A systematic review of the effect of mobile health on cardiac rehabilitation among coronary heart disease patients
Hui Liu, Xio Xio, Chun-Mei Lu, Dong-Ln Ling, Rui-Hong Wei*
a Cardiac Intensive Care Unit (CCU), Department of Cardiology, The Second Affiliated Hospital of Shantou University Medical College, Shantou,Guangdong 515041, China
b Obstetrics Department, Southern Medical University Affiliated Shenzhen Maternity and Child Healthcare Hospital, Guangzhou, Guangdong 510515,China
cDepartment of Nursing, The First People’s Hospital of Foshan Affiliated to Sun Yat-Sen University, Foshan, Guangdong 528000, China
dUrology Surgical Department, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong 510260, China
eNursing Department, The Second Affiliated Hospital of Shantou University, Shantou, Guangdong 515041, China
Abstract: Objective: Mobile health (mHealth) provides an innovative and effective approach to promote prevention and management of coronary heart disease. However, the magnitude of its effects is unclear. The aim of this systematic review was to examine the impact of mHealth-based cardiac rehabilitation outcomes among coronary heart disease patients.Methods: Medline, CINAHL, Embase, PubMed, Google Scholar, NICE, and Cochrane library were searched for randomized controlled trials published between January 2002 and March 2017 which compared mHealth with conventional cardiac rehabilitation programs among coronary heart disease patients.Results: Eight articles were included in this review. The impact of mHealth interventions on physical activity, medicine adherence,smoking cessation, level of anxiety, and quality of life was inconsistent among the articles.Conclusions: Further research is needed to conclusively determine the impact of mHealth interventions on cardiac rehabilitation outcomes. The limitations of the included studies (e.g., inadequate sample size, failure to address the core components of cardiac rehabilitation programs, and lack of theory-based design) should be taken into account when designing future studies.
Keywords: coronary heart disease · mobile health · text messaging · mobile applications · cardiac rehabilitation
1. Introduction
Coronary heart disease (CHD) is the most common type of cardiovascular disease (CVD) that causes stable angina, unstable angina, myocardial infarction,and sudden cardiac death.1CVD is the biggest killer in the world. In 2012, about 17.5 million people died from CVD, representing 31% of all global deaths; of these deaths, nearly half (7.4 million) died due to CHD.2The burden of CVD is growing globally, especially in lowand middle-income countries. According to Schwalm et al.,3more than 80% of CVD-related deaths occur in middle-income countries.
Cardiac rehabilitation (CR) has been strongly recommended for patients with CHD by international clinical practice guidelines.4CR promotes secondary prevention of CHD and provides a cost-effective and comprehensive framework that can reduce mortality by up to 25% while also improving patients’ physical activity, increasing life expectancy and health-related quality of life (HRQoL), and lowering hospital readmission rates and medical resource use.5The Global Action Plan for the Prevention and Control of Non-Communicable Diseases (2013–2020), launched by the World Health Organization (WHO), recommended that all CHD patients should have access to nationally determined sets of rehabilitative health services.6
However, according to Turk-Adawi et al.,5CR programs are underutilized when compared with revascularization or medical therapy to treat CHD patients.Worldwide, only 38.8% of countries have CR programs:specifically, 68.0% of high-income countries and 23%of low- and middle-income countries. In addition, only about half of eligible patients participate in CR programs in developed countries, and this number is much less in developing and underdeveloped countries.7Patients taking part in CR programs have a low rate of adherence and often drop out during the process.
Many factors and barriers contribute to the current situation of CR utilization and impact on outcomes of CR programs.7At the same time, adherence to CR programs is often challenged by the complexity of medication regimens and by the difficulty in making lifestyle and behavioral changes such as adherence to exercise,healthy diet, smoking cessation, and healthy weight control.8Therefore, it is important to implement innovative approaches aimed at removing these barriers and motivate patients to enroll and sustain the CR programs.
In this regard, mobile health (mHealth) may be an effective way to overcome some of these barriers and offer an effective alternative model for CR programs.9The WHO defines mHealth as any medical and public health practice that is supported by mobile devices,such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices.10In health care systems, mHealth is often delivered by way of short message services (SMSs), paging, mobile applications (apps), media capabilities, and video conferencing.11According to Neubeck et al.,12almost 2 billion people own and use smartphones. More than 50%of adults globally are predicted to own a smartphone by 2018. This rapid growth in the use of mobile phone provides opportunities to conduct mHealth.
