Regulatory effects of the combination of berberine and ginsenoside Rb1on high-fat diet-induced nonalcoholic fatty liver disease viaAMPK pathway and improved pharmacokinetics
Lv Zhou,Xiu-Teng Zhou,3,Wen-Bo Xu,Yi-Dan Chen,Ni-Hua Wang,Jiao Guo*,Hui Fan*
1Guangdong Research Center of Metabolic Diseases of Integrated Western and Chinese Medicine,Guangdong Pharmaceutical University,Guangzhou,510006,China.2Joint Laboratory between Guangdong and Hong Kong on Metabolic Disease,Guangdong Pharmaceutical University,Guangzhou,510006,China.3National Resource Center for Chinese Materia Medica,China Academy of Chinese Medical Sciences,State Key Laboratory Breeding Base of Dao-di Herbs,Beijng,100700,China.
Abstract Objective:We evaluated the protective effects of berberine(BBR)combined with ginsenoside Rb1(G-Rb1)on high-fat diet(HFD)-induced nonalcoholic fatty liver disease(NAFLD)in rats and futher investigated the underlying mechanisms.Methods:Rats were fed an HFD for 6 weeks and then randomly divided into four groups and treated with BBR(50 mg/kg),G-Rb1(100 mg/kg),BBR(50 mg/kg)+G-Rb1(100 mg/kg),or fenofibrate(40 mg/kg).Histological examination of liver tissue was performed.In human hepatocellular carcinoma cells HepG2,protein expression of AMP-activated protein kinase(AMPK)and acetyl-CoA carboxylase was detected by western blotting,and the mRNA expression of carnitine palmitoyl transferase 1 and 3-hydroxy-3-methyl glutaryl coenzyme A reductase was detected by quantitative PCR.Pharmacokinetic assessments included analysis of bioavailability of BBR and G-Rb1in vivo and G-Rb1metabolism by intestinal bacteria in vitro.Results:Compared to the single-use group,BBR combined with G-Rb1significantly ameliorated hepatic fat accumulation in HFD-induced obese rats,as demonstrated by reduced hepatic triglyceride content,and histological evaluation of liver sections.Activation of hepatic AMPK and phosphorylation of acetyl-CoA carboxylase were significantly elevated in hepatocytes treated with both BBR and G-Rb1.Consistent with the activation of AMPK,the mRNA expression of carnitine palmitoyl transferase 1 was stimulated,while the mRNA expression of 3-hydroxy-3-methyl glutaryl coenzyme A reductase was suppressed.Pharmacokinetic analysis revealed that BBR increased the bioavailability of G-Rb1in Sprague-Dawley rats.Additionally,BBR prevented degradation of G-Rb1in fecal solution in vitro.Conclusion:BBR combined with G-Rb1improved NAFLD through the AMPK signaling pathway,and BBR enhanced G-Rb1bioavailability via promoting the intestinal absorption of G-Rb1.This combination may be a useful therapeutic agent for NAFLD.
Keywords:Nonalcoholic fatty acid liver disease,AMP-activated protein kinase,Berberine,Ginsenoside Rb1,Pharmacokinetics,Intestinal bacteria
Background
Nonalcoholic fatty liver disease (NAFLD) is characterized by increased fat accumulation in hepatocytes and is the most common liver disease worldwide[1-4].The growing prevalence of NAFLD parallels the rise in obesity and type 2 diabetes mellitus,which are projected to increase dramatically over the next decade[5].Currently,there are no specific therapeutic drugs for NAFLD.Most researches have centered on compounds derived from traditional Chinese herbs such asberberine(BBR)[6],ginseng saponin [7],and oleanolic acid[8].
Abnormal lipid metabolism is the primary pathological cause of metabolic diseases,such as type 2 diabetes and NAFLD disease.The beneficial anti-hyperglycemic and lipid-regulating effects of traditional Chinese herbs have been prominent since ancient times.Mingyibielu of Han Dynasty of China(the third century A.D.)was the first ancient book that recorded the effect of Huanglian(Coptis chinensis)on“quenching thirst",the thirst of Chinese medicine,with symptoms of frequent drinking and urination,which is similar to diabetes in modern Western medicine.Bencaojingjizhu of the Liang Dynasty of China(480 A.D.-498 A.D.)also stated that Huanglian(Coptis chinensis)was commonly used to cure phlegm and thirst.Sanqi(Panax notoginseng)was known as a top-grade medicine in the ancient book Shennongbencaojing of Eastern Han Dynasty of China(the third century A.D.)and has been shown to reduce blood lipid levels by enhancing the Qi of the spleen and transporting dampness.
