Effects of physicochemical factors on development and survival of Opisthorchis viverrini uterine eggs
Chanisala Sereewong, Monticha Chaiyasaeng, Naiyana Senasri, Jukkrid Chaiyos, Smarn Tesana✉
1Food-borne Parasite Research Group, Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
2Department of Fisheries, Faculty of Natural Resources, Rajamangala University of Technology Isan Sakonnakhon Campus, Sakonnakhon 47160,Thailand
Keywords:Opisthorchis viverrini Physicochemical Development Survival Uterine eggs
ABSTRACT Objective: To investigate the maturity development of miracidia in uterine eggs from four portions of the Opisthorchis viverrini uterus and environmental factors possibly affected in maturation and infectivity of distal part uterine eggs. Methods: Uteri of adult worms were divided into 4 equal parts. Development of eggs was determined under light microscope. Only embryonated eggs were used to evaluate the effects of physicochemical factors: temperature,salinity, acidity, ultraviolet A, B, C. Infection success was evaluated by feeding treated eggs to intermediate host snails and determining by using a PCR approach. Results: Eggs obtained from the uterus closest to the ovary (regions 1 and 2) failed to develop in vitro. Eggs from region 4 of the uterus (close to the genital pore) were used to study effects of physicochemical factors. The highest survival and infection success was in groups of eggs kept at 30 ℃ (95.20%).The calculated period of loss infection success (LI50 and LI95) on miracidia in distal uterine eggs by exposure to UV-A, UV-B and UV-C were 73 and 1 523 d; 8 and 20 d; 1 and 2 d,respectively. Lethal concentrations (LC50 and LC95) of salinity, HCl and NaOH on miracidia in distal uterine eggs were 45.43 and 120.09 ppt, 0.01 and 0.25 M; 0.01 and 0.11 M, respectively,after 24 h exposure. Conclusions: Opisthorchis viverrini eggs display a high tolerance to environmental conditions, especially after snail host eating for infection.
1. Introduction
As many as 7 million Thai people are estimated to be infected with Opisthorchis viverrini (O. viverrini), with the highest prevalence in northeastern Thailand[1,2]. People become infected by eating fermented, undercooked or raw cyprinid fish dishes harboring infective encysted metacercariae[3]. After ingestion, the excysted larvae migrate into the biliary tree to develop to sexual maturity and produce uterine eggs within 2 weeks[4]. In northeastern Thailand,Bithynia siamensis goniomphalos snails serve as the first intermediate host[5,6]. They become infected by ingestion of fully mature eggs containing miracidia.
Distal uterine eggs of mature O. viverrini contain developing miracidia[4]. Eggs were laid in feces of definitive hosts need two weeks to become mature before they can infect snails[7]. A previous study on Clonorchis sinensis found that immature eggs from the proximal uterus (close to the ovary) could not develop outside the uterine tube[8], a situation that might also occur in O. viverrini. In the natural environment, eggs passed in feces and contaminating natural water bodies encounter environmental factors such as temperature changes, salinity, ultraviolet radiation, and changes in water pH.All of these may affect development of eggs to maturity and their survival. There are many environmental factors that can impinge on maturation of trematode eggs. Factors such as temperature and salinity influenced egg development and miracidial emergence of the digenean, Himasthla militaris. The eggs developed normally in the range 2.1 to 3 ppt salinity but in a salinity of 34 ppt, miracidial emergence was markedly inhibited[9]. Embryonation ceased at 12 ℃ ;egg development as well as miracidial emergence occurred at 20 ℃and 30 ℃, but both processes progressed at a slower rate at 20 ℃ than at 30 ℃. The eggs of Fasciola hepatica developed well at 27 ℃ when exposed to light[10]. Miracidia of Echinostoma caproni were not tolerant of salinities over 1 ppt but could survive in a pH range 3 to 11 and lived longer at 5 ℃[11]. Ultraviolet affected eggs and rhabditoid infectivestage larvae of canine hookworm. Irradiation for one hour at a distance of 10 cm reduced the hatching eggs by half. Those larvae hatched did not develop to the infective stage[12].
