Neuroprotective Effect of Bu-Shen-Huo-Xue Extract against High Glucose-induced Apoptosis in PC12 Cells
Shao-Yang Zhao , Xin Dong, Peng-Fei Tu, Ke-Wu Zeng*, Xue-Mei Wang
1Research Studio of Integration of Traditional and Western Medicine, First Hospital, Peking University, Beijing, China.2State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing,China.
Background
In recent years, Diabetic Encephalopathy (DE), a complication of Diabetes Mellitus (DM) that occurs in the Central Nervous System (CNS), has received widespread attentions. DE may be associated with several risk factors such as the age, duration and glycemic control of diabetes[1, 2]. The clinical manifestations of DE are morphological and physiological changes in the brains and resultant cognitive dysfunction. Hyperglycemia,oxidative stress and chronic inflammation may be the important pathogenic causes of DE [3]. Although an increasing number of experiments have shown that long-term sustained hyperglycemia can accelerate the brain aging, the precise pathogenesis of DE is unexplored.
Apoptosis is a programmed cell death controlled by genes in order to maintain the stable of microenvironment.The proteins involved apoptosis include the Bcl-2 family,caspases, p53, etc. Bcl-2 family is divided into two categories, one that promotes cell death, such as Bax, and the other resists apoptosis, such as Bcl-2. They can regulate apoptosis via caspase signaling pathway, which is a cascade amplification reaction of irreversible finite hydrolysis substrate of caspase [4-6]. In addition, studies have shown that High Glucose (HG)-induced apoptosis is associated with mitochondrial dysfunction, DNA damage and excessive production of Reactive Oxygen Species(ROS) [7]. A study of diabetic mice showed that Adenosine 5’-monophosphate (AMP)-activated Protein Kinase (AMPK), an intracellular energy and stress receptor, could inhibit ROS-mediated apoptosis.Moreover, increasing evidences have demonstrated that inhibition of Mitogen-Activated Protein Kinases (MAPKs)could be benefit for the inhibition of neuronal apoptosis.Another report showed that c-Jun N-terminal Kinase(JNK), Extracellular Regulated Protein Kinases (ERK),p38 MAPK signaling pathways may potentially regulate neuronal apoptosis [8,9].
Based on traditional Chinese medicine theory,kidney essence deficiency and blood stasis are considered as basic pathogenesis of DE. Therefore, the strategy of nourishing kidney and activating blood is commonly used in clinical application for DE. Previous studies could confirme that modified Wu-Zi-Yan-Zong prescription(MWP), a prescription aiming at nourishing kidney, can improve cognitive impairment and protect neurons by reducing Aβ toxicity [10, 11]. Bu-Shen-Huo-Xue (BSHX)prescription, in which leech is added based on MWP, is specifically formulated to nourish kidney and activate blood. Recently studies have shown that BSHX prescription can improve DM patients’ memory deterioration syndrome, suggesting a powerful neruoprotective properties [12]. However, the detailed pharmacological mechanism for BSHX still remains unclear. In this study, we aim to observe the protective effect of BSHX extract to HG-induced PC12 cells and explore potential molecular mechanisms.
Methods
Materials and reagents
All herbs were purchased from Kang Ren Tang Chinese Medicine Co., Ltd. (Beijing, China) and authenticated by Dr. P. F. Tu, pharmacognosist, according to Chinese Pharmacopoeia (The Pharmacopoeia Commission of PRC,2010). 3- [4, 5-Dimethylthiazol-2-yl] 2,5-diphenyltetrazolium bromide (MTT) was from Sigma Chemical Co. (Saint Louis, MO, USA). Lactate dehydrogenase (LDH) assay kit was purchased from Nanjing Jiancheng Bioengineering Institute. (Nanjing,Jiangsu, China). Acridine Orange (AO)/Ethidium Bromide (EB) double stain kit and Hoechst 33258 were obtained from Solarbio Co., Ltd. (Beijing, China).Mitochondrial membrane potential assay kit with JC-1, 2,7-dichlorofluorescein diacetate (DCFH-DA) were purchased from Beyotime Institute of Biotechnology(Shanghai, China). Primary antibodies for caspase-3,cleaved caspase-3, PARP, cleaved PARP, cytochrome C,Bax, Bcl-2, p38, p-p38, ERK, p-ERK, JNK, p-JNK and α-tubulin were obtained from Cell signaling Technology(Boston, MA, USA).
