CO2鼓泡法可控制备单层中空CaCO3微球:染料负载及细胞生物成像
刘 江 沙 峰,2 杨廷玉,2 马 良,2 张建斌*,,2,3
(1内蒙古工业大学化工学院,呼和浩特 010051)
(2内蒙古自治区CO2捕集与资源化工程研究中心,呼和浩特 010051)
(3中国矿业大学,江苏省煤基CO2捕集与地质储存重点实验室,徐州 221008)
Nowadays,CaCO3with nanometer-to-micrometer dimensions is today one of the absolutely most addressed materials in the view of its extensive applications[1].Under ambient condition,it exists in mainly three polymorphs,including calcite,aragonite,and vaterite,in decreasing order of thermodynamic stability.Among them,vaterite species have been intensively studied as templates or carriers due to their good biocompatibility,and easy production and removal under mild conditions[2-3].Simultaneously,hollow structural CaCO3particles have recently spurred great interests because of their many beneficial properties such ashigh porosity,largesurfacearea,good biocompatibility,and their potential applications in controllable drug delivery,artificial cells,lightweight fillers,catalysis,and confined reactors[4-8].As a result,it is essential to study the design and synthesis of functional nano-meter and micro-meter hollow structural vaterite materials.
There are two mainly methods,as far as we know,including biomimetic method and CO2bubbling method,to synthetize specific CaCO3products.The biomimetic method ties to simulate nature′s ability to regulate the size,shape and phase of CaCO3in the presence of various organic templates and additives[9-14].Surfactants[15-20], synthetic polymers[21-26], biomolecules[27-28],polymer-surfactant mixtures[29-32]and amino acids[33]have also been used as biomimetic agents for the controlled growth of CaCO3crystals to achieve different morphologies,polymorphs and sizes.Specially,porous or hollow CaCO3spheres in micrometer scale have been obtained by using triblock copolymer[34],soluble starch[35],polymer[36],phytic acid[37],double hydrophilic block copolymer(DHBC)[38-40],DHBC/surfactant system[41],and polymer/surfactant system[42-44].Although biomimetic synthesis is easy to implement as it basically involves the mixing of CO32-and Ca2+solutions,various additives must be required to make particularhollow CaCO3spheres,making against industrialscale production for production cost.Another method,namely CO2bubbling method,is simple,effective,and most used in industrial process for the production of nano-sized CaCO3particles[45-50].Despite existing research had further improved the efficiency and yield CO2bubbling method,the polymorphisms are mainly cubic or rhombohedral calcite[51-52],even in the presence of additives[53-54]or elevated pressures and moderate temperature CO2[55].Yet,Chuajiw et al.[56]found that the vaterite and aragoniteCaCO3productswereprecipitated from Ca(OH)2suspensions and CO2when amino acids with formula H2N(CH2)n-1COOH were added (n=4 and 6),respectively.Recently,other amino acid such as aspartic acid(Asp),glycine(Gly),alanine(Ala),proline(Pro),lysine(Lys),leucine(Leu),and glutamate(Glu)were used as directing agents for the crystallization of CaCO3precipitates,and the crystalline products presented as different morphologies such as rhombohedral,small needle-like efflorescent bundles,spiraling,dumbbell-like,spindly,and monodisperse spherical[57-65].However,the construction of hollow sphere CaCO3microspheres using amino acid by the CO2bubbling method is still a challenge.
To the best of our knowledge,the CO2bubbling method was the most commonly used in the industrial production of CaCO3,mainly affording cubic or rhombohedral calcite rather than valuable hollow sphere vaterite.Here,the controllable fabrication of hollow sphere vaterite microspheresusing L-enantiomers methionine (L-Met)was firstly reported based on the above-mentioned methods.In this system,only by adjusting the L-Met adding amounts,nearly 100%hollow sphere vaterite was obtained at room temperature and atmospheric pressure.On the other hand,RhB has been demonstrated to exhibit an aggregationenhanced emission while aggregating at the surfaces ofnanoparticlesorbeing confined in nanoscale domains[66-68].The hollow sphere vaterite incorporated some L-Met possesses functionalized surfaces and may present excellent biocompatibility[69-70]. Taking into account the above mentioned advantages,a luminescent composite material(RhB@hollow-CaCO3)has also been fabricated and used as a contrast agent for optical imaging in cell.Notably,the resulting products incorporated some L-Met,representing added applicationsforloadingdyesand even drugdelivery.Furthermore,this finding on good biocompatibility of A-549 lung cancer cells(A-549 LCCs)and HO8910 ovarian cancer cells(HO8910 OCCs)extended to the otherprocessesofdrug delivery and cellbioimaging[71-74].
1 Experimental
1.1 Materials
All the starting analytical grade reagents were acquired from commercial sources and utilized without further purification.L-Met was purchased from Aladdin(Shanghai)Chemical Reagent Co.,Ltd.Ca(OH)2and DMSO were purchased from Sinopharm Chemical Reagent Co.,Ltd.Compressed CO2(99.999%,V/V)was purchased from the Standard Things Center(China).Doubly distilled water with conductivity lower than 0.1 mS·cm-1(25℃)was used.A-549 lung cancer cells(A-549 LCCs)and HO8910 ovarian cancer cells(HO8910 OCCs)were purchased from American type culture collection(ATCC)and reserved at liquid N2condition.
1.2 Preparation of hollow sphere CaCO3 microspheres
The crystallization of CaCO3was carried out by CO2bubbling into 100 mL Ca(OH)2solution,which already mixed with appropriate amounts of L-Met at room temperature.In a typical experiment,1.0 g LMet was added into 100 mL Ca(OH)2-saturated suspensions,which was loaded in 250 mL flask with three necks.After dissolving via ultrasonic agitation,CO2with gas flow velocity of 50 mL·min-1was bubbled into the mixed solution at room temperature for 30 min.Then,the as-obtained precipitate was separated with natural filtration,washed with deionized water,and dried at 120℃for 12 h.Additionally,L-Met concentration,CO2gas flow velocity,and reaction temperature were further systematically investigated.