In this study, we investigated the maturity development of miracidia in uterine eggs from 4 portions of the O. viverrini uterus. In addition,effects of environmental factors possibly involved in maturation and infectivity of uterine eggs from the distal part of the uterus were investigated. These factors include temperature, salinity, ultraviolet radiation, pH of water.
2. Materials and methods
2.1. Adult O. viverrini preparation
Eight Syrian golden hamsters were infected orally with 50 O.viverrini metacercariae harvested from naturally infected cyprinid fish. Infected animals were provided with water and food ad libitum and maintained for 2 months, were sacrificed to obtain parasites from livers. The worms were washed several times in sterile physiological saline. Animal use was approved by the Animal Ethics Committee of Khon Kaen University under National Council Research of Thailand guidelines (Ethics clearance number 0514.1.75/42).
2.2. O. viverrini egg preparation
Uteri of the adult worms were divided equally into 4 regions between the ovary and the genital pore (Figure 1) and eggs were dissected from each region in Petri-dishes containing sterile normal saline solution.Eggs were maintained at room temperature (25±3) ℃. The eggs were sampling to observe on 0 (control), 1, 3, 5, 7, 9, 11, 13 and 15 days post dissection for miracidial development and their viability were determined by staining with trypan blue. Eggs were laid by adult worms maintained in normal saline for 24 h at room temperature were used as positive control.
2.3. Snail sample preparation
Snails (Bithynia siamensis goniomphalos) were collected from natural water reservoirs in Khon Kaen Province, northeast Thailand. After collection, those snails were examined individually for larval trematode infection by the cercarial shedding method every alternate week for two months. Only non-infected snails were used for the studies.

Figure 1. Photograph of O. viverrini adult showing uterus was equally divided to 4 regions.
2.4. Observation of survival of O. viverrini miracidia
Survival and development of eggs from different regions of the uterus were investigated. The criteria for egg developmental stages were determined following a previous study[4].
The survival of eggs was determined by using 0.4% trypan blue staining. Dead eggs were permeable and could uptake the dye,therefore appearing blue. Dead miracidia or zygotes appeared as bubbles or vacuoles within degenerating eggs.
2.5. Incubation of O. viverrini eggs at various temperatures
From results of maturity development, all distal uterine eggs (region 4) could develop. They were maintained in plastic cups (250 eggs/cup; 5 replicates for each temperature) with 10 mL distilled water at -20 ℃, -15 ℃, 0 ℃, 15 ℃, 30 ℃, 45 ℃, 60 ℃, 75 ℃ and their viability determined at days 0 (control), 1, 3, 5 and 7.
2.6. Incubation of O. viverrini eggs at different salinities
Distal uterine eggs were maintained in plastic cups (50 eggs/cup;5 replicates for each concentration) with 10 mL of saline of the following concentrations: 0 (control), 25, 50, 75, 100, 125, 150 ppt.Viability was determined after 24 h exposure.
2.7. Incubation of O. viverrini eggs under ultraviolet radiation
Distal uterine eggs were maintained in a glass box with 2 mL distilled water and exposed to UV-A (17,758 lux, FL 15T8/BL, Thai Toshiba Lighting Co., Ltd., Pathumthani, Thailand), UV-B (9377.59 lux,TL20W/01 RS SLV/25, Philips Lighting Holding B.V., Pila, Poland) or UV-C (4,371.2 lux, UV F17T8, Koninklijke Philips N.V., Eindhoven,Holland). Survival of eggs was observed at day 0 (control), 1, 2, 5,7, 9, 14, 20 and 30. Five replicates were used, 50 eggs each, for each ultraviolet treatment.
2.8. Incubation of O. viverrini eggs in acid and base solutions
Distal uterine eggs (region 4) were maintained in a glass box with 2 mL of 0 M (control), 1 M, 0.1 M, 0.01 M and 0.001 M HCl. Five replicates were used, 50 eggs each, at each concentration of HCl and viability determined after 24 h exposure.