In recent years, some studies have been conducted to explore whether mHealth can help overcome some barriers to CR and evaluate the effectiveness of different kinds of mHealth tools in CR. Although there is increasing research in this area, published studies are still limited. The mHealth tools used in these studies mainly include text messages (TMs), mobile apps,patient monitoring devices, digital assistants, wireless devices, or a combination of these tools. TMs are the most reported tool. Some studies have combined TMs with apps and other mHealth tools. Study designs have included randomized controlled trials (RCTs),quasi-experimental studies, and observational cohort studies. A dominant number of studies have examined medication adherence as the main clinical outcome,while other studies examined outcomes such as physical activity, lifestyle change, quality of life, psychological status, number of hospitalizations, hospital readmission rate, adverse events, and cost. Findings in these studies are inconsistent, and it remains difficult to draw conclusions on the effectiveness of mHealth on CR.
Therefore, the purpose of this systematic review was to examine the impact of mHealth-based CR outcomes among CHD patients. The specific aims were to(1) describe the current mHealth-based CR among CHD patients and (2) discuss the impact of these interventions on CR outcomes.
2. Methods
2.1. Inclusion and exclusion criteria
The inclusion criteria for the literature search were as follows: (1) RCT, (2) mHealth as the only or main intervention, (3) CHD patients aged 18 years or older, (4)published in English, (5) published between January 2002 and March 2017.
The exclusion criteria were as follows: (1) interventions predominately conducted via e-mail, Internet,or telemonitoring devices, (2) studies that did not target CHD patients, and (3) studies only had abstracts or study protocols available.
2.2. Search strategy
To identify the relevant literature on this topic, a systematic and comprehensive search was conducted using Medline, CINAHL, Embase, Google Scholar, NICE, and the Cochrane library. The search terms included “coronary heart disease,” “cardiovascular disease,” “myocardial infarction,” “heart disease,” “coronary artery disease,”“acute myocardial infarction,” “Acute myocardial infarction(AMI),” “angina,” “ischemic heart disease,” “unstable angina,” “CHD,” “CVD,” “Coronary Atherothrombotic Disease (CAD),” “Myocardial infarction (MI),” or “Ischemic heart disease (IHD).” These terms were used in conjunction with the terms “CR,” “secondary prevention*,”or “rehabilitation*” and then conjunction with “mobile phone,” “smartphone,” “cellular phone,” “mHealth,” “text messaging,” “text message,” “short messaging service,”“SMS,” “mobile app,” “mobile application,” “telehealth,”“e-health,” “telecare,” “telemedicine,” or “mHealth.”
2.3. Study selection and data abstraction
All studies identified in the search were independently assessed by two researchers (first author and corresponding author), based on their reading of the titles and abstracts (if available), against the inclusion and exclusion criteria. Forty-five studies were included, and the full versions of these studies were retrieved. The same two researchers independently read all 45 studies. A meeting was then convened to reach a consensus on selection, and the input of a third independent researcher was sought if consensus could not be reached. Ultimately, eight studies met the eligibility criteria and were included in the systematic review. Data were independently extracted from each study by the researchers using a standardized form. The search findings and process are shown in Figure 1.
Risk of bias was evaluated using the tool outlined in the Cochrane handbook for systematic reviews of interventions. The tool assessed the risk of selection bias,performance bias, detection bias, attrition bias, and reporting bias. Two reviewers independently assessed the rigor of the included studies. Discrepancies were discussed and then reconciled. The number of included studies was insufficient to detect publication bias via funnel plot asymmetry. Heterogeneity was explored using the I2statistic. The heterogeneity of the mHealth interventions and outcomes precluded a meta-analysis.Hence, a systematic review with descriptive synthesis was performed, with quantitative results from the individual studies presented to support the narrative.
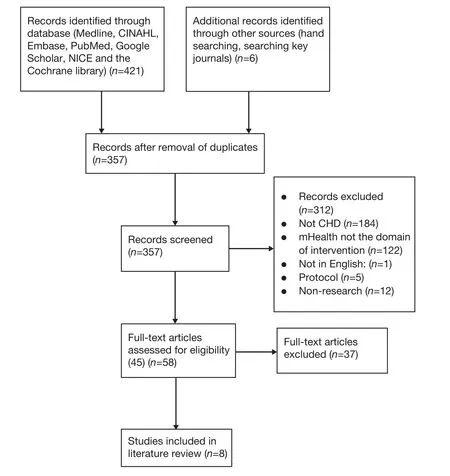
Figure 1. Search findings and process.