Preparation of BSHX extract
BSHX consists of seven medicinal components (Table 1).For BSHX extract preparation, Tsizi (Cuscuta chinensis Lam.), Gouqi (Lycium barbarum L.), Fupenzi (Rubus chingii Hu), Wuweizi (Schizandra chinensis (Turcz.)Baill.), Cheqiancao (Plantago asiatica L.), Shuizhi(Hirudo nipponica Whitman.) and Yinyanghuo(Epimedium brevicornu Maxim.) were mixed in proportion of 8:8:4:1:2:1:8 and immersed in a total volume of 10 times (v/w) distilled water for 30min, then boiled for 1 h and boiled again for 1h in a total volume of 5 times (v/w) distilled water. The supernatants were combined and dried by lyophilization. The final dried extract was 25% (w/w) of the weight of the raw herbs and a voucher specimen was deposited in the Modern Research Center for Traditional Chinese Medicine,Peking University Health Science Center, Beijing, China.
Cell culture
Rat pheochromocytoma cell lines (PC12 cells) were obtained from Peking Union Medical College Cell Bank(Beijing, China) and grown in Dulbecco's Modified Eagle Medium (DMEM) (Macgene, Beijing, China)supplemented with 10% fetal bovine serum (Biowest,France) and 1% Penicillin-Streptomycin (100×, Macgene,Beijing, China) in a humidified incubator containing 95%air and 5% CO2at 37 °C.
Sample treatment
In preliminary test, PC12 cells were treated with BSHX extract (20, 50, 100 mg/L) alone for 48 h to evaluate the cytotoxicity. For neuroprotective effect analysis of BSHX extract against high glucose (HG)-induced neurotoxicity,PC12 cells were co-treated with BSHX extract (20, 50,100 mg/L) and HG (75 mM) for 48 h, followed by MTT assay for cell viability. All the other assays were done under the same condition with the range of BSHX extract concentration from 20 to100 mg/L.
Cell viability assay
PC12 cells were seeded into 96-well plates at a density of 3.5 × 103cells per well. After incubation with BSHX extract (with or without HG) for 48 h, the supernatant was removed and MTT solution (0.5 mg/mL) was added.After incubation at 37 °C for 4 h, MTT solution was removed and the cells were dissolved in dimethyl sulfoxide (DMSO) for shaking 5 min. The Optical density(OD) was measured at 570 nm using a micro plate reader.Cell viability (%) = [OD (treatment) – OD (blank)]/ [OD(untreated control) – OD (blank)] × 100%.