Distal uterine eggs were maintained in a glass box with 2 mL of 0 M(control), 1 M, 0.1 M, 0.01 M and 0.001 M NaOH. Five replicates were used, 50 eggs each, at each concentration of NaOH and viability determined after 24 h exposure.
2.9. Determining infection success of O. viverrini eggs in snails
Fifty O. viverrini eggs from each relevant experimental treatment were placed with individual snail (10 snails/group) in a plastic container containing 5 mL distilled water. The snails were stimulated by exposure to electric light at (28±3) ℃ in water baths for 24 h following a previous report[13]. This was done to encourage them to actively move to seek food and thus encounter the parasite eggs. After that, each snail was washed and reared in a new plastic container for 24 h. The infection success was determined by PCR using species-specific primers for O. viverrini. DNA was extracted from the whole soft body of the snail. The negative controls were snails that had not been exposed to O. viverrini eggs.
Total genomic DNA was extracted from soft tissue of snail individually using cetyltrimethyl ammonium bromide solution (CTAB) buffer (2% w/v CTAB, 1.4 M NaCl, 0.2% v/v β-mercaptoethanol, 20 mM EDTA, 100 mM Tris HCl, pH 8.0, 0.2 mg/mL proteinase K) and phenol-chloroform phase extraction. The soft tissue was blended by grinder with 600 μL of CTAB buffer, incubated at 55 ℃ for 3-4 h or overnight. The lysate was phase-extracted with phenol-chloroform (1:1) then centrifuged for 10 min at 12 000 g. Then the supernatant was transferred to a clean tube and extraction repeated with phenol/chloroform/isoamyl alcohol (25:24:1) and centrifuged at 12 000 g for 10 min twice.The DNA in the supernatant was precipitated in isopropanol for 20 min at 4 ℃ and centrifuged for 10 min at 12 000 g at 4 ℃. After that DNA was washed in 75% ethanol and absolute ethanol, centrifuged for 5 min at 12 000 g at 4 ℃. After drying the DNA pellet, it was dissolved in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0). Purity and concentration of DNA was measured using a spectrophotometer(NanoVue, GE Healthcare Ltd, Buckinghamshire, UK) at absorbances of 260 and 280 nm. The ratio values (OD260/OD280)from 1.7 to 2.0 indicated that DNA was adequately purified from contaminating protein. Extracted DNA was diluted to a working concentration of 10 ng/μL and kept at -20 ℃ until PCR assays were performed.
Specific primers were used to amplify the target of 330 base pairs(bp), OV-6F (5’-CTG AAT CTC TCG TTT GTT CA-3’) and OV-6R (5’-GTT CCA GGT GAG TCT CTC TA-3’)[14]. The PCR was performed using a DNA Thermal cycler (GeneAmp® PCR System 9700, Applied Biosystems, Foster City, CA), carried out with a total volume of 10 μL containing 0.04 μL TaKaRa Ex Taq 250 U, 1 μL dNTP mixture, 1 μL 10× Ex Taq buffer, 3 μL DNA sample, 3 μL distilled water, and 5 pmol of each primer. The PCR procedure was performed with cycling conditions as follows: initial denaturing 94 ℃10 min, denaturing 94 ℃ for 1 min, annealing 55 ℃ for 1 min, primer extension 72 ℃ for 1 min, and final denaturing 72 ℃ for 1 min for 35 cycles.
DNA from an uninfected snail was used as the negative control.DNA extracted from an O. viverrini adult was used as the positive control. After amplification, the PCR products were analyzed by 1.5% agarose gel electrophoresis in TBE buffer, pH 8.0. The PCR products on the agarose gel were stained with ethidium bromide(0.5 μg/mL) in 1× TBE buffer for 10 min, and then washed with distilled water for 15 min. Finally, the gel was visualized under UV illumination, and a picture captured using a Bio Doc-It™ Imaging System (Jena Analytik AG, Jena, Germany). One-hundred bp DNA ladder (Vivantis Technologies Sdn. Bhd. Revongen Co, Malaysia)was used as a standard marker for size determination. This ladder consists of 10 fragments between 100 and 1 000 bp in multiples of 100 bp.