3. Results
3.1. Characteristics of the current mHealth studies
The key characteristics of the included studies are summarized in Table 1. All the studies included in this systematic review were RCTs; two were conducted in the USA, two in Australia, three in Europe, and one in New Zealand. The study duration ranged from 28 days to 6 months. The sample size of the studies ranged from 48 to 710, totaling 1,980 participants. The mean age of the study participants was 59.52 years. The majority of the study participants (66.8%) were men.
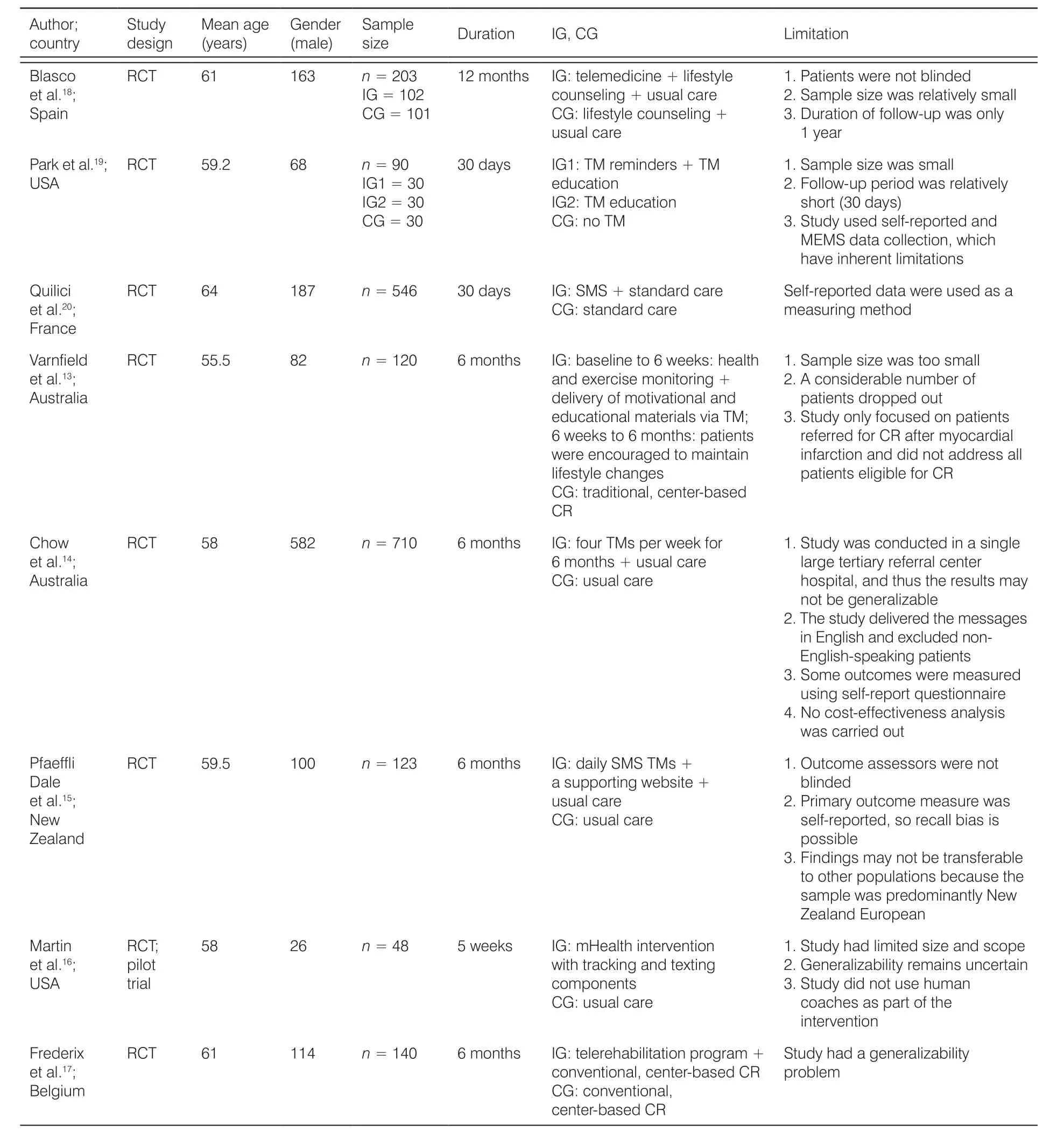
Table 1. Characteristics of the included studies.