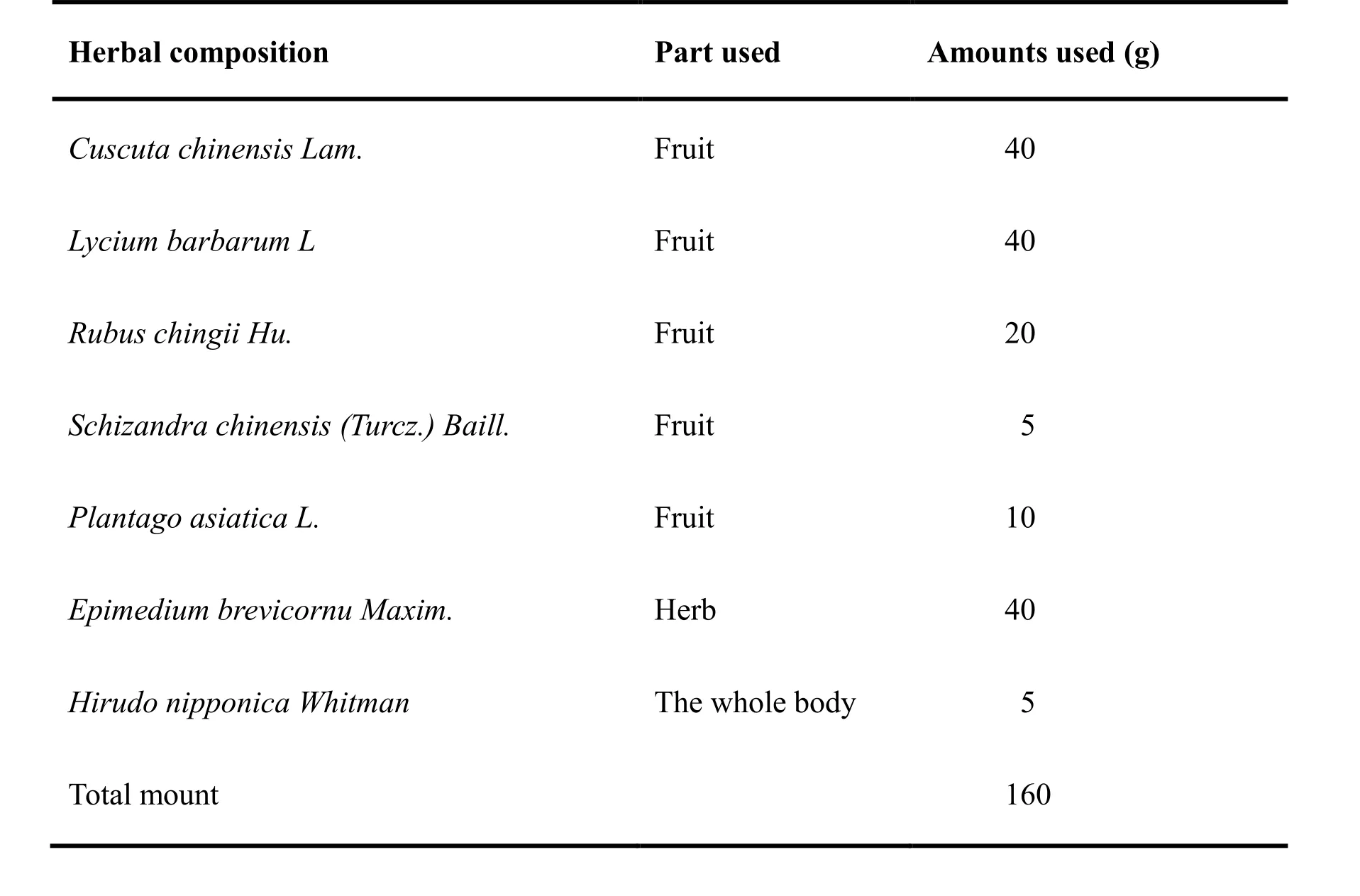
Table 1. Composition of BSHX
LDH assay
PC12 cells were cultured and stimulated to HG and BSHX extract treatment as described previously. Then,the LDH released from the cells was detected using a commercial LDH kit according to the manufacturer’s instructions. Absorbance was measured at 450 nm. LDH(U/L) = [OD (treatment) – OD (blank)] / [OD (standard)– OD (blank)] × 0.2 (mmol/L) × 1000.
