Meta-analysis of clinical efficacy and safety of the treatment of impaired glucose tolerance by Tianqijiangtang Capsule
Cheng-Ya Wu,Ke Li
1Beijing Shunyi Hospital of Traditional Chinese Medicine Hospital,Shunyi District,Beijing,China.
Abstract Objective:To evaluate the clinical efficacy and safety of Tianqijiangtang Capsule(TG)during the treatment of impaired glucose tolerance(IGT).Methods:Using Chinese words of“impaired glucose tolerance,abnormal glucose tolerance,IGT”and“TG”as search terms,China National Knowledge Infrastructure,VIP,Wanfang Data and Pubmed were searched from database inception until October 2017.All the controlled clinical researches on the treatment of IGT by TG meeting the inclusion and exclusion criteria were retrieved and analyzed by Review Manager 5.3 software.Results:A total of 7 articles including 1082 participants were enrolled.Meta-analysis showed that the OR value of inversion rate was 2.17,95%CI(1.65,2.84).Weighted mean difference(WMD)value of fasting plasma glucose was-0.25,95%CI(-0.39,-0.11).After 2 h,the WMD value of serum glucose was-0.73,95%CI(-0.96,-0.51),all of which were better than these of control group.The OR value of progression rate(type 2 diabetes mellitus)was 0.44,95%CI(0.32,0.59),less than the control group.All of the differences were statistically significant.No hepatic and renal toxicity case was reported.Only 1 article reported adverse reactions in the course of treatment.Conclusion:TG could treat IGT effectively,delay and even invert the progress of IGT,but its security still needed further discussion.
Keywords:Tianqijiangtang Capsule,Impaired glucose tolerance,Meta-analysis
Introduction
Impaired glucose toleration(IGT)indicating the blood glucose level is between 7.8 mmol/L and 11.1 mmol/L after the glucose tolerance test(GTT),which is one of the cause leading to diabetes or pathological changesin blood vesselsofheartand brain.Epidemiological survey revealed that the incidence of adults diabetes above 20 years old was 11.28%,and that of prediabetes was 15.19%[1].The phase of IGT exists in most type 2 diabetes mellitus(T2DM)patients.8%-11%IGT patients will progress to T2DM per year in our country[2].However,IGT possesses a high degree of reversibility[3].Effective intervention is vital for preventing T2DM.
Current research found that the key pathogenesis of IGT is the deficiency of both qi and yin[4].Tianqijiangtang Capsule(TG)consists of Huangqi (Astragali Radix), Tianhuafen(Trichosanthis Radix),Nüzhenzi(Ligustri Lucidi Fructus),Shihu (Dendrobiicaulis),Shanzhuyu(Corni Fructus),Mohanlian (Ecliptae Herba),Wubeizi(Galla Chinensis),etc.,which can tonify qi and yin,remove heatto promote salivation,strengthen the kidney to stop nocturnal emission.TG has the merit of multiple targets and less adverse effects,reducing blood glucose and improving symptoms and quality of life at the same time,therefore,it is propitious to a long-term medicine intervention therapy for IGT [5]. Previous multi-center,random,double blind,and placebo control revealed that TG can delay the progress of IGT to T2DM,in which above 50%patients inverted to NGT[6].The downside is lacking the methods of evidence-based medicine to evaluate its curative effect.Therefore,the present study is to evaluate the efficacy and safety of clinical controlled trials about TG in treating IGT,and provide reference for clinical rational drug use.
Material and Method
Inclusion criteria
Study type.Clinical randomized controlled trials of TG in the treatment of IGT.
Object of study.In line with the diagnostic criteria of IGT in 1999 WHO diagnosis and classification of diabetes diagnostic criteria[7]:fasting blood glucose(FPG)<7.0mmol/L,and 2 Hours Plas Ma Glucose(2hPG)after taking 75g glucose in glucose tolerance test(OGTT)≥7.8 mmol/L and<11.1 mmol/L;(2)patients newly diagnosed with IGT still meet the diagnostic criteria for IGT after one month of induction.
Intervention.The control group was given general lifestyle interventions or placebo or oral administration of other anti-diabetic drugs.The treatment group was given general lifestyle intervention plus TG(produced by Heilongjiang Baoquan Pharmaceutical Co., Ltd. Zhunzi Z20063799).