1.3 Preparation of RhB@hollow-CaCO3
By soaking of hollow sphere CaCO3microspheres(Sample-F)(50 mg)in an aqueous solution of rhodamine B (RhB,100 mg·L-1,10 mL)for 24 h at room temperature,the dye was well absorbed by hollow sphere CaCO3microspheres,as appeared of the supernatant was nearly complete fading.The mixture was centrifuged,the solid sample was washed with deionized water several times and dried.According to the weight calculating before and after dye loading,the dye-uptake amount was about 1.8%(w/w).
1.4 Characterization of CaCO3microspheres
The size and morphology ofsampleswere investigated using a Quanta FEG 650 scanning electron microscope(SEM)at an accelerating voltage of 20 kV,and a FEI Tecnai G2F20 high-resolution transmission electron microscope (HRTEM)at an accelerating voltage of 200 kV.Energy dispersive spectrometer(EDS)elemental mapping analyses were carried out on the SEM equipped with an EDX apparatus.Powder X-ray diffraction (PXRD)patterns of the samples were collected on a PANalytical Empyrean sharp shadow system X-ray diffractometer equipped with Cu Kα radiation(λ=0.154 059 8 nm)over the 2θ range of 5°~80°operated at working voltage of 40 kV and working current of 40 mA.Fourier transform infrared spectroscopy (FTIR)were recorded in the 4 000~400 cm-1region using two KBr pellets on a NEXUS-670 Fourier transform infrared spectrophotometer at room temperature.Fluorescence spectra for the compounds were collected on a Hitachi F-7000 FL Spectrophotometer equipped with a 150 W xenon lamp as the excitation source at room temperature.The pore size distributions of samples were determined by TristarⅡ3020 automated gas adsorption analyzerfollowing the Brunauer-Emmett-Teller (BET)method of nitrogen adsorption-desorption at 77 K.The N2adsorptiondesorption isotherms of the samples were recorded at 77 K with a Micromeritics ASAP 2020 after degassing in vacuum at 200℃for 4 h.The BET surface areas were calculated according to a multipoint BET method.Moreover,the pore size distributions were determined from desorption branch of nitrogen isotherms by the Barret-Joyner-Halenda(BJH)method.
1.5 In vitro cytotoxicity test
In vitro cytotoxicity tests of A-549 lung cancer cells(A-549 LCCs)and HO8910 ovarian cancer cells(HO8910 OCCs)were detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diprenyltetrazolium bromide (MTT)method.Firstly,A-549 LCCs and HO8910 OCCs were respectively inoculated into 96-well plates at cell density of 8000 per well,and cultured 24 h under the condition of 5%(V/V)CO2at 37℃.And the culture medium of two kinds of cells was continued culturing 24 h under the same culture conditions after adding RhB@hollow-CaCO3with 0,25,50,100,and 200 μg·mL-1concentration gradient in culture medium.Then,removing the culture medium of two kinds of cells,adding 20 μL MTT solution into per individual well,and culturing 4 h in incubator.Lastly,the absorbance values(OD)of individual well were recorded at 630 nm by a microplate reader after adding 150 μL dimethyl sulfoxide (DMSO)and oscillating 10 min.And the cell survival rate was calculated according to the Eq.(1):
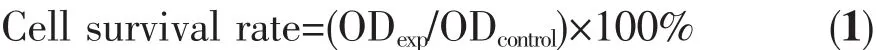
The inhibition rate was calculated using the Eq.(2)[75]:
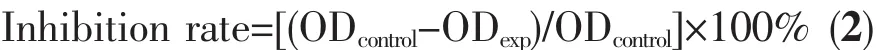
where ODcontrolis the OD value of control group and ODexpis the OD value of experimental group.
1.6 In vitro fluorescence imaging test
A-549 LCCs and HO8910 OCCs were respectively inoculated into 6-well plates,and cultured overnight.After adding RhB@hollow-CaCO3composite,in which the concentration in culture medium was set at 0.2 mg·mL-1,two kinds of culture medium were continued culturing for 4 h,respectively.Then,two kinds of culture medium were washed by phosphoric acid buffer for three times,the cell imaging capacity was confirmed using ahigh contentimaging system(PerkinElmer Operetta),and the fluorescence imaging were recorded at 375 nm excitation wavelengths by confocal laser scanning microscopy(CLSM).
2 Results and discussion
2.1 Incorporation of L-Met in CaCO3crystals
CaCO3crystals were prepared in the presence of L-Met using the CO2bubbling method,and the morphology of crystals was characterized by SEM and TEM (Fig.1 and 5).As shown in Fig.1,the morphology of CaCO3crystals was various under different L-Met adding amounts,and represented as rather uniformly shaped crystals with two primary kinds of morphology.Fig.1C showed a specific cube-like shape obtained in a mixed solution of 0.4 g L-Met and 100 mL Ca(OH)2-saturated suspensions,while Fig.1D~1H presented a representative microspheres when L-Met adding amounts were greater than 0.6 g.Interestingly,the cube-like particles were solid,while the microspheres were hollow sphere (Fig.1C and 1F,respectively).At the same time,the size of the cube-like particles was approximately 4.8 μm×4.6 μm,while the diameters of microspheres were in the range of 1.8~4.1 μm.Surprisingly,there were irregular CaCO3crystals without L-Met(Fig.1A).This illustrated that L-Met could polish the crystallization of CaCO3and regulate the morphology from“irregular-to-cube-to-hollow sphere microsphere”.