3.2. Intervention characteristics
The key characteristics of the mHealth interventions are summarized in Table 2. All the eight studies applied mobile phone technology. Among these studies, half(four out of eight; 50%) used TM as the single intervention. Two of the studies combined TM with mobile application13,14One study combined TM with Internet service,15and one study combined TM with telecoaching.16All the studies utilized a central monitoring center.Seven allowed for automatic data transfer between the participants and the monitoring center, whereas one was semiautomatic, which required the participants to manually input and transfer their data to the monitoring center. Five of the studies had trained nurses and physicians in their central monitoring centers who regularly monitored the participants’ status; the remaining study did not specify who/what monitored the data. Four of the studies provided real-time feedback to patients within 24–48 hours, while other studies did not. Four of the eight studies used a theory such as social cognitive theory, self-efficacy theory, or behavioral change theory as a framework for the intervention. The frequency of sending message is varied among studies, ranging from three times a day to once a week. Four of the studies assessed the patients’ experiences with using mobile phones for health-related outcomes.
3.3. Assessment of risk of bias
The results of the risk of bias assessments are depicted in Figure 2. Overall, the methodological rigor of the included studies was moderate. Although two studies had a low risk of bias in most categories, the majority of the studies had a high risk of bias in at least two categories. In addition, the majority of the studies failed to provide enough information to allow for a complete assessment of their risk of bias (i.e., unclear risk of bias).Blinding is difficult to conduct in these studies, especially for participants. Therefore, performance bias existed in all studies. Outcome assessment can be blinded,although only three out of the eight studies blinded the outcome assessor.
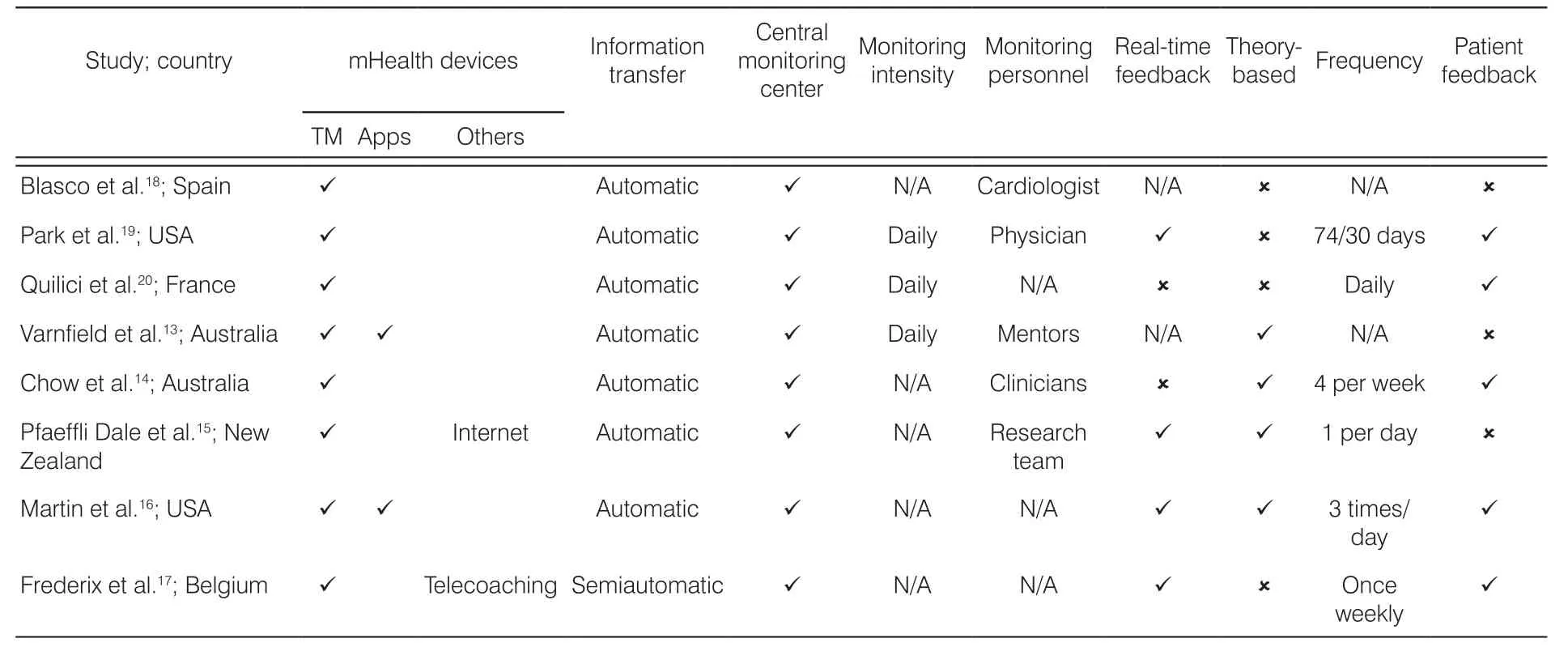
Table 2. Intervention characteristics.