Hoechst 33258 staining and AO/EB staining
For Hoechst 33258 staining, the cells were fixed by 4%paraformaldehyde for 30 min. Then the cells were washed with Phosphate Buffer Saline (PBS) for 3 times and incubated with Hoechst 33258 working solution (1 μg/mL)in the dark for 30 min at room temperature. Images were captured using a fluorescence microscope (IX73,Olympus, Japan) under excitation wavelength 352 nm /emission wavelength 461 nm. For AO/EB staining,removing medium from 96-well plates and adding AO/EB mixture (100 μg/mL AO and 100 μg/mL EB mixed) for 5 min in the dark at room temperature. Then the cells were washed with PBS for 3 times. The cells were visualized using a fluorescence microscope under excitation wavelength 490 nm / emission wavelength 530 nm for AO staining and excitation wavelength 520 nm /emission wavelength 590 nm for EB staining.
Intracellular ROS detection
For intracellular ROS detection, PC12 cells were seeded into 6-well plates at a density of 5×105and subjected to HG and BSHX extract treatment for 24 h. After incubation 24 h, PC12 cells were washed with serum-free dulbecco's modified eagle medium for 3 times, and added into 10μΜ 2’, 7’-dichlorofluorescin diacetate (DCF-DA),which is oxidized to fluorescent 2’, 7’-dichlorofluorescin(DCF) by hydroperoxides, at 37 °C for 30 min in the dark.Then, the cells were washed with cold PBS for 3 times and resuspended in PBS. The intracellular level of ROS was detected by measuring the mean fluorescence intensity by flow cytometry (BD FACSCaliburTM, USA).The excitation/emission wavelengths were at 488 / 525 nm. A minimum of 10,000 events were counted per sample. The values were expressed as the mean absorbance normalized to the percentage of control value.
Mitochondrial membrane potential assay
Mitochondrial depolarization was assessed using a commercial JC-1 assay kit. After treated with HG and BSHX extract, the cells were incubated with JC-1 working solution (1 ×) at 37 °C for 15 min in dark. Then,removing the supernatant and washing the cells for 2 times with JC-1 buffer solution (1 ×). The cells were visualized using a fluorescence microscope under excitation wavelength 460 nm / emission wavelength 530 nm for JC-1 monomer (detected in cells with depolarized mitochondria), and excitation wavelength 520 nm /emission wavelength 590 nm for JC-1 polymer (present in polarized mitochondria). The cells with green(monomeric) fluorescence were considered to have depolarized mitochondria; the cells with red (polymeric)fluorescence were considered to have normal mitochondria.
Western blot analysis
PC12 cells were collected and lysed in cold RIPA buffer with a 1× cocktail protease inhibitor. The supernatant was collected by high-speed centrifugation at 12,000 rpm for 10 min at 4 °C. Protein concentrations were determined by Bradford assay. Cytosol and mitochondria-enriched fractions were isolated with the Mitochondria Isolation Kit (Peerce, Rockford, USA) for cytochrome c analysis.For other proteins, total cell lysates were separated by SDS-PAGE (10% -15%) and transferred to Polyvinylidene Fluoride (PVDF) membranes. The polyvinylidene fluoride membranes were then blocked with 5% nonfat milk and incubated with the indicated primary antibodies at 4 °C for overnight. The membranes were washed with PBST (phosphate-buffered saline,0.1% Tween 20) for three times and incubated with secondary antibodies at room temperature for 2 h. Then,the membranes were washed with PBST for another three times and visualized using enhanced chemiluminescent substrate and scanned with the Kodak Digital Imaging System (Gel Logic 2200Pro, Kodak, USA).
Statistical analysis
All values are expressed as mean ± standard deviation(S.D.). Significance of the differences between mean values was determined by one-way analysis of variance(ANOVA) using the Statistical Package for Social Sciences (SPSS 16.0 software). P < 0.05 compared with control group was considered statistically significant.