Fig.1 SEM images of CaCO3obtained at various adding amounts of L-Met
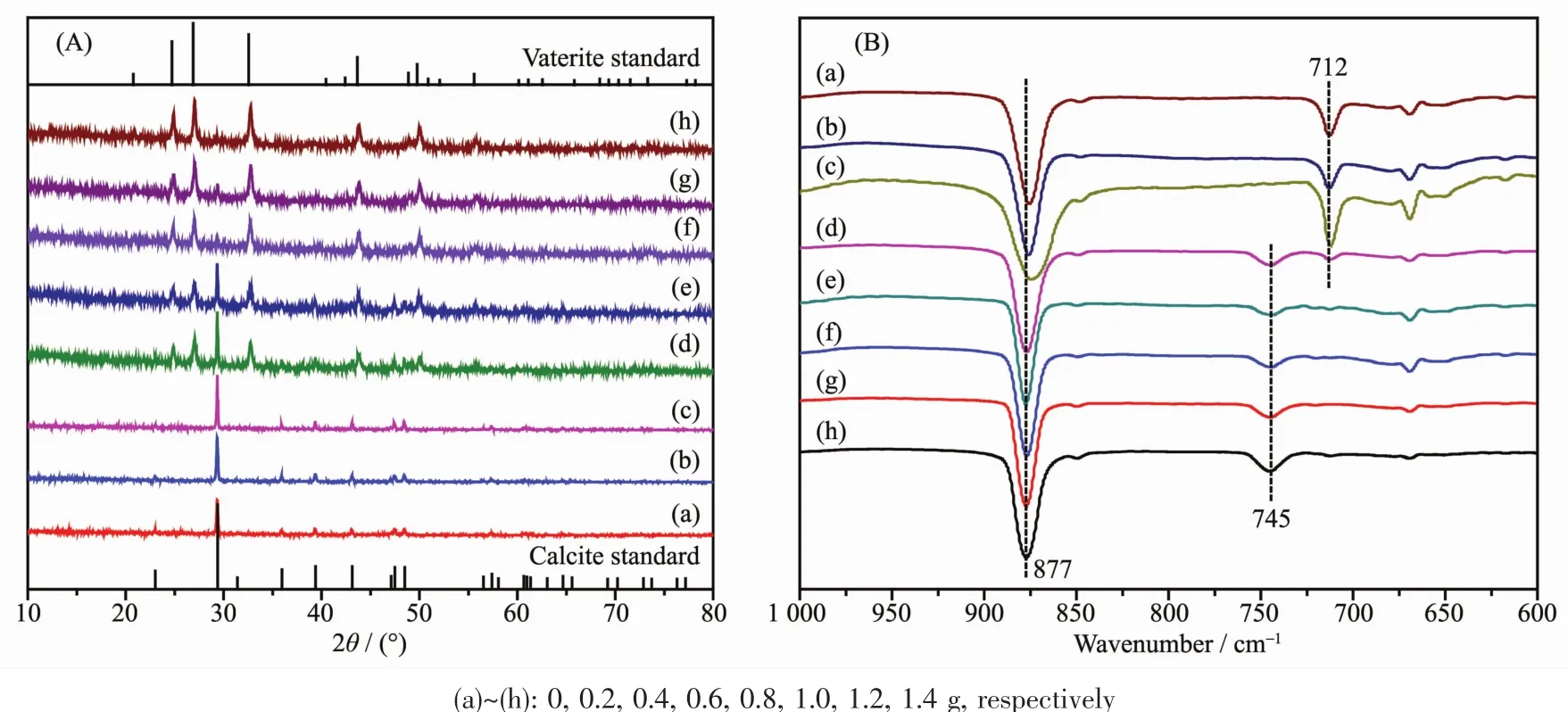
Fig.2 XRD patterns(A)and FTIR spectra(B)of CaCO3obtained at various adding amounts of L-Met
Polymorphs of CaCO3crystals were examined carefully using XRD and FTIR spectral techniques,the patternsand spectra were shown in Fig.2.Generally,CaCO3mainly contains three anhydrous polymorphs (calcite,aragonite and vaterite),which depended on the processing conditions.Calcite is the most stable form and vaterite is the least stable one in thermodynamics[76].However,vaterite is an ideal candidate as a template for protein particles because it is decomposable at relatively mild conditions[77].Here,an exciting result that L-Met could modify CaCO3crystals polymorphs changing from calcite to vaterite was presented in Fig.2A.From Fig.2A,all peaks of the XRD pattern were consistent with a pure calcite crystal structure in samples A~C,and mixed calcite and vaterite in samples D~H.The relative percentage of calcite and vaterite can be calculated from their characteristic XRD peak intensities according to the following equations[78].
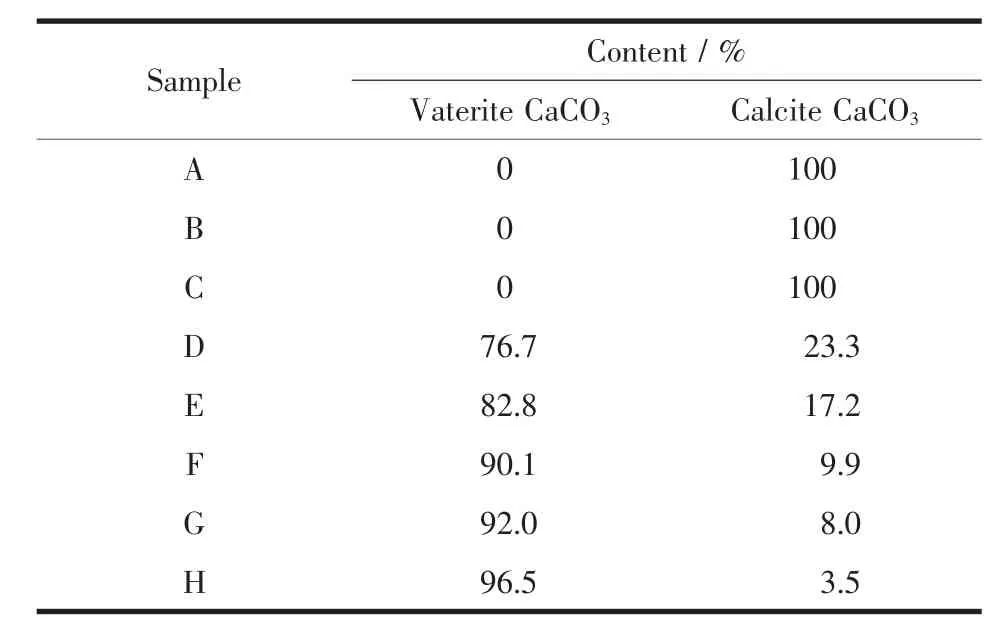
Table 1 Semi-quantitative analysis for different polymorphs of CaCO3samples

where XVand XCare the molar fraction of vaterite and calcite,respectively.Peak intensities of(110)plane(I(110))and (104)plane(I(104))represent vaterite and calcite,respectively.According to this semi-quantitative analysis,with the increasing L-Met adding amounts,the relative percentage of calcite decreased while vaterite gradually increased (Table 1).From Fig.2B,the absorption peaks at 877 and 712 cm-1in samples A~C were characteristics of pure calcite(PDF#05-0586)[9],and the peaks at 877 and 745 cm-1(samples D~H)were characteristic of pure vaterite(PDF#72-0506)[79].
