various cell lines: HL-60, MCF-7, WEHI-3B, K562, HeL and CEM-SS.
3.5. Purification and characterization of p-hydroxybenzamide
The p-hydroxybenzamide (Figure 2) was isolated as white powdered compound from butanol fractions obtained after solvent partitioning of methanol extract of A. aaptos, followed by a repeated column chromatography on silica gel and Sephadex LH20.Spectroscopic data of p-hydroxybenzamide were recorded as below:IR υmaxcm-1(KBr disc): 3369 and 3188 (NH2group), 3080 (O-H),1650 (C=O), 1638 (C=C), 1317 (C-O), and 1198 (C-N stretching),EIMS m/z (rel. int): 137 [M]+(31.69), 107 (100.00), 93.05 (17.58),77.0 (62.98), 63.00 (52.79).
1H-NMR (CD3OD, 600 MHz)δppm: 7.39 (d, 7.8 Hz, 2H, H-2 and H-6), 5.61 (d, 7.8 Hz, 2H, H-3 and H-5), 1.89 (s, 2H, NH2).
13C-NMR (CD3OD, 150 MHz) δppm: 110.9 (C-1), 142.2 (C-2 and C-6), 100.9 (C-3 and C-5), 152.1 (C-4 (OH)), 166.0 (C=O).
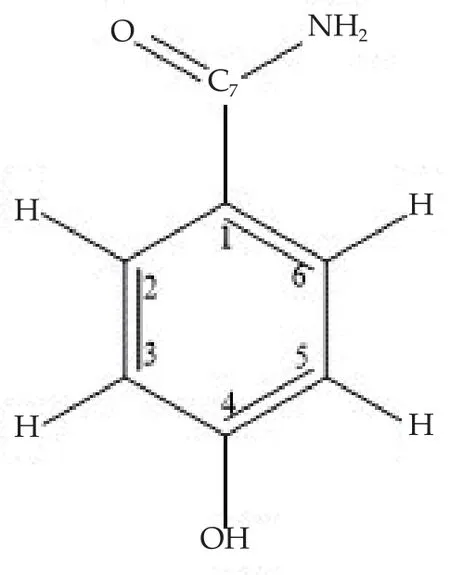
Figure 2. p-Hydroxybenzamide.
4. Discussion
The births of hundreds of new metabolites have been discovered every year. Although more than 5 300 different natural products have been purified from marine sponges and their associated microorganisms, sponges still remain as an important marine organism for the discovery of new bioactive natural products. Many substances such as bioactive alkaloids, sterols, terpenes, amino acid derivatives, cyclic peptides, peroxides, unusual nucleosides(most probably halogenated) and FCs have been identified from sponges and their associated microorganisms. Most of these natural products that come from sponges have shown a wide range of pharmacological activities such as antiviral, anticholestrolemic,anthelmintic, anticancer, antifungal, antiprotozoal, anti-in flammatory,immunosuppressive, antifouling activities, antimalarial, antitumour,cardiovascular agent, antihelminthic, muscle relaxant agent and neurosuppressive[49-51].
Subsequently, FCs have been widely distributed in marine sponges as they play significant functional and structural roles in plasma membrane and biogenesis. Lipids and FA have performed an important role in stress resistance by maintaining proper membrane function so as to to endure the tough environmental changes that bring effects to marine sponges. Sponges with higher composition of storage lipids, phospholipids, sterols, n-3 and n-6 polyunsaturated fatty acid (PUFA) have displayed the highest level of resistance to ocean warming and ocean acidification[52]. Besides, high levels of polyunsatured long chain fatty acids (C24–C30), high branched and odd-chain fatty acids in marine cold-water sponges of genus Latrunculia, namely Latrunculia bocagei Ridley and Dendy, 1886,and Latrunculia biformis, to some extent have been needed for cell membrane integration so as to survive in low temperatures ocean[53].In physiological function, long-chain n-3 polyunsaturated fatty acids(n-3 LC-PUFA), likewise have the potential to decrease in flammation in both in vitro and in vivo studies. Furthermore, the n-3 LC-PUFA eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid(DHA, 22:6 n-3) inhibit interleukin-1β and interleukin-6 production in human macrophages. It has been proposed that n-3 LC-PUFA may play a prominent role as alimentary therapeutic component for the inhibition and treatment of the inflammatory ailments[54].In addition, prescribing DHA with UA-lowering medicine may enhance the regulation of blood glucose levels in diabetic patients with high level of UA[55]. EPA and DHA also have improved some cardiovascular risk factors[56].