3.4. Effectiveness of mHealth interventions
The impact of the following mHealth interventions on CR outcomes was assessed in two or more of the studies:physical activity, medicine adherence, smoking cessation, level of anxiety, quality of life, clinical events, and patient satisfaction. Outcomes that were measured in only one study were not selected because they could not be compared across studies.
3.4.1. Physical activity
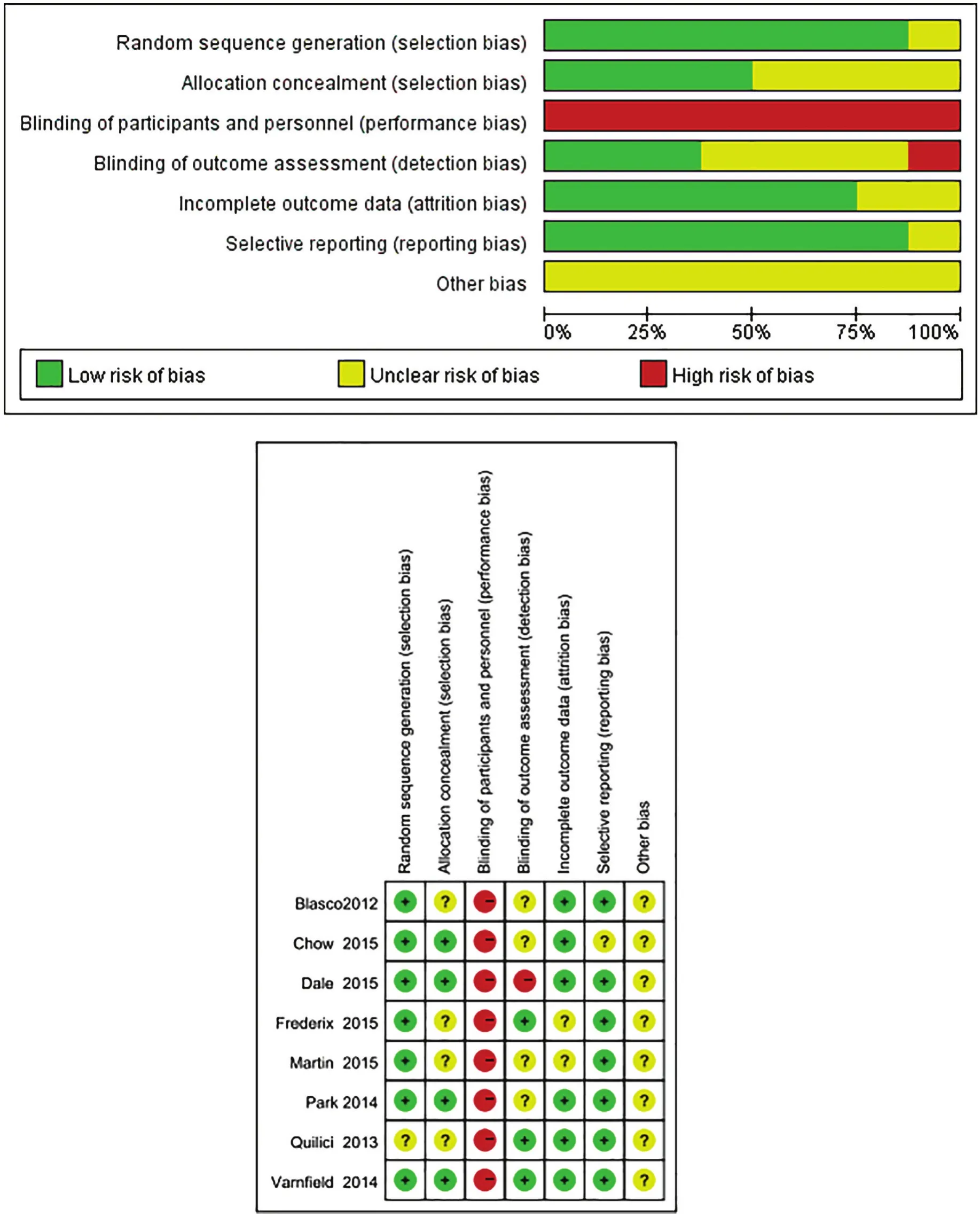
Figure 2. Bias risk assessments.