Considering that pH value of solution could influence both the degreeofprotonation ofthe carboxylic groups and the CaCO3supersaturation in the solution,the varieties in pH values of the whole reaction process were recorded,as shown in Fig.3.Before CO2was bubbled into the mixed solution,the pH values of the solution decreased from 12.74 to 8.92 with the increasing L-Met adding amounts.At low L-Met adding amounts(0,0.2 and 0.4 g),with the introducing of CO2,the pH value of the solution decreased gradually within the first 10 min;then it decreased significantly between 10 and 16 min,and decreased slowly at the last 14 min (Fig.3A~3C).When L-Met adding amounts changed from 0.4 to 1.4 g,the change trend of solution pH was similar to low L-Met adding amounts.After reaction,all the pH value of solution were in the range of 6.26~6.61.The variation of pH values with L-Met adding amounts is related to the ionization degree of L-Met since L-Met contained carboxylic acid groups.More L-Met adding amounts,more activating carboxylic acid groups to neutralize the alkaline Ca(OH)2-saturated suspensions.However,the controlled crystal growth depends on the degree of interaction between the carboxylic acid groups in the additives and the Ca2+ions in solution.Hence,the more the carboxylic groups get ionized,the more sites are available for binding with Ca2+ions which therefore inhibits crystal growth in all directions.And the degree of ionization of the carboxylic groups increases with the increasing pH value[80]but so does the solution supersaturation,due to the hydrocarbonate/carbonate buffer[23].At high solution pH value,the high solution supersaturation overwhelms the degree of ionization of the carboxylic acid groups,which leads to uncontrolled crystal growth and the occurrence of largersized,irregularshaped particles.On the contrary,the ionization degree of the carboxylic acid groups,comparing supersaturation,made dominant in low solution pH value,which promoted the formation of well-defined superstructures.This was why irregular calcite were obtained in low L-Met adding amounts(Fig.1A and 1B)while hollow sphere microsphere vaterite (Fig.1D~1H)were prepared in high L-Met adding amounts.
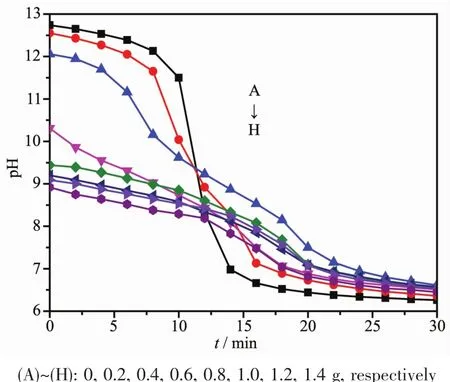
Fig.3 Changes of pH values with various L-Met adding amounts
Hollow CaCO3microspheres with nanometer-tomicrometerscalehaveattracted moreand more attentions owing to their unique physical and chemical properties,and their potential applications in controlling release systems such as drugs,cosmetics,dyes,and inks[7-8].For this reason,the hollow sphere CaCO3microsphere obtained in 1.0 g L-Met adding amount,as a representative,were further characterized by EDS,TEM and N2adsorption-desorption isotherm.As shown in Fig.4,as-obtained hollow sphere CaCO3microsphere mainly contained Ca,O,C,N,and S elements(O 48.08%,Ca 31.39%,C 10.72%,N 2.97%,and S 6.84%).This implied that L-Met participated in the crystallization of hollow sphere CaCO3microspheres and already incorporated into hollow sphere CaCO3microspheres.As hollow sphere CaCO3microspheres had potential applications in loading guest molecules,the hollow sphere vaterite incorporating LMet could become an ideal candidate as a dye carrier for cell bioimaging.TEM images indicated that hollow sphere vaterite microspheres with rough surface were aggregated by particulates,which were less than 200 nm (Fig.5A).It could also be seen that the interior of hollow microsphere were relative bright under the electron beam,suggesting lower density of substance(Fig.5A).Additionally,the lattice spacings of 0.273 5 and 0.221 9 nm were corresponded to the(102)plane of vaterite and (103)plane of calcite,respectively(Fig.5B).