Results
BSHX extract protects PC12 cells against HG-induced cell injury
To investigate whether BSHX extract could protect PC12 cells from HG insult, we treated PC12 cells with a range of BSHX extract concentrations (20, 50 and 100 mg/L)for 48 h under HG (75 mM) conditions. Assessing cell viability using the MTT assay showed that HG insult caused a significant decrease in the viability of PC12 cells (cell viability was approximately 56.9 ± 6.7%) and that BSHX extract treatment significantly prevented cell death in a concentration-dependent manner. The cell viability increased to 87.8 ± 9.2% when the cells were co-treated with BSHX extract (100 mg/L) (Figure.1A).Lactate Dehyrodgenase (LDH) is released from damaged cells and can therefore also be used as an indicator of cell toxicity. Consistent with the results of the MTT assay, we found that HG insult significantly increased LDH release from PC12 cells and that BSHX extract treatment prevented LDH release in a concentration-dependent manner (Figure. 1B), These results suggest that BSHX extract increases cell viability in HG-induced PC12 cells.
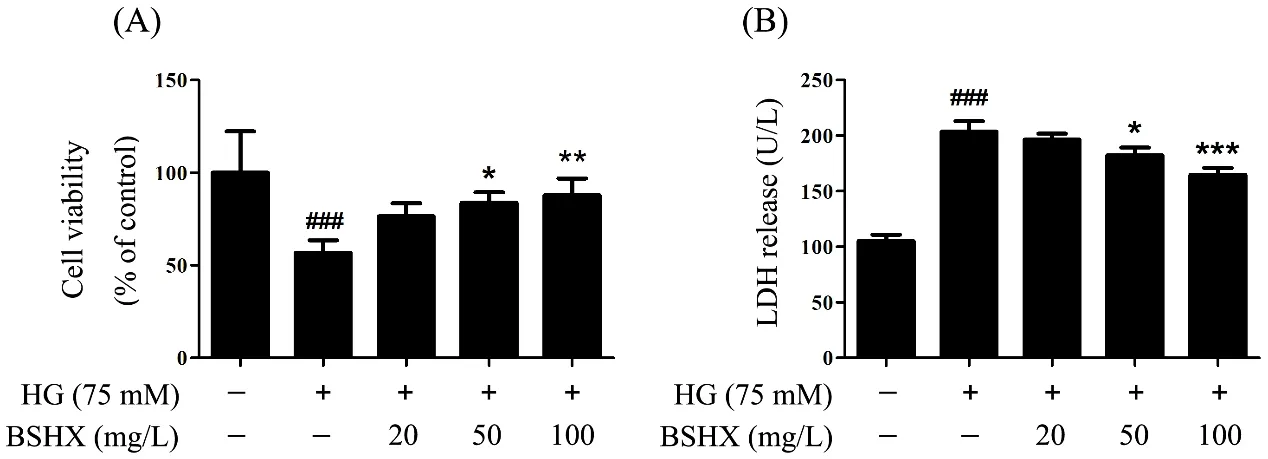
Figure.1 BSHX extract protects PC12 cells from HG-induced injury.
BSHX extract inhibits PC12 cell apoptosis via the caspase-9/3-dependent pathway
To determine the effect of BSHX extract on cell apoptosis,we used DNA dye Hoechst 33258 as a sensitive assay for apoptosis. The nuclei of normal cells stained by Hoechst 33258 showed uniform blue fluorescence, while apoptotic cells that nuclear chromatin cloud pyknosis showed hyper-chromatic and dense fluorescent particles.Percentage of apoptosis was calculated according to the number of apoptotic cells vs the total number of cells.Our data revealed that HG (75 mM) insult dramatically increased the proportion of apoptotic cells (51.7 ± 4.3%)and BSHX extract treatment significantly protected against HG-induced apoptosis in PC12 cells (Figure.2A).These results were further supported by AO/EB double staining analysis. AO enters both normal and apoptotic cells and emits green fluorescence whereas EB only enters apoptotic cells and emits red fluorescence [13].The AO/EB assay showed that the percentage of apoptotic cells significantly increased following HG (75 mM) insult (47.8.7 ± 2.6%) and decreased upon BSHX extract treatment in PC12 cells (Figure.2B), suggesting that BSHX extract could effectively inhibit the apoptosis of PC12 cells induced by HG.