BBR,an isoquinoline alkaloid isolated from Huanglian(Coptis chinensis),has been shown to exhibit protective effects against diabetes and dyslipidemia[9].Although the mechanismsofaction differed from those of 3-hydroxy-3-methyl glutaryl coenzyme A(HMG-CoA)reductase inhibitors such as statins,their effects on decreasing the levels of serum cholesterol,triglycerides(TG),and low-density lipoprotein-cholesterol were comparable to those of statins[10-12].Lower level of TG was observed in patients with hypercholesterolemia treated for 3 months with BBR.This work suggested that BBR activates AMP-activated protein kinase(AMPK),which translated into the phosphorylation of acetyl-CoA carboxylase(ACC)and then a subsequent increase in fatty acid oxidation and decrease in fatty acid synthesis,and ultimately a decrease in TG synthesis[13-15].The mechanism of BBR in decreasing the TG level was similar to that of metformin[16].Ginsenoside Rb1(G-Rb1),an active tetracyclic triterpenoid saponin obtained from dried Renshen(Radix Ginseng)or Sanqi(Radix Notoginseng)root,has been used to treat and prevent various diseases for several millennia[17].In previous studies,G-Rb1significantly reduced liver cell lipid accumulation in high-fat diet(HFD)-induced obese animals.This effect was likely mediated by an increase in the AMP/ATP ratio,leading to activation of the AMPK signaling pathway[18].
The combination of Chinese herb is a feature of traditionalChinese medicine.The combination of Huanglian (Coptis chinensis) and Sanqi (Radix Notoginseng)is frequently used in Chinese medicine to manage diabetes and obesity.Thus,it is important to understand whether synergistic effects exist between the active componentsofBBR and G-Rb1.Available evidence does not clarify whether this occurs through any possible mechanisms[19].Therefore,we evaluated the mechanism from the perspectives of energy metabolism pathways and pharmacokinetics.
Materials and methods
Materials
BBR(purity≥95%)and G-Rb1(purity≥95%)were purchased from Tianjin Science and Technology Co.,Ltd.(Tianjin,China) and dissolved in 0.5% sodium carboxymethyl cellulose solution when in use.Fenofibrate 200 mg capsules(Liping,Tianjin Science and Technology Co.,batch number:130220)were also dissolved in 0.5% sodium carboxymethylcellulose solution to give a fenofibrate concentration of 40 mg/mL.TG, glucose, test Bradford protein, and alanine aminotransferase(ALT)assay kits were purchased from the Nanjing Jianchen Bion-engineering Institute Co.,Ltd.(Nanjing,China).The antibodies for p-AMPK,AMPK,pACC,and ACC were from Abcam(Cambridge,UK).
Animals model
Male Sprague-Dawley rats(n=36,180-220 g)were purchased from the Guangdong Medical Laboratory Animals Center(Guangdong,China).Animals were housed in individual cages(60±5%humidity and a 12h dark-light cycle with free access to drinking water).Control animals were fed a standard diet.Experimental animalswere fed an HFD (Guangdong Medical Laboratory Animals Center)for 6 weeks to prepare the rat model of NAFLD.The control and experimental animals were then divided into six groups(n=6 each):standard diet without drug treatment(Control group);HFD group without drug treatment(HFD group);HFD with BBR 50 mg/kg(BBR group);HFD with 100 mg/kg G-Rb1(G-Rb1group);HFD with BBR 50 mg/kg+G-Rb1100 mg/kg(BBR + G-Rb1group);andfenofibrate40mg/kg(Fenofibrate group).Drug treatments,where relevant,were administered for 4 weeks.The control and model groups were administered saline in the same volume as the daily drug treatments.At the end of the experimental period,the rats were sacrificed after 12 h fasting,and the liver was excised and frozen immediately in liquid nitrogen and stored at-80°C until further analysis.
Serum biochemical analysis
After 2 h of blood coagulation from the rats’orbital venous plexus,it was centrifuged at 3000 rpm for 15 min,and the supernatant was taken to obtain serum samples.Then TG,ALT,and glucose were measured in serum samples from rats via their respective kits and according to the manufacturer’s instructions.
Determination of TG levels in the liver
A total of 500 mg of liver tissue was collected from each rat,added to a chloroform to methanol(1:1)mixture to a final volume of 5 mL,and homogenized.Homogenates were placed at 4°C for 24 h,centrifuged at 3500 rpm for 15 min,and an aliquot of supernatant was taken(20 μL).The remaining sample was allowed to stand at 25°C for more than 12 h.After trichloromethane and methanol were volatilized,TG content in the liver was determined using a triglyceride kit.
Histology examination
Liver tissue was embedded in paraffin,sliced,and stained with hematoxylin and eosin(HE).The sections were then observed for hepatic steatosis and inflammation grading and scored as described in the NAFLD histopathological diagnosis reference in February 2006,Chinese Medical Association credits Fatty LiverDisease liverand Alcoholic liver Disease Study group revised“NAFLD treatment guidelines.”
Cell culture
Human hepatocellular carcinoma cells HepG2 were cultured in Dulbecco Modified Eagle’s medium containing 10%fetal bovine serum.The medium was changed every 3 days.The cells were grown to 80-90%confluence,and then sub-cultured into 6-well plates at 2×105cells per well for subsequent experiments.Cells were divided into the following groups:normal control group,25 μg/mL BBR,100 μg/mL Rb1,and 25 μg/mL BBR+100 μg/mL Rb1.Cells were incubated for 24 h at 37 °C with 5%CO2.