Index of outcome.The main outcome measures were reservation rate(NGT)=NGT/(NGT+T2DM+IGT),T2DM=T2DM/NGT+T2DM+NGT,fasting serum glucose,serum blood glucose 2h after sugar load.
Exclusion criteria
1.Repeated publication of the literature;2.Data of the literature can’t be extracted;3.Serious liver and kidney dysfunction and severe infection; 4.Conference papers, academic papers, animal experiments.
Document retrieval strategy
The randomized controlled trials of TG in the treatment of IGT were searched in databases such as CNKI,VIP,Wanfang Database,Superstar Mobile Library,PubMed and Cochrane Library.The search time are from database construction to October 2017.TheChinesesearchtermsinclude“abnormal glucose tolerance”,“impaired glucose tolerance”,“impairedglucosetolerance”[and]“Tianqijiangtang Capsule”,the English terms include“IGT”,“Tianqijangtang”.
Data Extraction and Quality Evaluation
Two researchers independently screened the literature,extracted data and evaluated quality.If there was a disagreement,the corresponding author drew the conclusion.The data extracted includes:1.basic information of the research,including the first author,publication year;2.the basic characteristics of the study object,including the number of the treatment group and the control group,intervention measures,intervention time,observation indicators;3.outcome measures and outcome measures;Data were evaluated using the Cochrane Handbook 5.0.1"Bias Risk Assessment",with 7 main items:(1)random allocation method;(2)allocation scheme was hidden;(3)blind method used to the study participants and treatment plan implementers;(4)blind method used to survey result for measurers;(5)integrity of the outcome data;(6)report research results selectively;(7)other sources of bias.
Statistical method
Meta-analysis was performed using Review Manager 5.3 software provided by the Cochrane Collaboration.The odds ratio(OR)and its 95%confidence interval(CI)were used as the curative effect statistics in the count data.The weighted mean difference(WMD)and 95%CI of the measurement data were used as the curative effect analysis statistics in measurement data.The I2test was used to test the heterogeneity of the results.When I2<50%,the fixed effect model was used for the analysis.When I2>50%,the random effect model was used to analyze the published bias.Use the inverted funnel plot to determine publication bias.
Results
Search and screen the results
58 papers were searched initially,12 repeated papers and 26 unrelated ones were removed by reading the titles and the abstracts.By further reading the full text,6 papers not meeting the observational index,2 conference papers,3 academic dissertations,2 original article were excluded,finally 7 paper were included(6 Chinese paper and 1 English paper)for meta-analysis.Documentretrievalprocess was shown in Figure 1.
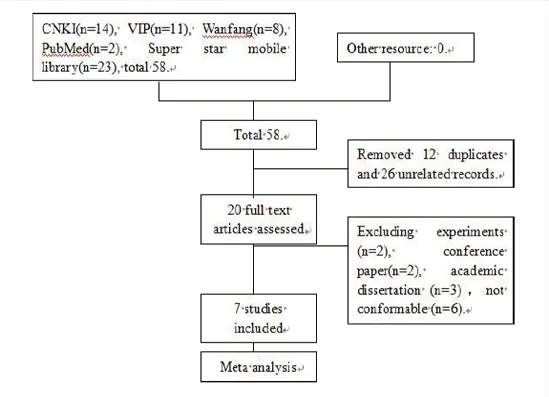
Figure 1 Document retrieval process
The basic characteristics of the studies included
A total of 1082 subjects were enrolled in the 7 articles,556 cases were in the treatment group and 526 cases in the control group.The patients age ranged from 40 to 85 years old.Take five tablets at a time orally with three times a day.Intervention time of 1 article[9]was 2 years,that of 1 article[8]was 8 weeks,that of 1 article[12]was 16-40 weeks,and that of the remaining 4 articles[10-11,13-14]was 1 year.The observation index of five articles[9-12,14]referred to the reversal rate(NGT)and progress rate,and observation indexes of six articles[8-13]refer to FPG and 2hPG.1 article[8]had incomplete data.Detailed characteristics included in the study were shown in Table 1.