2.10. Statistical analysis
Lethal concentrations or lethal effects of 50% and 95% were calculated using probit analysis. The differences between groups were tested by one-way ANOVA and pair-wise comparisons by Duncan’s multiple range test between 2 groups.
3. Results
3.1.Survival and development of O. viverrini eggs from 4 regions of uterus
Samples of eggs from all regions of the uterus were assessed for viability using trypan blue at initiation of cultivation (day 0, Figure 2). Viability was in the range 74.4%-99.2%. Viability decreased sharply in regions 1 and 2 on day 3 compared to the control group(laid eggs from adult worms maintained in normal saline) (P<0.05).All eggs from regions 1 and 2 were dead by day 5. But the viability of embryonated eggs in regions 3 and 4 decreased only gradually,not differing significantly from the control group (P>0.05). The viability of eggs in region 3 after 3 days of incubation was lower than in region 4 and in controls. The eggs in region 3, region 4 and the control group slightly decreased in viability till 15 days, as assessed by trypan blue staining (Figure 2).

Figure 2. Average percentages of viable eggs obtained from 4 regions of the uterus, incubated at room temperature for 0-15 days compared to laid eggs from adult worms.
3.2. Development of eggs from 4 uterine regions.
Immature eggs (type A), found only in region 1 (17.4%), contained small spherical granules, the vitellocytes and the zygote appeared as a grey-brown mass. The diameter of type B was less than that of type A. Eggs of type B were found only in regions 1 (53.8%)and 2 (11.2%). Eggs of types C and D exhibited an operculum and an abopercular knob. Type C was found in regions 1 (25.2%),2 (53%) and 3 (3.2%). In type D the embryo was multicellular and surrounded peripherally by vitellocytes. Type D was found in regions 1 (3.6%), 2 (29.6%) and 3 (9.6%). Type E had an eggshell with muskmelon-like appearance and a clearly evident operculum,shoulder, and knob, and was found in regions 2 (8.8%), 3 (58.4%)and 4 (4.6%). Eggs of type F were mature. The miracidium in these was clearly visible. Eggs of this type were more common in region 4 (95.4%) and some of them in regions 3 (28.8%) and 2 (1.8%) of the uterus.
3.3. Survival of miracidia in eggs at various temperatures
The highest percentage survival was in groups of eggs kept at 30 ℃(95.2%). All eggs died at -20 ℃ and at 75℃, according to trypan blue staining (Figure 3). No snails became infected from eggs kept at -20 ℃,-15 ℃ or 75 ℃, according to PCR analyses.
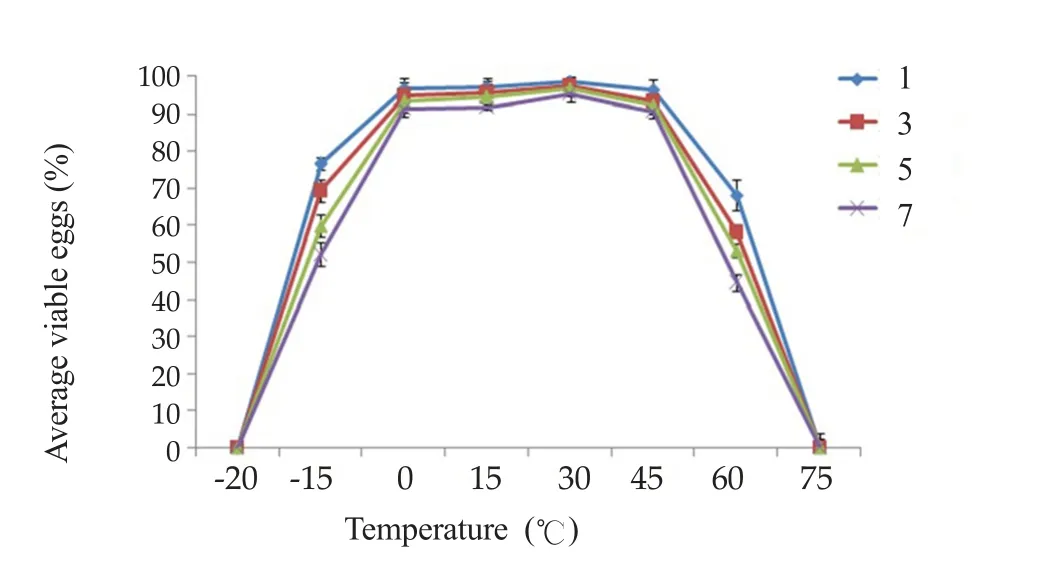
Figure 3. Average percentages of viable eggs incubated at various temperatures for 1-7 days.