Western blot analysis
The groupings and drug concentrations used for western blot analysis were the same as those used for cell culture.Total protein was extracted from HepG2 cells according to the manufacturer’s instructions(Beyotime Institute of Biotechnology,Jiangsu,China).Protein concentration was measured using the bicinchoninic acid protein assay kit(Bioteke,Beijing,China)with bovine serum albumin as the standard.Protein(80-120 μg)was separated in a 12%SDS polyacrylamide gel and electro-transferred onto polyvinylidene difluoride membranes(Bio-Rad,Hercules,CA,USA).Membranes were blocked with 5%(w/v)skim milk or 5%bovine serum albumin for 2 h at room temperature and then incubated with mouse or rabbit polyclonalantibodies (p-AMPK,t-AMPK,β-actin,p-ACC,t-ACC)with light shaking overnight at 4°C.The membranes were washed three times for 5 min each with 15 mL of TBST(10 mM Tris–HCl,150 mM NaCl,and 0.1%(v/v)Tween-20)and then incubated with secondary antibody at room temperature for 2 h.Protein was visualized with enhanced chemiluminescence and images were generated with a MiniChemi610(Beijing sage creation,Beijing,China).Image band intensities were quantified using Image J software(NIH,Bethesda,MD,USA),and protein expression was normalized to β-actin signals.
Quantitative real-time PCR
The groups and drug concentrations used in this method were consistent with those used in cell culture.Total RNA was isolated from rat tissues and HepG2 cells using MaxRecovery™ BiooPure™ RNA Isolation Reagent(Bioo Scientific,Austin,TX,USA).After quantification,RNA wasreverse-transcribed to cDNA using the ReveraidTMFirst Strand cDNA Synthesis kit(Fermentas,Vilnius,Lithuania).Amplification was performed in a total volume of 20 μL containing the cDNA template,primers,and Maxima SYBR qPCR Master Mix(2×)(Thermo Scientific,Waltham,MA,USA).PCR conditions were 95°C for 10 min,followed by 40 cycles of 95 °C for 15 s and 62 °C for 1 min,with a final dissociation stage.The samples were run on a PikoReal 96 Real-Time qPCR detection system(Thermo Fisher).Primer sequences were as follows:
Carnitine palmitoyl transferase 1(CPT-1):
Forward(5′→3′):GCCGCTCGTTCACTCCA;
Reverse(5′→3′):AGCCGCAGATGTCAATCCC;
HMG-COA:
Forward(5′→3′):TAACTCAGAGGGTTAAGAT;
Reverse(5′→3′):ACTGCCAGAGGGAAAC.
Pharmacokinetic analysis
Thirty-nine Sprague-Dawley rats were housed in a standard cage and fed with typical rat food and with free access to drinking water.After fasting for 12 h,via gavage,rats were received BBR(100 mg/mL),G-Rb1(300 mg/mL),and BBR(100 mg/mL)+G-Rb1(300 mg/mL),respectively,with 13 mice each group.After 5,15,and 30 min and 1,2,3,4,6,8,10,12,24,and 48 h,orbital blood(5 mL)was collected into heparinized centrifuge tubes and serum was obtained by centrifugation at room temperature (5000 rpm for 10 min). Column chromatography was conducted to collect the samples,which were evaporated before ultra-performance liquid chromatography(UPLC).UPLC was conducted using a UPLC TMBEH C18column(50 × 2.1 mm,1.7 μm),guard column VanGuardTMPre-Column,mobilephaseof methanol-water(5:95,v/v),flow rate of 1 mL/min,column temperature of 25℃,injection volume of 20 μL,and negative ion scan mode.We determined the following pharmacokinetic indicators:t1/2α(h),t1/2β(h),Ka(1/h),t1/2Ka(h),AUC(0-t)(mg/L·h),AUC(0-∞)(mg/L·h),Tmax(h),Cmax(mg/L),CL/F(L/h/kg).
Fecal microbiota incubation in vitro
The appropriate amountfresh fecesfrom normal Sprague-Dawley rats was dissolved in PBS buffer and centrifuged at 25°C(1000× g for 10 min).The supernatant was removed for use as the bacterial solution.Samples were divided into the blank control group,100 μg/mL of G-Rb1group,and 100 μg/mL of G-Rb1+100 μg/mL of BBR group.Ice-cold PBS containing BBR and G-Rb1were incubated in a 37°C water bath for 2 h.The solution wasthen added to an extractcontaining water-saturated n-butanol:ethyl acetate in a 3:1 ratio,vortexed,and centrifuged at 4°C(12,000 rpm for 10 min).The supernatant was removed and dried,and then reconstituted with the initial mobile phase.This sample was vortexed,centrifuged,and loaded.Drug content was determined by High Performance Liquid Chromatography.