Quality evaluation in the study included
Four papers[8,12-14]mentioned randomization,but the specific methods of randomization were not explained.Two articles[9,11]used random numbers table to carry on grouping,and one article[10]use stratified block randomization method to dived groups.One of the articles[9]conducted allocation concealment by a randomized distribution card.One article[10]conducted allocation concealment by sealed letters.Only one article[8]did not mention the blind included was shown in Figure 2.
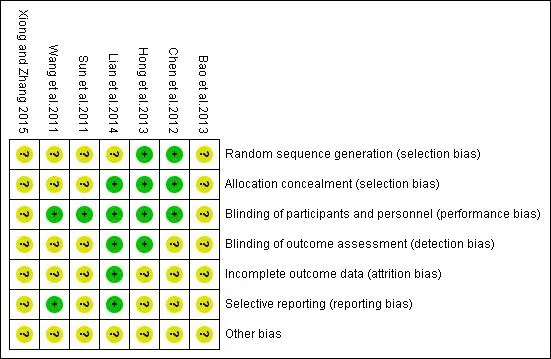
Figure 2 Quality evaluation in the study included
Meta Results method,and the others were all double blind.Therefore,the performance bias and measurement bias were both assessed as a low risk.Two articles[12,14]had follow-up visit and others were not mentioned.
NGT.TG+lifestyle VS placebo+lifestyle:a total of 4 studies[10-11,13-14],heterogeneity test revealed that P=0.68,I2=0%,no heterogeneity,so a fixed effect model was used.Meta-analysis results showed TG had better curative effect in reversing IGT with the same lifestyle intervention(P<0.001),OR=2.17,95%CI(1.65,2.84)(Figure 3).