Many fatty acids have been originated from unusual biosynthetic pathways, thus this displays unusual characteristic of unsaturated patterns, exhibit terminal and/or mid-chain branching. They may happen as mono-, di- and tri- unsaturated and cover an extensive carbon-number range normally C24-C30. Marine sponges also are a rich source of brominated, α-methoxylated, acetylenic, branched fatty acids as well aspolyoxygenated fatty acid amides[3,50,57].However, based on the result obtained in this study, it has been found that Aaptos sp. only contained common saturated and monoenoic fatty acid and it is comparable with Ircinia spinulosa[58]. However,its FC content is different compared to marine sponges (Latrunculia)and seaweed (Sargassum granuliferum, Ulva armoricana, and Solieria chordalis, Gracilaria sp.) which has high PUFA content[8,53,59,60].
Nevertheless, from this study, the five major of FAMEs with each concentration patent in each extract were: behenic acid; ethyl acetate> methanol > chloroform > hexane extract, cis-10-heptadecenoic acid; hexane > chloroform > methanol ≥ ethyl acetate, palmitic acid; chloroform > hexane > ethyl acetate > methanol, and cis-10-pentadecenoic acids; hexane > methanol ≥ chloroform > ethyl acetate. To summarize, chloroform, ethyl acetate and methanol extracts have shown similarity in higher proportion of straight chain saturated fatty acid, showing 28.5%, 46.4% and 40.9% (total of three highest values) of total FAME content respectively. However, FA composition in hexane extract was more dominated by unsaturated FC with a proportion of 35.9%. The domination of palmitic acid in the chloroform extract of marine sponges has long been proven since 1980s and up to 2000s as reported by Carballeira & Maldonado,who discovered that chloroform/methanol extract of marine sponge Chondrilla nucula was rich in palmitic acid which accounted for 26.0% of its total FA[61]. Later, Lee et al. reported that the high concentration of saturated fatty acid isolated from Bahamas marine sponge was dominated by palmitic acid and octadecanoic acid[62].
In addition to the determination of FC, the DPPH free radical scavenging activity and total phenolic content of the respective extracts were also carried out. Among the extracts, the highest and lowest content of phenolics that are observed in sample K and H from Kapas Island coast, respectively. There were claims that the antioxidant activity of extracts should be proportional to its total phenolic contents[63]. However, this study has shown that extracts containing high phenolic contents did not always reveal high antioxidant activity. It has been found that ABCE and ABME have displayed high potential of DPPH free radical scavenging (67.6 ±0.1)% and (8.2 ± 0.1)%, respectively. The total phenolics content of ABCE and ABME were (10.50 ± 0.01) and (6.50 ± 0.04) mg/g GA, respectively. This may be due to the fact that different phenolic compounds have different responses in the experiment conducted by Folin-Ciocalteu[64]. Hence, the molecular antioxidant response of phenolic compounds varied remarkably, depending on their chemical structures[36]. Therefore, the antioxidant activity of an extract cannot be predicted solely on the basis of its total phenolic content.This prediction indicated that there are other factors than the total phenolics which can play a major role in the antioxidant activity of tested materials. Besides that, interference from other chemical components presented in the extract, such as sugar or ascorbic acid also could lead to this possible outcome[65].
Phenolic compounds are rarely distributed in marine sponges. Most of the phenolic compounds obtained from marine sponges were produced by their microbial symbionts such as cytotoxic phenolic bisabolanesesquiterpenoid dimers disydonol A-C which have been isolated from an endophytic Aspergillus sp. (sponge Xestospongia testudinaria, Weizho Is., South China Sea)[66] and two antifungal and antioxidative sesquiterpene phenols, (+)-curcupheno1 and(+)-curcudiol, from both deep and shallow water collections of the sponge Didiscus flavus van Soest[67,68]. Being said that, this study also wish to report the isolation of a phenolic composition,p-hydroxybenzamide from methanol extract of A. aaptos. However,the result from the isolation only showed low free radical scavenging activity (22% inhibition) with IC50>10 mg/mL. Hydroxybenzamides are important and well-known phenolic compounds. Owing to the wide range of biological activities of their derivatives, considerable interest has been placed on derivatization of these compounds.Benzamides derivatives were reported to display cerebroprotective activity, anti-leishmanial activity, antibacterial and antifungal,antipsychotic, and have potential for treating atheroschlerotic and cardiovascular diseases[69-73].