Statistical analysis
Statistical analysis was conducted using SPSS 17.0 software (SPSS,Inc.,Chicago,IL,USA),with experimental data expressed as the mean±standard deviation.If the variance was homogeneous,one-way analysis of variance was used to compare pharmacokinetic parameters between groups for significant differences.If significant differences were identified,the least significant difference method was applied for comparison between pairs.If the variance was heterogeneous,Tamhane's T2 pairwise comparison was used.P-value<0.05 indicates statistically significant difference.
Results
BBR combined with G-Rb1reduces fat accumulation and improves glucose and lipid metabolism disorder state
After sacrifice of the model rats,the removed liver was observed to be pale,while the cut surface was canary yellow,indicating moderate or severe fatty liver steatosis.Varying degrees of recovery were observed in all treatment groups,with the liver of the BBR+G-Rb1group appearing as bright red and similar to the normal liver,showing better effects than any single drugs(Figure 1).
HE staining was conducted to evaluate pathological changes in the liver tissue(Figure 2).Compared to the control group,the HFD group tissue sections contained many fat vacuoles,liver cells showed foamy enlargement,and the field of vision of tissue sections appeared before diffuse fat vacuoles.All treatment groups had improved fat vacuoles.The BBR+G-Rb1group had significantly improved fat vacuoles,fat vacuoles recovery,and clear and liver sinusoidal cord tissue,which was superior to the effects of any single drugs.
The level of TG was also tested.Rats in the HFD group showed elevated concentrations of TG in both the serum and liver.Compared with HFD group,the BBR,G-Rb1,and BBR+G-Rb1groups showed significantly lower in the levels of TG in both the serum and liver(P=0.038,P= 0.04,P = 0.031,respectively).Moreover,the combination of BBR and G-Rb1showed advantage in liver TG over the single drug(P=0.039,P=0.042,P=0.028,respectively)(Figure 3A,B).
The HFD group also showed elevated ALT,indicating impaired liver function.Compared with HFD group,either BBR or Rb1 could improve liver enzyme ALT(BBR group vs HFD group,P=0.037;Rb1 group vs HFD group,P=0.022).The combination group also improved the ALT(P=0.025),but did not showed advantage over the single drug(P>0.05 for both)(Figure 3C).
Fasting blood glucose concentrations in the HFD group were also markedly higher than that in the control group(P = 0.021),indicating hyperglycemia and insulin resistance.Compared with HFD,fenofibrate increased the level of glucose significantly(P=0.026),which is consistent with the results of a previous study[22].However,BBR+G-Rb1significantly reduced fasting blood glucose concentrations(P=0.027).Moreover,the combination of BBR and G-Rb1had better effects than single G-Rb1(P=0.041)(Figure 3D).
BBR combined with G-Rb1increased AMPK and ACC protein phosphorylation
To explore whether the lipid-lowering mechanism of BBR+G-Rb1involves activation of the AMPK cascade in HepG2 cells,western blot analysis was conducted to measure the protein expression of AMPK and AMPK phosphorylation. Expression of p-AMPK/AMPK significantly increased in the three treatment groups(BBR,Rb1,BBR+Rb1groups)compared with that in the control group(P=0.028,P=0.045,and P=0.01 respectively).The BBR+G-Rb1group showed greater enhancement of AMPK phosphorylation than the other groups(Figure 4A).Because it is well-established that peripheralAMPK activation promotes fatty acid oxidation by inactivating ACC [21],weevaluated whether BBR+G-Rb1induces ACC phosphorylation in HepG2 cells.Consistent with the increased AMPK phosphorylation,ACC protein phosphorylation levels in the BBR+G-Rb1group were 3-fold greater than that in the normal control group(P=0.015)(Figure 4B).Effect of BBR combined with G-Rb1on mRNA levels of carnitine palmitoyl transferase 1(CPT-1)and HMG-CoA To further explore whether BBR+G-Rb1acted through AMPK,RT-qPCR wasusedtodetectthemRNA expression of a key enzyme involved in fatty acid oxidation,CPT-1,and cholesterol biosynthetic enzyme,HMG-CoA.The three treatment groups(BBR,G-Rb1,and BBR + G-Rb1)showed differentlevels of improvementin CPT-1 mRNA levels(Figure5A).Compared to the control group,each group(BBR,G-Rb1,and BBR+G-Rb1)significantly inhibited HMG-CoA expression(P=0.029,P=0.033,P=0.018).However,the effect of BBR+G-Rb1was greater than that of the other groups(Figure 5B).Thus,BBR+G-Rb1had greater effects on the expression of enzyme mRNA than the two individual agents.