T2DM.TG+lifestyle VS placebo+lifestyle:a total of 4 studies were included [11-12, 13-14],heterogeneity test suggested that P=0.69,I2=0%,no heterogeneity,so a fixed effect model was used.The results of meta-analysis showed that TG had the better curative effect in delaying IGT progression to T2DM with the same lifestyle intervention(P<0.001),OR=0.44,95%CI(0.32,0.59)(Figure 4).
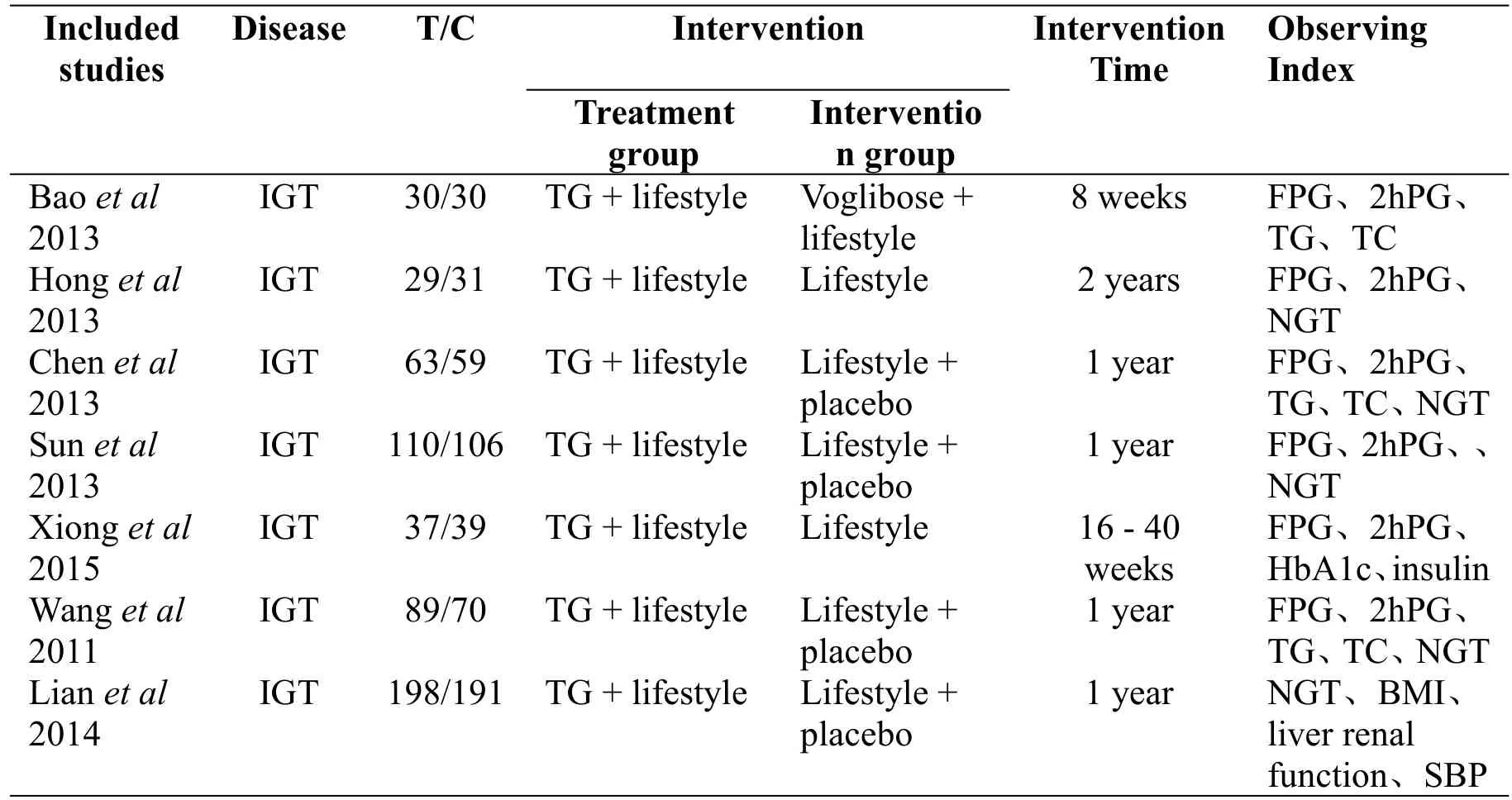
Table 1 Characteristics of the studies included
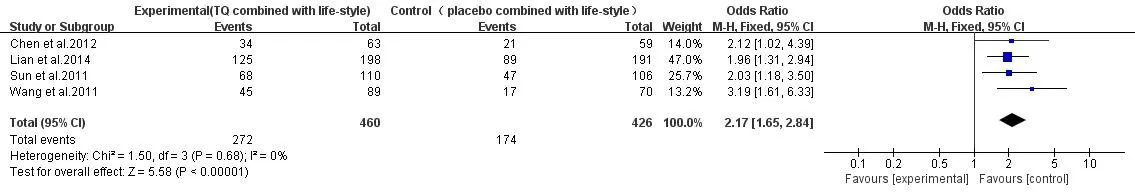
Figure 3 NGT
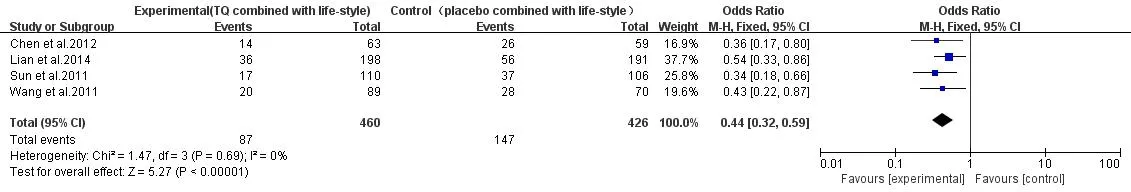
Figure 4 T2DM
FPG.FPG was reported in 6 articles[8-13],of which 3 articles[10-11,13]described placebo+lifestyle VS TG+lifestyle,an article[8]described TG+lifestyle VS Voglibose+lifestyle,two articles[9,12]described lifestyle VS TG+lifestyle.Placebo+lifestyle VS TG +lifestyle was studied,the heterogeneity test suggested P=0.16,I2=45%,statistical heterogeneity is small,so the fixed effect model was selected.Meta-analysis results showed that TG had a better curative effect in reducing fasting blood glucose of IGT based on the same lifestyle intervention(P=0.0004),WMD=-0.25,95%CI(-0.39,-0.11)(Figure 5).
2hPG.3articles[10-11,13]described placebo+lifestyleVS TG + lifestyle,heterogeneity test suggested that P=0.35,I2=5%,heterogeneity is small,so the fixed effect model was adopted.Meta-analysis results showed that combination of TG with 2hPG had better curative effect with the same lifestyle intervention(P<0.001),WMD=-0.73,95%CI(-0.96,-0.51)(Figure 6).