The cytotoxic activity screenings of marine sponge crude extract has attracted the attention of the marine natural product research community. For example, Seleghim et al. discussed in his study,that out of 215 Brazilian sponge crude extracts, 11% have displayed cytotoxic activity against MCF-7 breast cancer cell, while 18 %against HCT-8 colon cancer cells, and 8% against B16 murine melanoma cancer cells[74]. Previously, the crude methanolic extract of A. aaptos was collected at different dates and locations exhibited cytotoxic activity against HL-60, MCF-7, CEM-SS, HeLa, HT-29 and L929[9].
Compared to other fractions such ABHE, ABEE and ABEE, the sample ABCE exhibited potential antiviral activity. A previous study also reported that 2 g/mL of methanol-methylene chloride extract of Aaptos sp. from Abrolhos, Brazil could inhibit 76% of HSV-1 replication in Vero cells[30]. It has been reported that several derivatives of myristic acid and palmitic acid have been effective in inhibiting herpes viruses[75], whereas lauric acids inhibit the arena virus production[76]. Recently, fatty acid esters of antiviral drug entecavir were prepared by going through the process of esterification by reacting n-tetradecanoic acid, n-hexadecanoic acid, and n-octadecanoic acid with entecavir to produce novel lipidic prodrug of entecavir for parenteral sustained delivery[77]. In response to that experiment, octacosanoic acid was found to be cytotoxic against HL-60 cell line[19]. Whereas, Ito et al[78] suggested that palmitoleic acid induced the change in the lipid composition of tumour cells hence resulting in the damage of the cells. As stated by Ito, palmitic acid could trigger an increase of dose-dependent in apoptosis of MG 63 cell line[79]. Other FCs, 4-Me-6E, 8E-hexadecadienoic is reported to reduce the viability of MCF-7 breast cancer cells in a dose dependent manner (up to 63%) and the gene expression of two lipogenic enzymes, the acetyl CoA carboxylase and the fatty acid synthase[80].Thus, this study suggested that the fatty acid constituents as the results shown might have some contribution in the cytotoxic and antiviral properties of the extracts, in addition to the aaptaminoids.Previously, we reported that 3-(phenethylamino)dimethyl(oxy)aaptamine isolated from this A. aaptos induced the apoptosis and contributed in anti-HSV-1 activity[16]. Besides, the isoaaptamine from A. aaptos was also reported to induce T-47D cells apoptosis and autophagy via oxidative stress making it is a good candidate for breast cancer treatment[81]. In another study, nucleosides (Ara-A and Ara-C, mycalamide A, mycalamide B), sesquiterpene hydroquinones(Avarol), cyclic depsipeptides (papuamide A, B, C, and D,microspinosamide), alkaloid (4-methylaaptamine, dragmacidin F,manzamine A), phenolic macrolides (hamigeran B) were among the antiviral substances that belong to marine sponges[82]. In 2015,a broad spectrum of natural antiviral drugs have been reviewed by Martinez et al[83], only mycophenolic acid has a short chain fatty residue. In the chemical structure of mycophenolic acid, 4-methyl-4-hexenoic acid residue was attached to a phthalanylmoeity that was fused to an oxoisobenzofuran-6-yl.
Conflict of interest statement
We declare that we have no con flict of interest.
 Asian Pacific Journal of Tropical Biomedicine2018年11期
Asian Pacific Journal of Tropical Biomedicine2018年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- α-Mangostin and apigenin induced the necrotic death of BT474 breast cancer cells with autophagy and inflammation
- Antidiabetic effect of combination of fractionated-extracts of Andrographis paniculata and Centella asiatica: In vitro study
- Bacillus sp. SAB E-41-derived extract shows antiaging properties via ctt1-mediated oxidative stress tolerance response in yeast Schizosaccharomyces pombe
- Enhanced cytotoxic effect on human lung carcinoma cell line (A549) by gold nanoparticles synthesized from Justicia adhatoda leaf extract
- Protective efficacy of Nigella sativa oil against the harmful effects of formaldehyde on rat testicular tissue