Five studies reported objective or self-reported physical activity levels.13-17Mobile-based CR was compared with usual care in all the five of these studies.Among the five studies, two studies reported positive outcomes, while the other three demonstrated that there was no significant difference between groups.The studies used different tools to measure physical activity levels. Two studies used a daily step count,while the other three studies used a 6-minute walk test (6MWT), a questionnaire, and a metabolic equivalent of task (MET), respectively. Chow et al.14reported that the total physical activity in the intervention group was 936.1, while that in the control group was 642.7(P = 0.003). Physical activity was assessed by the Global Physical Activity Questionnaire (GPAQ). In the GPAQ, participants reported time (in hours and minutes)spent doing work activities in a typical week. Martin et al.16reported that participants receiving TMs walked 2,534 more daily steps than those who did not receive TMs (95% CI: 1,318–3,750; P < 0.001). However, the other three studies reported that there was no significant difference between groups. Varnfield et al.13stated that the 6MWT distance improved at 6 weeks and was maintained at 6 months in both groups. Between-group differences in changes in 6MWT were not significant at 6 months. Frederix et al.17reported that, in the intervention group, the total number of daily steps increased from baseline (median: 7,448; interquartile range [IQR]:24) to both 6 weeks (median: 7,799; IQR: 37) and 24 weeks (median: 8,233; IQR: 32); however, none of the changes were significant (P = 0.24). In the control group, the total number of daily steps showed an initial increasing trend from baseline (median: 5,678; IQR:13) to week 6 (median: 6,630; IQR: 11), but declined afterward (median: 5,265; IQR: 17; P = 0.85).
3.4.2. Medication adherence
Four studies measured medical adherence,8,15,18,20The results of the four studies are inconsistent. Two of the studies reported no significant difference between the groups,8,18while the other two reported positive results.Quilici et al.20reported that SMS intervention significantly improved self-reported aspirin adherence (odds ratio [OR]: 0.37, 95% CI: 0.15–0.90; P = 0.02; number need to treat [NNT] =23). According to Pfaeffli Dale et al.,15the intervention group reported a significantly greater medication adherence score (mean difference:0.58, 95% CI: 0.19–0.97; P = 0.004).
3.4.3. Smoking cessation
Two studies measured the percentage of patients who quitted smoking.14,18The results were inconclusive.Blasco et al.18reported that there were no betweengroup differences in smoking cessation (80.7% vs 81.0%, P = 0.964; risk ratio [RR] = 1.0; 95% CI: 0.9–1.1), while Chow et al.14stated that the current smoking rate in the intervention group is 88/339 (26.0%) and that in the control group is 152/354 (42.9%; 95% CI: 0.61[0.48–0.76]; P < 0.001).
3.4.4. Level of anxiety
Three studies measured the level of anxiety.13,15,18The outcome in terms of anxiety levels was inconclusive.One study reported a reduction in anxiety scores in the intervention group, as measured using the Depression Anxiety Stress Scales.13Another study stated that there were no significant differences between the intervention and control groups, as measured using the State-Trait Anxiety Inventory at the initial visit.18Another study,conducted by Pfaeffli Dale et al.,15reported a negative effect on total hospital anxiety in the intervention group,which reported significantly greater anxiety than the control group at 6 months (mean difference: 1.18, 95%CI: 0.28–2.08; P = 0.01).
3.4.5. Quality of life
Three studies measured HRQoL using different evaluation tools.13,17,18One study used the short-form 36 health survey (SF-36), one used EuroQol Five Dimensions Questionnaire (EQ5D) index, and the other used the HRQoL questionnaire, respectively. The results of these three studies were inconsistent. Blasco et al.18reported no significant differences between the scores obtained in the SF-36. Varnfield et al.13stated that the HRQoL was significantly better in the intervention group than in the control group. Frederix et al.17reported that patients in the intervention group showed a significant improvement in the physical subscale of the perceived HRQoL from baseline (mean: 2.23, standard deviation[SD]: 0.08) until the end of the study period (mean:2.52, SD: 0.07; Friedman’s test: χ2= 15.4, P < 0.001).Between-group analysis confirmed that, globally,HRQoL improved more in the intervention group than in the control group (U = 2,407, Z = 2.805, P = 0.01).