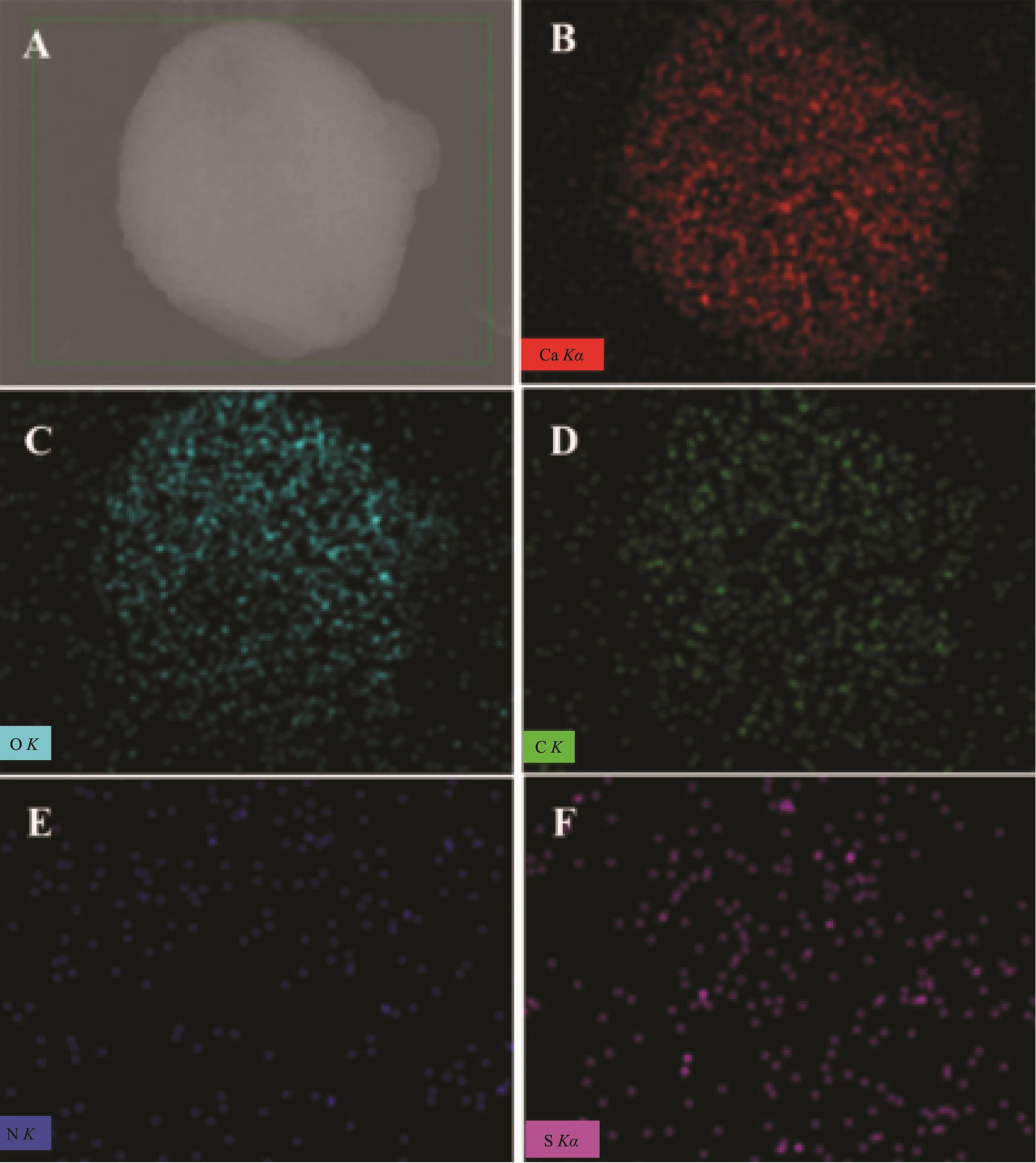
Fig.4 EDS elemental mapping images of CaCO3sample obtained in 1.0 g L-Met adding amount

Fig.5 TEM images of CaCO3sample obtained in 1.0 g L-Met adding amount
N2adsorption-desorption isotherm results were presented in Fig.6.Without L-Met adding,the irregular CaCO3crystals had a 39 m2·g-1specific surface area,and the average pore size was in the range of 2.1~6.8 nm,mainly distributed at 4.4 nm (Fig.6A).When LMet adding amount was 1 g,the hollow sphere CaCO3microspheres exhibited specific surface area of 51 m2·g-1,and the average pore size was in the range of 3.6~8.3 nm,mainly distributed at 5.8 nm(Fig.6B).The hollow structures of CaCO3microspheres with greater average pore size,which were obtained in 1 g L-Met adding amount,were generated by particulate aggregating under the help of L-Met.This kind of hollow sphere CaCO3microspheres not only provides as ideal carriers for loading guest molecules but also suggests using for cell bio-imaging agent or controlling release systems in organisms.

Fig.6 N2adsorption-desorption isotherm and pore size distribution of as-prepared CaCO3samples:(A)0 g L-Met adding amount and(B)1 g L-Met adding amount
2.2 Effect of CO2flow rate
Fig.7 presented the SEM images of CaCO3microspheres obtained at different CO2flow rates.Regardless of the varying CO2flow rates,the CaCO3microspheres with rough surface were identical in morphology,but the microspheres diameter increased with the increasing CO2flow rates.XRD patterns,as shown in Fig.8a,showed that microspheres were mixture of vaterite and calcite,and the relative percentage of vaterite decreased while calcite increased according to the semi-quantitative analysis (Table 2).The result was further demonstrated by FTIR spectra(Fig.8b),in which all samples had three characteristic peaks at 875,746 and 711 cm-1indicating that the mixture of calcite and vaterite was formed[81].Meanwhile,the
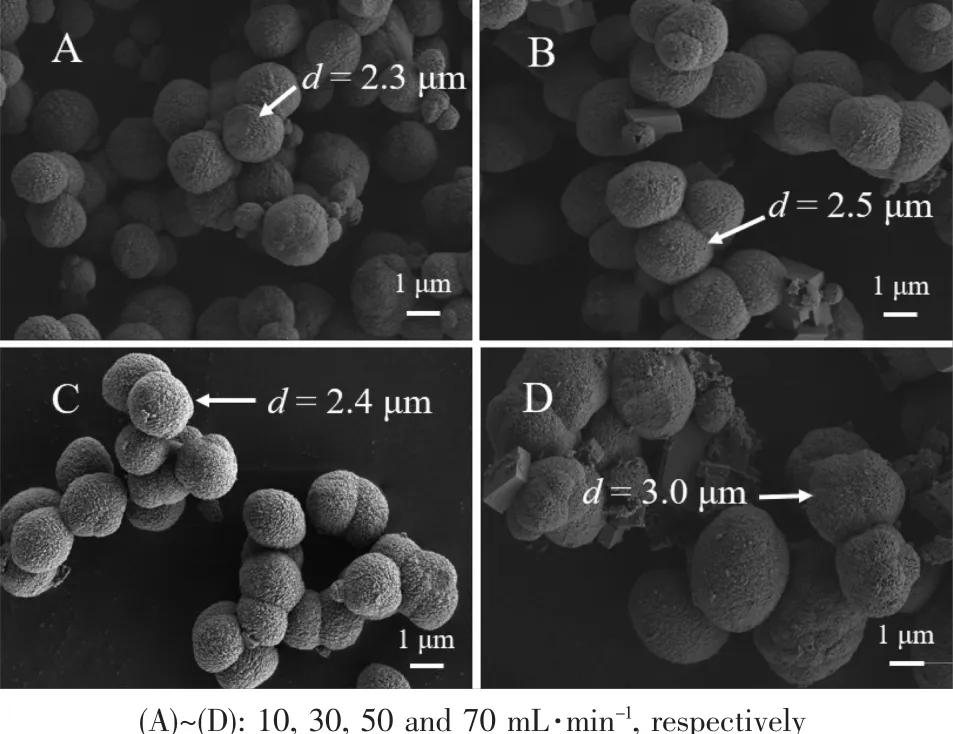
Fig.7 SEM images of CaCO3obtained from reaction systems with 1.0 g L-Met under different CO2 flow rates

Fig.8 XRD patterns(A)and FTIR spectra(B)of CaCO3obtained from reaction systems with 1.0 g L-Met under different CO2flow rates
change ofpH valuesofreaction system under different CO2flow rates were recorded in Fig.9.With the increasing CO2flow rate,the solution pH value decreased markedly at the end of reaction,which could be attributed to the CO2introducing amount and mass transfer increasing at the same reaction time.