Pharmacokinetic studies of BBR and G-Rb1
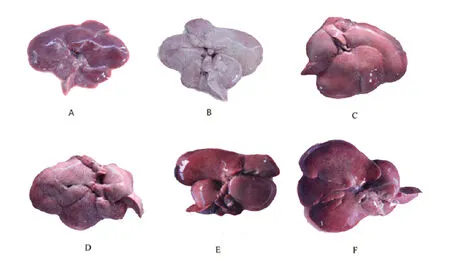
Figure 1 liver morphology after different treatment
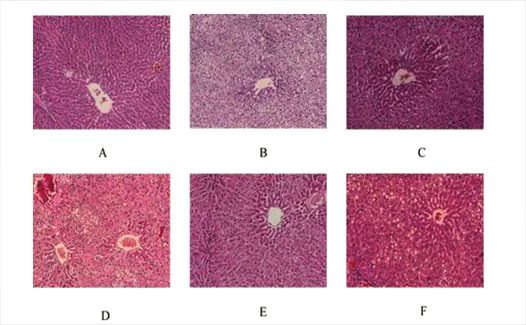
Figure 2 Hematoxylin and eosin-stained liver sections
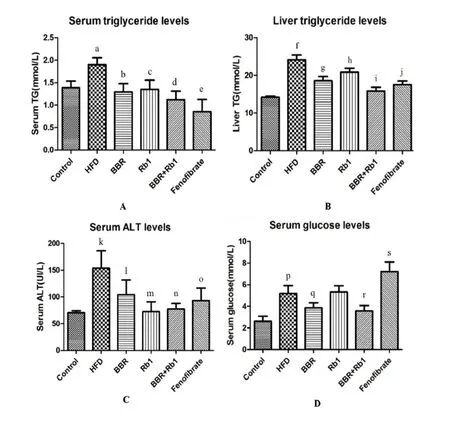
Figure 3 BBR combined with G-Rb1 reduced serum levels of glucose,triglyceride,alanine aminotransferase,and liver triglyceride
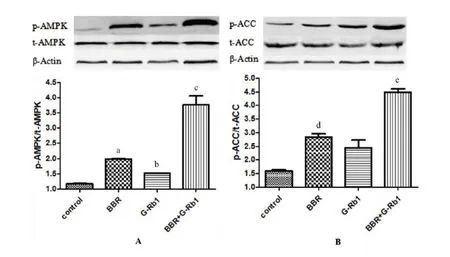
Figure 4 BBR combined with G-Rb1increased AMPK phosphorylation
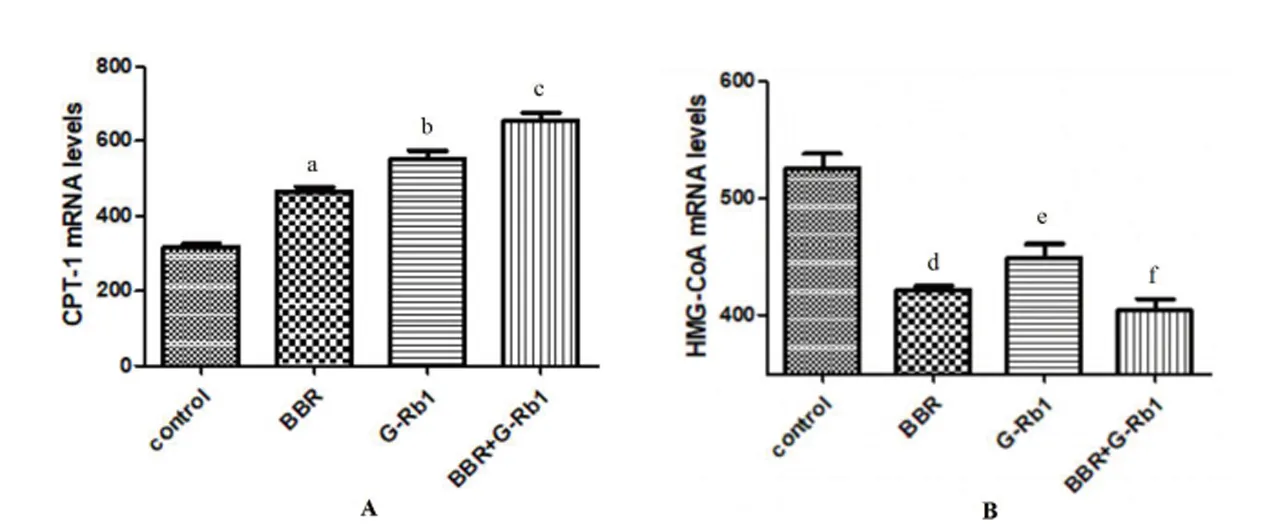
Figure 5 Effect of BBR combined with G-Rb1on the level of the mRNA of CPT-1 and HMG-CoA
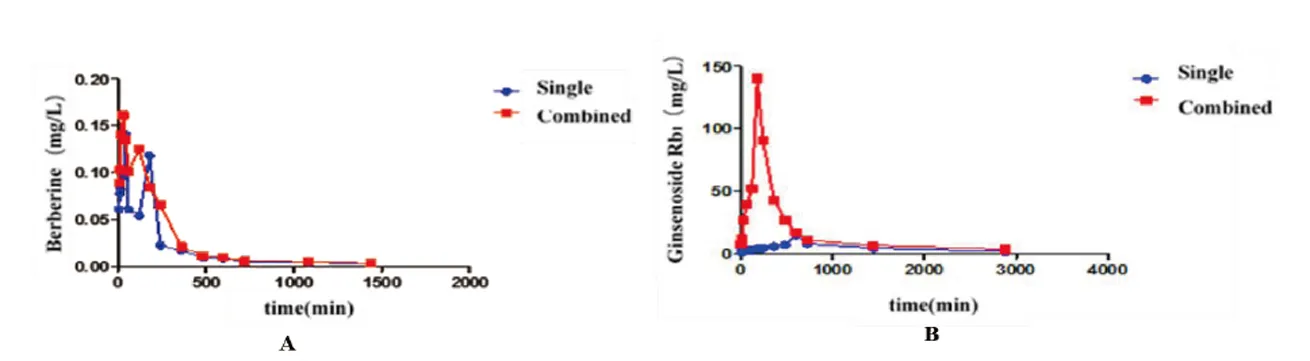
Figure 6 Concentration-time curve of berberine and ginsenoside Rb1
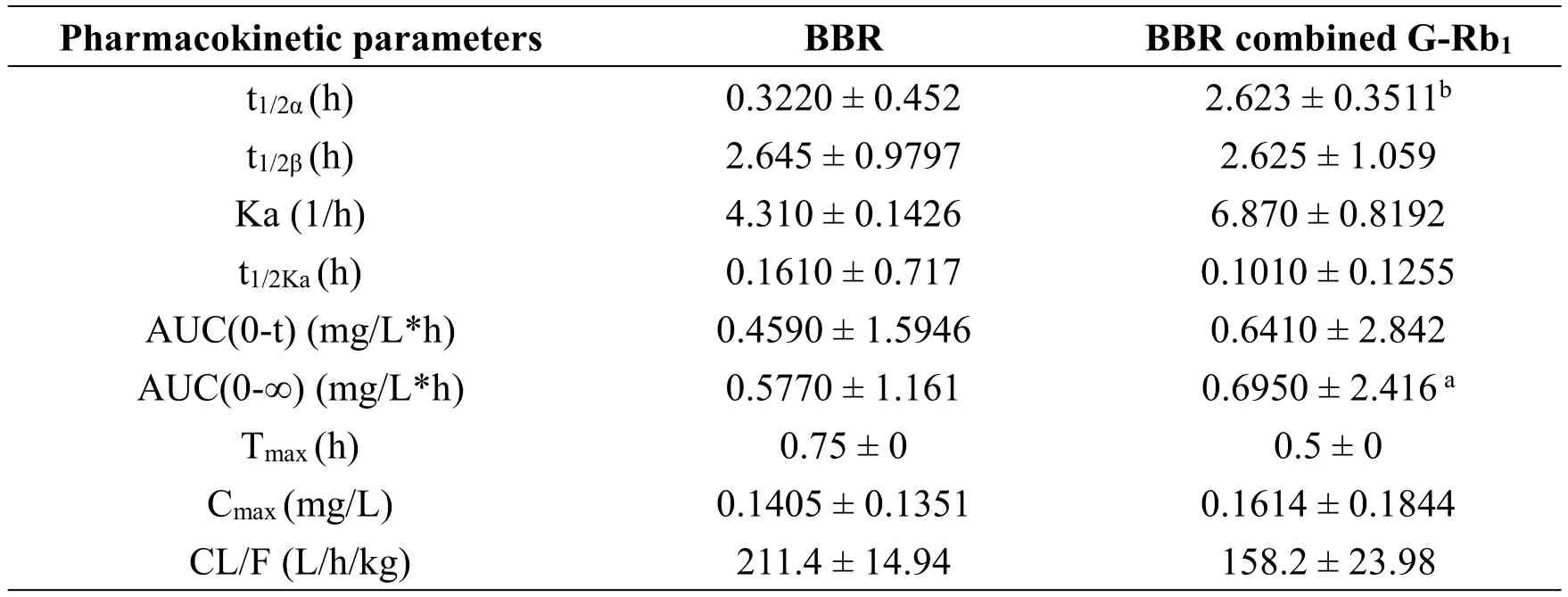
Table 1 Pharmacokinetic parameters of BBR
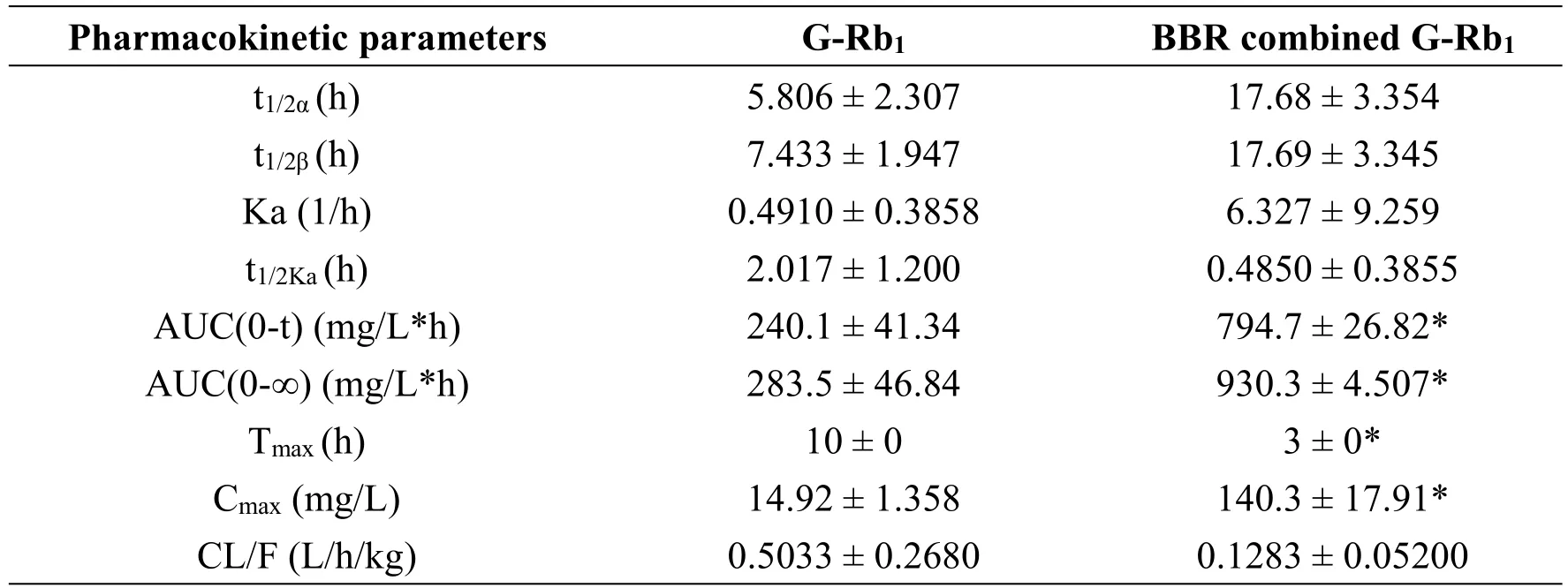
Table 2 Pharmacokinetic parameters of G-Rb1
To determine whether the combination of BBR+G-Rb1affected the bioavailability of each individual agent,we evaluated the concentrations of each compound in rat serum to determine the concentration-time profile for each agent when administered alone or in combination.The internal standard used for UPLC-MS determination of the