3.4.6. Clinical events
Two studies reported clinical events.14,15Chow et al.reported that a further five patients died. Pfaeffli Dale et al. reported that 13 (intervention: n = 8; control: n = 5)serious adverse events occurred during the trial. However, none of these events were study related.
3.4.7. Patient satisfaction
Five studies reported the satisfaction of participants.8,14,16,17,20The results of the five studies indicated that most of the participants were satisfied with the mHealth intervention. Park et al. reported that, of the 53 patients in the experimental group who completed the mobile phone intervention, most were satisfied with receiving a TM.8Quilici et al.20reported that, at the end of the study, 92% of the patients in the intervention group reported satisfaction and believed that the SMS support service was valuable. Chow et al.14stated that the large majority reported that the TM support program was useful (91%), easy to understand (97%),and motivating with respect to changes in diet (81%)and physical activity (73%). Martin et al.16reported that participants largely expressed feelings of satisfaction and enthusiasm for trial participation. Frederix et al.17reported that, in general, the patients were very satisfied(30/69, 44%) or satisfied (35/69, 51%; total: 95%; 65/69,very satisfied/satisfied) with the rehabilitation program.
4. Discussion
To our knowledge, this is the first systematic review to examine the use of mHealth specifically for delivering and monitoring structured, individualized, prescriptive CR in a CHD population. Eight RCTs (=1,980) were included in the review. Due to the heterogeneity of the outcomes, it was not possible to conduct a metaanalysis. The results regarding the impact of mHealth intervention on CR outcomes were inconsistent. Future research is needed to elucidate the effectiveness of mHealth in CR programs.
The characteristics of the included studies showed that a majority of studies used TM as the main intervention approach. Positive results regarding medication adherence, smoking cessation, and physical activity improvement have been observed from some of these studies. The majority of the TM studies used personalized TM content, such as participants’ names, medication names or dosages, catered timing based on the individual’s prescription, individualized message copy related to the participant’s condition, motivational text correlated with the participant’s indicated goals, and content matching the participant’s individual barriers.Most TM studies requested participants to respond to TMs. The frequency and content of the TMs are different in each study. The frequency of message sending varied between 3 and 21 times each week. In general, most studies indicated that TM as a mHealth tool is effective in improving the outcomes of CR programs among CHD patients. Positive results are observed in studies with the following characteristics: content of the message highly related to individual’s needs and according to the individual’s prescriptions; the frequency of sending message is relatively high; the design and content of the message are based on theory, and the messages contain some motivational words.
Although TM is the dominant approach to mHealth used in CR programs, mobile apps provide more functions than TMs do alone. According to Teyhen et al.,21apps can help collect and analyze data in real time and offer interactivity, gaming, and feedback. In the past 10 years, apps have become popular in health promotion.22However, studies on the use of apps for CR among CHD patients are limited. Some studies have combined apps with TM and other mHealth tools. In this systematic review, only two trials used apps as the intervention.
According to Beatty et al.,23the design of apps for CR among CHD patients should be based on behavior change theory and should contain the core components of CR and cater to the needs of individuals. In addition,the design and reporting of clinical trials of mobile apps for CR should follow the Consolidated Standards of Reporting Trials guidelines for mHealth interventions.24Two of the included studies did not specifically address behavior change strategies in their design. However,they did incorporate behavior change strategies, including short- and long-term goal setting, motivational messages, and reminders. Applying principles from behavior change theories in the design of mobile interventions for CR may significantly increase the likelihood of success.25In addition, mobile technology may provide an opportunity to deliver real-time cues to promote behavior change.
Among the included studies, only one explicitly reported that the content of message was developed based on national guidelines.13When designing the content of the mHealth intervention, most research did not include the core components of CR programs; some of them concentrated only on exercise.
The evaluation of the effectiveness of CR programs has changed from focusing on serious cardiovascular events such as death, heart failure, and stroke to patient-centered outcomes that are influenced by physical, mental, and social health. Thus, the impact of a mobile intervention on health outcomes must be examined at multiple levels, including participation in CR sessions, physical activity, exercise capacity, cardiovascular risk factors, patient-reported health status,cost, and clinical events. This systematic review showed that the evaluation tools used in these studies are inconsistent and so it is difficult to compare the results.