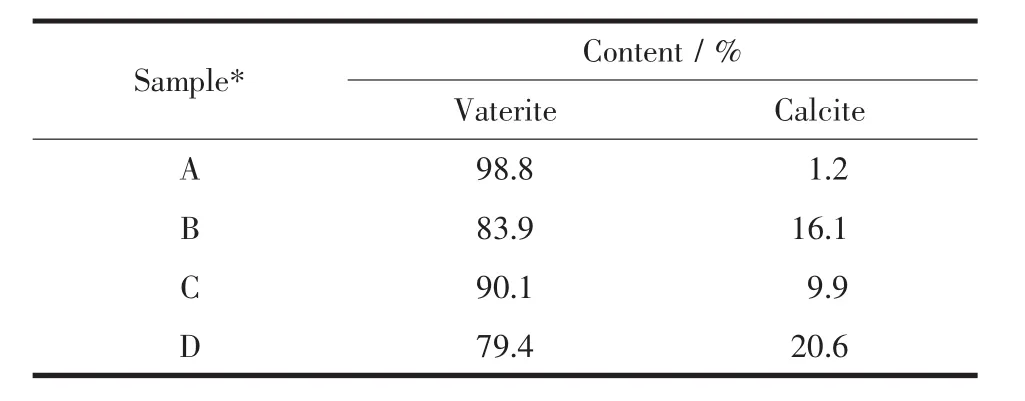
Table 2 Semi-quantitative analysis for different crystal forms of CaCO3obtained at 1.0 g L-Met under different CO2flow rates
As shown in Fig.10,the morphology of CaCO3crystals was affected by reaction temperature.Although hollow sphere CaCO3microspheres which were assembled from micro-meter/nano-meter scale CaCO3crystal particles were obtained at all experimental temperatures,the surface was getting more and more rough.Simultaneously,the size of hollow sphere microspheres also grew larger when the experimental temperature increased gradually.Interestingly,new morphologies of symmetrical slices appeared at 45 and 55℃(Fig.10C and 10D).At the same time,XRD patterns and corresponding semi-quantitative analysis showed that as-obtained CaCO3contained vaterite and calcite,and content of vaterite increased while that of calcite decreased gradually with the increasing temperatures(Fig.11a and Table 3).Furthermore,the vibrational bands at 876 and 746 cm-1,together with 876 and 712 cm-1in FTIR spectra (Fig.11b),were characteristic of vaterite and calcite,respectively[82-83].It could be seen that the pH value changing trend of reaction system almost similar no matter the increasing temperature(Fig.12).

Fig.9 Change of pH values in reaction system with 1.0 g L-Met under different CO2flow rates

Fig.10 SEM images of CaCO3obtained from reaction systems with 1.0 g L-Met under different temperatures
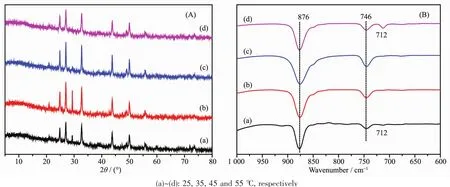
Fig.11 XRD patterns and FTIR spectra of CaCO3obtained at 1.0 g L-Met under different temperatures
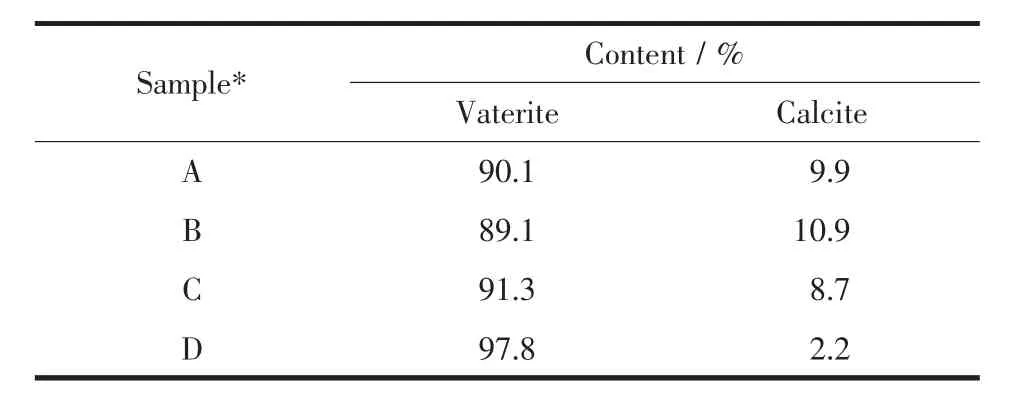
Table 3 Semi-quantitative analysis for different crystal forms of CaCO3obtained at 1.0 g L-Met under different temperatures
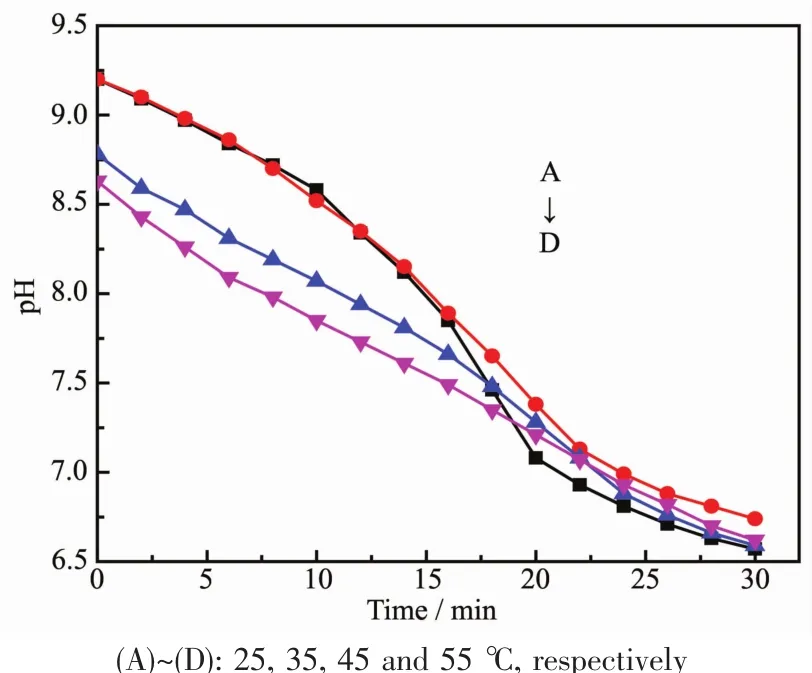
Fig.12 Change of pH values in reaction system with 1.0 g L-Met under different temperatures