Evaluation of publication bias
The funnel-plot was used to analyze the publication bias.As shown in the figure below,the figure is almost symmetrical without publication bias.
Safety
No toxicity of liver and kidney was reported in all articles,one article[11]reported mild gastrointestinal symptoms during treatment,and its safety needs further study.
Discussion
So far,there are no systematic reviews of TG for the treatment of IGT in the retrieved databases,so this study was actively evaluated in accordance with evidence-based medicine.Aftercompleting the evaluation of this study,PRISMA[15]was used for self-evaluation,basically conform to the reporting standards and methodological quality assessment entries.The meta-analysis showed that TG can reduce fasting blood glucose and blood glucose after 2h glucose load of IGT,and can reverse and delay the progression of IGT to T2DM,only one article reported mild gastrointestinal reactions,and the security needs to be further explored.

Figure 5 FPG
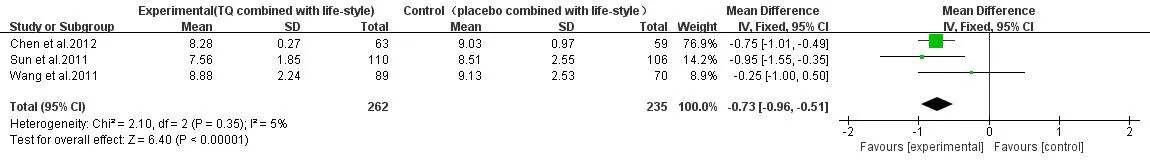
Figure 6 2hPG
Pre-diabetic population is a reserve army for type 2 diabetes,and IGT isan importantpartof pre-diabetes.Effectively intervening blood glucose in IGT population is of great significance for the prevention and treatment of type 2 diabetes.At present,the further progress is mainly controlled by drugs and lifestyles such as diet and exercise.While hypoglycemic drugs has a large side effects.Many patients discontinue metformin hydrochloride treatment because of nausea and diarrhea and other gastrointestinal reactions intolerance[16].IGT is the onset of diabetes,and Chinese medicine has its own unique advantages in inhibiting the progress of IGT.TG is a pure Chinese herbal medicine compound,of whichHuangqi(AstragaliRadix)andRenshen(Ginseng Radix Et Rhizoma)can tonify Qi,Nüzhenzi(Lucidi Fructus)and Tianhuafen(Ecliptae Herba)and Digupi(Lycii Cortex)can clear heat,Mohanlian(deficient heat) can nourish Yin,Tianhuafen(Trichosanthis Radix)[17],allherbswork as tonifying Qi,nourishing Yin,promoting the secretion of saliva,and strengthening the kidney to stop nocturnal emission.It can reduce blood glucose and regulate lipid clinically,and improve insulin resistance.The basic experiments showed that TG may play a role in hypoglycemic and lipid-lowering by up-regulating MAPK pathway and GluT4 gene,down-regulating inflammatory cytokines to improve insulin sensitivity, promoting liver glycogen synthesis and reducing liver glycogen output[18].
The deficiency of study is that it only included articles published in the database,not the publishing articles or gray literature,which may result in publication bias.The second is that there are only a few articles in this study and the sample size is relatively small.At present,the number of clinical RCT test of TG in the treatment of IGT is small,and interventions were mostly compared with placebo,only in one literature,intervention was compared with positive control drug.The use of any drug has a certain effect compared with non-taking of drugs.However,whether the drug has clinical advantages over western medicine is the key to explore the value ofthis traditionalmedicine.More multicenter,randomized,double-blind and positive control drugs of clinical trials will have greater significance in order to make better use of TG in clinical services.
 TMR Integrative Medicine2018年2期
TMR Integrative Medicine2018年2期
- TMR Integrative Medicine的其它文章
- Regulatory effects of the combination of berberine and ginsenoside Rb1on high-fat diet-induced nonalcoholic fatty liver disease viaAMPK pathway and improved pharmacokinetics
- Analysis of 610 cases inpatients with chronic heart failure
- Application of sinomenine in rheumatic diseases
- Progress in the study of diabetic retinopathy