3.4. Survival and development of miracidia in eggs in various salinities
All eggs incubated in salinity of 150 ppt died. Among the remaining groups, those incubated at 125 ppt had the lowest survival rate(1.8%). The highest survival was at 25 ppt (76.8%). No groups kept at salinities of 75 ppt or higher could infect snails. The calculated lethal concentrations (LC)50and LC95of salinity on miracidia were 45.43 and 120.09 ppt, respectively (Figure 4).
3.5. Survival and development of miracidia in eggs exposed to ultraviolet radiation
The eggs could survive in UV-A up to day 30. In UV-B all eggs died by day 14 and in UV-C the eggs had died by day 2 (Figure 5). Snails could not be infected beyond these same numbers of days.
The calculated period of loss infection success (LI)50and LI95values for miracidia in distal uterine eggs due to exposure to UV-A, UV-B and UV-C were 73 and 1 523 d; 8 and 20 d; 1 and 2 d, respectively.
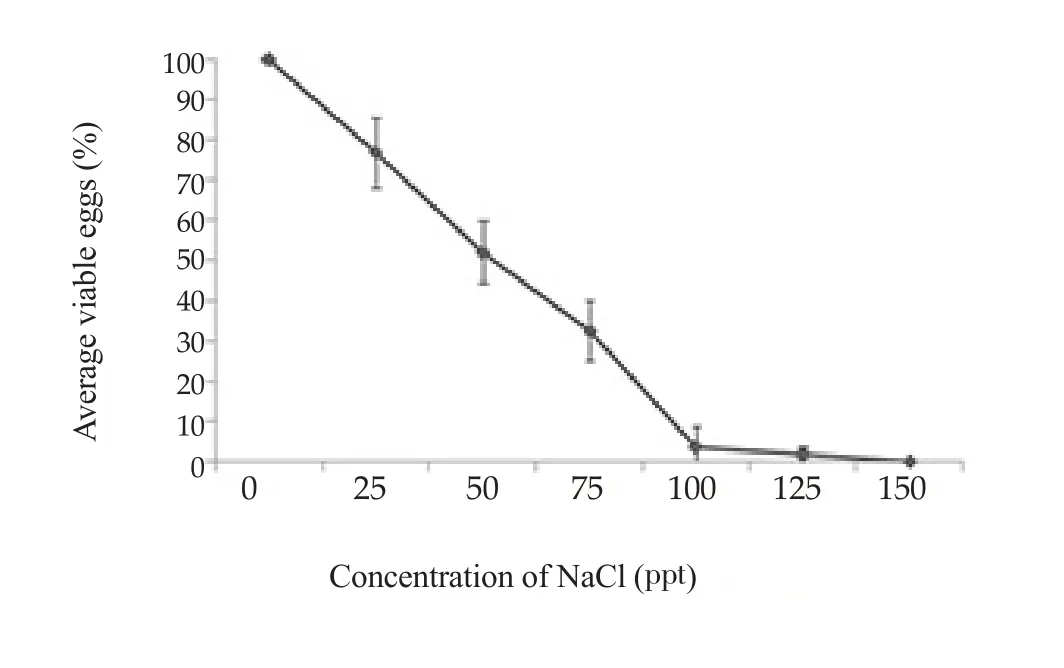
Figure 4. Average percentages of viable eggs after exposure to various salinity for 24 h.