2.3 Fluorescence behavior and EDS results of RhB@hollow-CaCO3
Due to existing the non-radiative energy transfer process which would quench the photoluminescence,a lot of organic dyes display rather weak luminescence emission in the solid state.In order to isolation of the dye molecules and restrain the non-radiative energy transfer process, we encapsulated/loaded RhB molecules into the hollow sphere of CaCO3microspheres.The photoluminescence behaviors of RhB and RhB@hollow-CaCO3were examined at room temperature in the solid state.As shown in Fig.13,the dye encapsulated/loaded composite RhB@hollow-CaCO3exhibits strong emissions at 586.4 nm upon excitation at 375 nm,whereas RhB displays rather weak emission at 642.8 nm.Compared with RhB,the emission peak of RhB@hollow-CaCO3exhibits obvious blue shift,which may be due to the interaction between the L-Met on the surface of microcrystal CaCO3and the RhB.This result demonstrates that the hollow sphere of CaCO3microspheres can be used as ideal carriers for loading guest RhB molecules and provided as a potential cell bio-imaging agent.
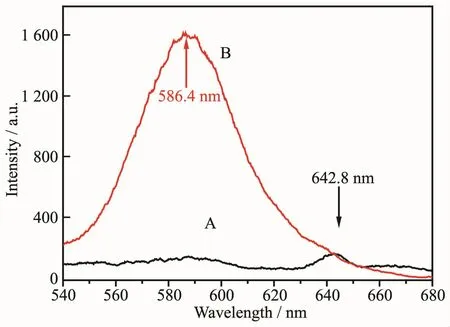
Fig.13 Emission spectra of RhB(A)and RhB@hollow-CaCO3(B)excited at 375 nm in the solid state
To further confirm this conclusion,EDS elemental mapping was conducted and the result is shown in Fig.14.Apparently,the element Ca is distributed uniformly in the framework of hollow-CaCO3and RhB@hollow-CaCO3.On account of the existence of L-Met,there are some N elements in the framework of hollow-CaCO3,whereas the N element in the framework of RhB@hollow-CaCO3is much more than that in hollow-CaCO3due to the loading of RhB.Furthermore,the N element is mainly distributed in the core of RhB@hollow-CaCO3,indicating that RhB is loaded in the cavity of hollow-CaCO3.