plasma concentrations of G-Rb1and BBR was the same whether the agents were administered as monotherapy or in combination.The pharmacokinetics of BBR did not differ significantly whether administered alone or in combination with G-Rb1(Figure 6B,Table 1).However,the G-Rb1pharmacokinetics in the combined group differed,with a peak concentration that was 10-fold higher and 7 h earlier than that in the monotherapy group and an area under the concentration-time curve AUC(0-t)that was 3.3-fold higher(Tmax=3,Cmax=140.3,AUC(0-t)=794.7)(Figure 6A,Table 2).This substantial change in shape of the concentration-time profile and individual pharmacokinetic parameters suggested that BBR augmented the oral availability of G-Rb1in vivo.
Effect of BBR combined with G-Rb1co-incubation in the fecal microbiota in vitro
To further explore whether BBR promoted the intestinal absorption of G-Rb1to improve its bioavailability,we co-incubated the drug with fecal microbiota in vitro.HPLC was used to detect changes in G-Rb1content.The results showed that G-Rb1content was significantly lower than that in the control group after incubation with bacteria solution for 2 h(P<0.001).However,compared to G-Rb1alone,the BBR +G-Rb1group showed significantly higher values(P=0.039,Figure 7).This indicates that BBR increases G-Rb1contents in the intestine through the fecal microbiota.
Discussion
The present study aimed to determine if there was a synergistic effect between BBR and G-Rb1,the active components in the Chinese herbs Huanglian(Coptis chinensis)and Renshen(Radix Ginseng)or Sanqi(Radix Notoginseng),respectively,and further elucidate the mechanism from the perspective of energy metabolism pathways and pharmacokinetics.In the HFD-induced NAFLD rat model,oral administration of BBR with G-Rb1for 4 weeks significantly attenuated hepatic steatosis,which was more effective than administration of either G-Rb1 or BBR alone and comparable to the effect of the positive control drug fenofibrate(Figure 1,2).The combination also had advantage in decreasing the liver TG and serum glucose over single drug(Figure 3B,D).Although either BBR or G-Rb1could improve the level of serum TG,the combination did not have better effect than any single drug(Figure 3A).This showed that combined BBR and G-Rb1maybe improved the hepatic triglyceride metabolism,but could not improve hyperlipidemia.The influence of BBR or G-Rb1on the ALT also could not be strengthened(Figure 3D).