Physical activity reduces the risk of secondary cardiovascular events in CHD patients. It can be evaluated using several methods. Patient recall is a common method for evaluating physical activity, although it is not as accurate as the real-time reporting of physical activity. In one study, mobile-reported physical activity correlated with both objectively measured physical activity and self-reported physical activity, but there was a high degree of variability in mobile-reported physical activity at similar levels of objectively measured activity.
Some trials used a self-report questionnaire to evaluate medication adherence. Quilici et al. found that the results of self-report medicine adherence are not according to the results from biological testing. Based on the self-reported data, only 3.6% of patients in the intervention group had stopped aspirin therapy, while Arachidonic Acid Induced Platelet Aggregation (AA-Ag)testing showed that 5.2% of patients had not adhered to the medical regime. This serves as a reminder that selfreporting of medication adherence might generate some discrepancies and that some biological testing should be used to validate the result. Self-reporting was the most common method of measuring adherence to treatment regimens across all the studies. This method is easy to conduct, but it might produce potential participant bias.26
According to Beatty et al.,23rigorous study with an RCT design should be used to evaluate the effect of mHealth programs. With regard to the characteristics of studies included in this systematic review, there were some methodological problems. First, the sample sizes in most of the studies are too small, which might decrease the external validity of the findings. Second,although blinding is an important way to guard against bias, particularly when assessing subjective outcomes,27among these selected studies, only a few studies used blinding. Although it is impossible to blind the investigator and participants, it is possible to blind the data collector and assessor. Bias might be generated in these studies. Third, when designing the content of the apps,most studies did not address the core components of CR programs; most of them concentrated only on exercise. In addition, the follow-up time of the programs was between 1 month and 6 months. Although Schulz et al.28stated that a 4-week follow-up is long enough for the effectiveness of an intervention program to become apparent for a life-long disease, the long-term effectiveness could not be observed in such a short period.
5. Conclusions
This systematic review provided an overview of mHealth programs in CR among CHD patients. Findings from these studies demonstrate that mHealth is a feasible and acceptable way to remove some barriers to CR programs, improve patients’ adherence to CR, and positively impact the outcomes of CR programs, including improved physical activity, improved health-related quality of life, smoking cessation, and cardiovascular risk factor management. However, the findings from these studies were inconsistent, and high-quality studies in this area are limited. Therefore, it is still difficult to draw conclusions on the effectiveness of mHealth for improving major clinical outcomes.
In the future, more rigorous research is needed in this area. First, future research should include appropriate sample sizes based on the calculation of effect sizes;RCT designs with long follow-up durations are needed to observe long-term outcomes. Second, multiple interventions, including TM, apps, and other mHealth tools,should be combined together. Third, only a few of the studies applied theory to support the development, testing, or implementation of the intervention. Therefore,future research should be based on some motivational theories, such as social cognitive theory, the health belief model, self-efficacy theory, or behavioral change theory.Fourth, according to the mHealth evidence reporting and assessment (mERA) guidelines,25user feedback about the intervention or user satisfaction with the intervention is an essential criterion, so the future research should pay more attention to the patients’ experience with CR programs. Fifth, more clinical outcomes, such as mortality and cost-effectiveness, should be evaluated in future research. Sixth, patients can lose their privacy when using apps or TMs to convey information or data,so future research should consider these security issues.
5.1 Limitations
This systematic review is the first to evaluate the effectiveness of mHealth in CR among CHD patients. There are several limitations to this review. The data were too heterogeneous to conduct a meta-analysis; so we used a narrative synthesis to establish the potential of mHealth to promote CR. In addition, many studies combined the use of multiple technologies, which made it difficult to tease out the unique contribution of the individual intervention components (i.e. TM, apps, and telemonitoring via smartphone).
Conflicts of interest
All contributing authors declare no conflicts of interest.
- Frontiers of Nursing的其它文章
- Family characteristics on self-reported toothache among Indonesian children aged 12–14 years
- Beneficial effects of auricular acupressure on preventing constipation in breast cancer patients undergoing chemotherapy: evidence from systematic review and meta-analysis
- Effects of parental involvement in infant care in neonatal intensive care units: a meta-analysis
- Family functioning integrated with diabetes self-management: a concept analysis
- Bibliometric analysis of literature regarding ostomy research based on the Web of Science database†
- Peer educator training program for enhancing knowledge on issues in the growth and development of adolescents and risk behavior problems in Indonesian context†