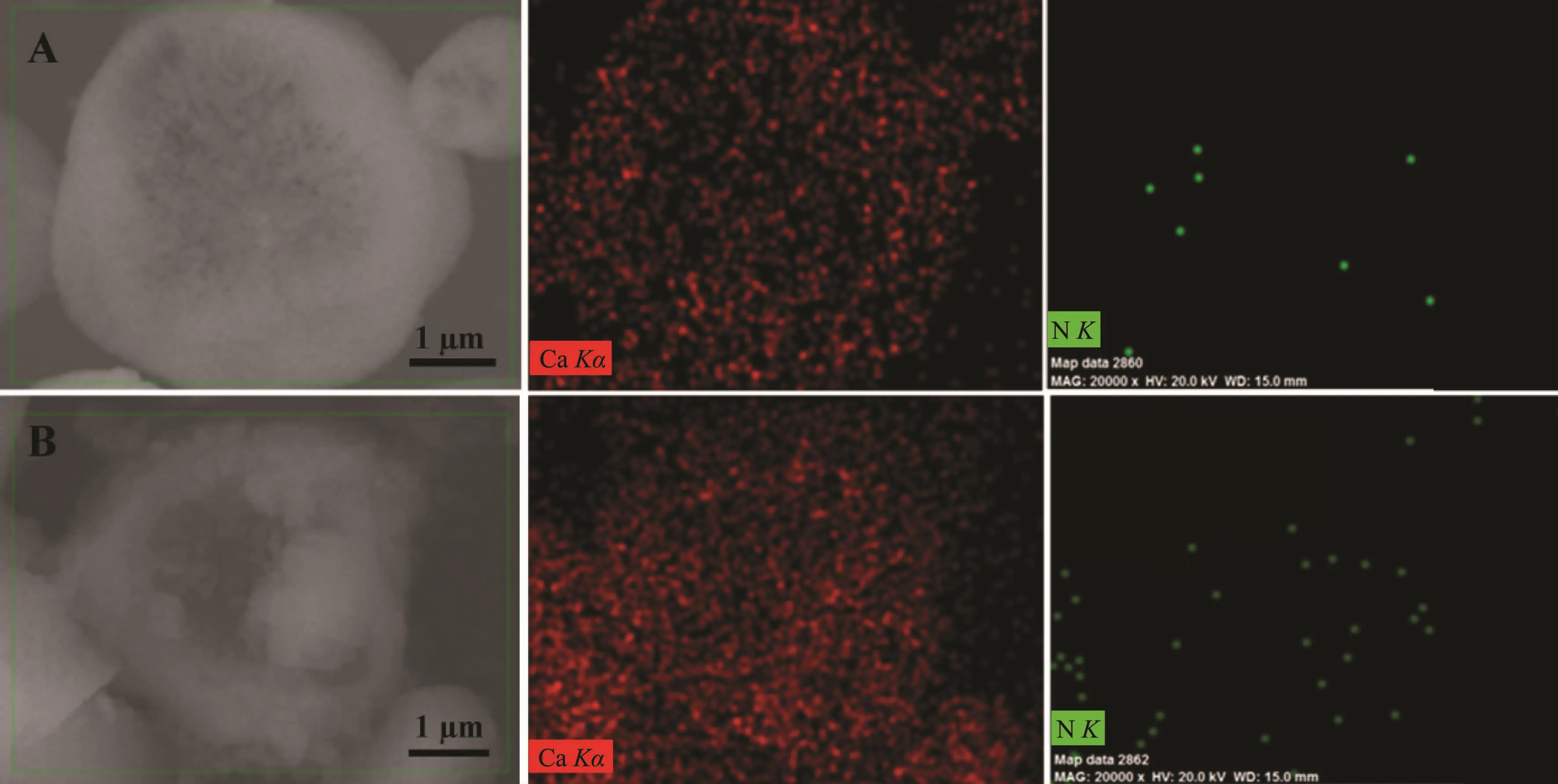
Fig.14 EDS elemental mapping results of Ca,N in hollow-CaCO3(A)and RhB@hollow-CaCO3(B)
2.4 Cytotoxicity of RhB@hollow-CaCO3

Fig.15 Viabilities of A549 cancer cells(A)and HO8910 cancer cells(B)in the presence of RhB@hollow-CaCO3composite as assayed by MTT
In the current study,the in vitro cytotoxic effects of RhB@hollow-CaCO3on A-549 LCCs and HO8910 OCCs were evaluated by MTT colorimetric assay and the results were shown in Fig.15.It could be seen that the cell viability of A-549 LCCs decreased slightly(from 100%to 96.73%)after 24 h of treatment with various concentrations of RhB@hollow-CaCO3(from 0 to 200 μg·mL-1).Specially,cell viability of A-549 LCCs was up to 96.73%when RhB@hollow-CaCO3concentration was set as 200 μg·mL-1.For HO8910 OCCs,the cell viability,comparing with the fresh HO8910 OCCs,almost invariable regardless of the changingRhB@hollow-CaCO3concentration.These results revealed that RhB@hollow-CaCO3has good biocompatibility to the A-549 LCCs and HO8910 OCCs,and would be further applied in the organism.
2.5 In vitro fluorescence imaging observation
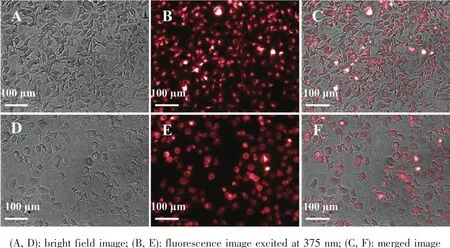
Fig.16 CLSM images of A-549 LCCs(A~C)and HO8910 OCCs(D~F)co-culture with RhB@hollow-CaCO3for 4 h
In view of RhB@hollow-CaCO3has strong fluorescent emission and good biocompatibility to the A-549 LCCs and HO8910 OCCs,the application of RhB@hollow-CaCO3as cell bio-imaging agent was evaluated using a high content imaging system.After 4 h of coculture,the red luminescence could be observed in A-549 LCCs and HO8910 OCCs (Fig.16),which was appeared in dot-like shape.This indicated that RhB@hollow-CaCO3had already internalized into A-549 LCCs and HO8910 OCCs.Therefore,it could be concluded that RhB@hollow-CaCO3have the potential as cell bio-imaging agent on the basis of their good biocompatibility,easy cell uptake,stable and strong luminescence.
3 Conclusions
In the currentwork,hollow sphere CaCO3microspheres have been synthesized from Ca(OH)2-Met(L)-CO2reaction system based on the CO2bubbling method.Nearly 100% hollow sphere vaterite was obtained by only adjusting the L-Met adding amounts.As-obtained hollow sphere vaterite incorporating part L-Met can load RhB molecules and a new luminescence composite RhB@hollow-CaCO3was fabricated.This composite exhibits good biocompatibility to A-549 LCCs and HO8910 OCCs,and emits a strong fluorescence.The improvementofphysicaland chemical properties of hollow sphere vaterite microspheres have great potential to be used in the field of cell bio-imaging,enzyme biocatalysis,amino acids supplementation as well as drug delivery.
Acknowledgements:We are grateful for the financial support provided by the NSFC of China (Grant No.21666027),the Program for Grassland Excellent Talents of Inner Mongolia Autonomous Region,the Natural Science Foundation of Inner Mongolia Autonomous Region(Grant No.2016JQ02),Key Laboratory of Coal-based CO2Capture and Geological Storage(Jiangsu Province,China University of Mining and Technology,Grant No.2016A06),the Inner Mongolia Science and Technology Key Projects,and Undergraduate Innovation Fund of Inner Mongolia University of Technology(2016).