Figure 5. Average percentages of viable eggs after exposure to ultraviolet radiation for 1-30 days.
3.6. Survival and development of eggs exposed to acid and base solutions
In acid, the eggs died in 1 M HCl, as determined by trypan blue staining and no infection was detected in snails by PCR analysis even in 0.1 M. In the basic solution, all eggs died at 1 M and 0.1 M NaOH. The concentrations are calculated by using Probit analysis. The loss infection success of 50% and 95% to snails which determined by PCR for HCl and NaOH were of 0.01 and 0.25 M;0.01 and 0.11 M, respectively.
4. Discussion
In this study the development and survival of O. viverrini eggs from each part of uterus have been described. Physicochemical factors including ranges of temperature, salinity, alkalinity, acidity and ultraviolet radiation have been tested.
Eggs gradually develop as they move along the uterus from the region of the ovary towards the genital pore. Eggs in the uterus proximal to the ovary were immature and could not complete maturation outside the uterus. A similar situation has been found in Clonorchis sinensis: eggs maintained in vitro could not mature and become infective[8].
Eggs incubated at temperatures between 0 ℃ and 60 ℃ could infect snails, with a peak at 30 ℃. The highest and lowest temperatures recorded in Thailand are 44.5 ℃ and -1.4 ℃, respectively(Meteorological Department of Thailand), well within the range of temperatures suitable for infection.
O. viverrini is prevalent in northeast Thailand, an area of surface salt with high salinity of water bodies. We found that eggs could remain viable and infect snails at salinities as high as 125 ppt and 75 ppt,respectively. The host snail in northeastern Thailand, Bithynia siamensis goniomphalos, exclusively occurs in areas with a salinity range of 0.05-22.11 ppt[15]. So eggs of O. viverrini can survive in the full range of salinities in which their hosts occur.
The ability of eggs to survive in rather high concentrations of HCl and NaOH shows that they have a high tolerance for some kinds of chemicals.
The eggs could remain viable in UV-A but died in UV-B by day 14 and in UV–C by day 2. UV-A has a relatively long wave-length at 315–400 nm and comprises 95% of the UV radiation reaching the Earth’s surface. But UV-A is less intense than UV-B and UV-C.UV-B (wavelength 280–315 nm) is very biologically active but cannot penetrate because it is easily absorbed. UV-C has a light spectrum in the range 100–280 nm. This is the most damaging type of UV radiation. However, it is completely filtered by the atmosphere and does not reach the earth’s surface. But in recent years, ozone in the atmosphere has decreased[16]. Ozone blocks out harmful ultraviolet radiation from the sun and its decline will mean more UV reaching the Earth’s surface.
Global warming is causing changes to the Earth’s climate: glaciers are melting, sea levels are rising, forests are dying, and the ozone in atmosphere is decreasing. While it may be assumed that survival of eggs of O. viverrini which will be affected by these changes, are shown to display a high tolerance to environmental conditions,because they may need to remain viable for long periods until encountered by a snail host.
Conflict of interest statement
The authors declare that there is no conflict of interest.
 Asian Pacific Journal of Tropical Biomedicine2018年9期
Asian Pacific Journal of Tropical Biomedicine2018年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Spatial distribution of sand flies (Diptera: Psychodidae; Larroussius group), the vectors of visceral leishmaniasis in Northwest of Iran
- Identification of commonly regulated protein targets and molecular pathways in PC-3 and DU145 androgen-independent human prostate cancer cells treated with the curcumin analogue 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one
- Add-on therapy of herbal formulation rich in standardized fenugreek seed extract in type 2 diabetes mellitus patients with insulin therapy: An efficacy and safety study
- Anticancer activity of crude acetone and water extracts of Tulbaghia violacea on human oral cancer cells
- Conocarpus erectus L., a plant with a high content of structural sugars, ions and phenolic compounds, shows antioxidant and antimicrobial properties promoted by different organic fractions