The underlying mechanisms of BBR and G-Rb1were differentfrom Fenofibrate,which may related to upregulation of the liver expression of PPARα,inhibition of inflammatory responses,increase in SOD,and exertion of resistance oxidation-related effects[23].Interestingly,we found both BBR and G-Rb1could promoted AMPK and ACC phosphorylation,increased the mRNA expression of CPT-1,and increased fatty acid oxidation while inhibiting HMG-CoA mRNA expression.Moreover,the effects could be enhanced after combination.This showed that,through different targets,both BBR and G-Rb1could influence the AMPK pathway,which was the key point of the combination.
AMPK is a ubiquitously expressed serine/threonine protein kinase that is activated by low cellular energy status and plays an important role in regulating hepatic lipogenesis[24-27].ACC and HMG-CoA are two target proteins of AMPK and are key enzymes in the synthesis of fatty acids and cholesterol,respectively.ACC is the rate-limiting enzyme in the synthesis of malonyl-CoA,which inhibits the activity of CPT-1,a key enzyme in fatty acid beta oxidation [28].Interestingly,the antidiabetic drug metformin improves obesity-related NAFLD by inhibiting hepatic apoA5 synthesis via the AMPK/LXRα signaling pathway[29].Therefore,AMPK represents an attractive target for the treatment of some liverdiseases[30].AMPK inhibitsfatty acid and cholesterol synthesis by inhibiting the expression of HMG-CoA, and then via phosphorylation and inactivation of ACC.This leads to decreased levels of malonyl CoA,a precursor of fatty acid synthesis and inhibitor of CPT1(Figure 8)[31].
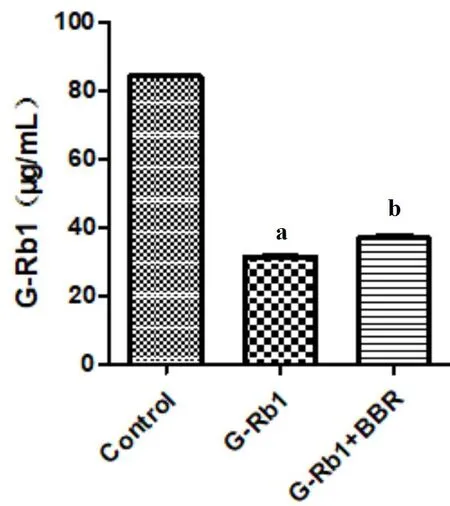
Figure 7 Effect of BBR(100μg/mL)combined with G-Rb1(100μg/mL)in the gut microbiota
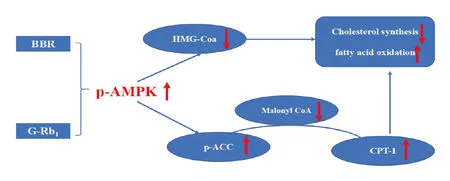
Figure 8 AMPK inhibits fatty acid and cholesterol synthesis
Previous studies showed that G-Rb1and BBR have low oralbioavailabilitiesand aremonomerswith poor bioactivity and plasma concentrations.BBR shows an extremely poor bioavailability,which may be as low as 4.0 ng/mL[32],while intestinal absorption of G-Rb1is only 3.9 ng/mL[33].We assessed whether combining these agents influences their bioavailability. In pharmacokinetic studies,BBR significantly increased the peak concentration and AUC of G-Rb1compared to G-Rb1administered alone.This indicates that one of the possible mechanisms for improving the activity of the combination was that BBR potentiated the bioavailability of G-Rb1in the intestine.Most traditional Chinese medicines are administered orally.The intestine is an important site of drug metabolism by the gut mucosa and intestinal bacteria.As described above,BBR increased the oral availability of G-Rb1.G-Rb1is a glycoside componentand undergoes gutmicrobiota-mediated de-glycosylation following oraladministration,with β-glucosidase being involved in the hydrolysisof glycosidic bonds[34].Thismay be a key factor compromising G-Rb1bioavailability and further studies are needed to examine this point.
Moreover,we found that BBR not only potentiated the oral bioavailability of G-Rb1in the intestine,but also inhibited the degradation of G-Rb1in the gut.Fecal incubation experimentsin vitro showed thatfecal bacterial solutions rapidly degraded G-Rb1after incubation for 2 h,while co-incubation with BBR was associated with an obvious increase in G-Rb1content after 2 h.This indicates that BBR alters the G-Rb1metabolic response to the gut flora.
Conclusion
Our study clarified that BBR combined with G-Rb1has a potent ability to reduce hepatic fat accumulation and may be useful as a therapy for NAFLD.The mechanism was related to the synactic effects on the AMPK signaling pathway and the increased G-Rb1bioavailability by BBR.