Then, we investigated the effect of BSHX extract on caspase-3/PARP pathway, an important pathway for mitochondria-related apoptosis [14]. Western blot revealed that HG (75 mM) insult significantly increased the expressions of active forms of caspase-3 and PARP and decreased the expressions of total caspase-3 and PARP in PC12 cells, and this effect was markedly reversed by BSHX extract treatment (Figure.2C),suggesting that BSHX extract effectively protected PC12 cells against apoptosis via regulation of mitochondria-dependent caspase-3/PARP pathway.
BSHX extract inhibits intracellular ROS production in HG-induced PC12 cells
Since HG could enhance the formation of intracellular ROS [15], we tested the level of intracellular ROS to determine whether BSHX extract could inhibit the generation of ROS. There was a 3.23-fold increase in mean fluorescence intensity observed in PC12 cells that were exposed to 75 mM HG for 48 h compared to control.In contrast, co-treatment with BSHX extract significantly decreased the mean fluorescence intensity to approximately 0.76-, 0.61-, and 0.56-fold of the model group, respectively, indicating that BSHX extract could inhibit the production of ROS induced by HG(Figures.3A, B).
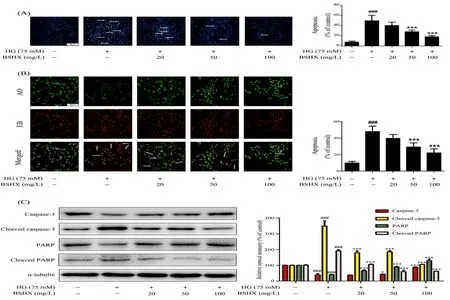
Figure.2 BSHX extract protects PC12 cells against HG-induced apoptosis via caspase-3/PARP pathway
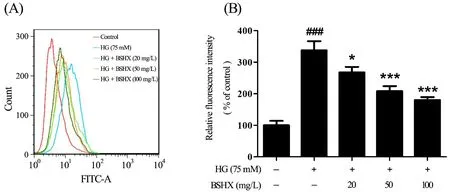
Figure.3 Effect of BSHX extract on ROS production in HG-treated PC12 cells
BSHX extract maintains mitochondrial homeostasis in HG-induced PC12 cells by regulating Bax and Bcl-2 expression
Mitochondrial membrane potential, is an important parameter of mitochondrial function used as an indicator of cell health. JC-1 is a lipophilic, cationic dye that can selectively enter into mitochondria and reversibly change color from green to red as the membrane potential increases. In healthy cells with high mitochondrial membrane potential, JC-1 spontaneously forms complexes known as J-aggregates with intense red fluorescence. On the other hand, in apoptotic cells with low mitochondrial membrane potential, JC-1 remains in the monomeric form, which shows only green fluorescence [16, 17]. The ratio of green fluorescence to red fluorescence can therefore reflect mitochondrial depolarization. In our study, the number of cells with depolarized mitochondria (green fluorescence) was significantly increased following HG (75 mM) for 48 h,and this increase was reversed by BSHX extract treatment in a concentration-dependent manner (Figure.4A).
We next detected the mitochondrial and cytoplasmic distributions of cytochrome c, a key player in activation caspase-dependent apoptosis pathway. We found that HG(75 mM) insult for 48 h induced a dramatic translocation of cytochrome c from mitochondria to the cytoplasm, and this relocation was significantly prevented by BSHX extract treatment (Figure.4B), indicating that BSHX extract could inhibit the release of cytochrome c from mitochondria.
Furthermore, Bcl-2 family proteins are important regulators of various apoptotic pathways. Bcl-2 can inhibit cell apoptosis caused by a variety of cytotoxic factors and is the inhibitor of cytochrome c release from mitochondria to cytoplasm. However, Bax, as a member of the Bcl-2 family, is a pro-apoptosis factor. When apoptosis is induced, Bax migrates from the cytoplasm to the mitochondria and destroys the mitochondrial integrity[5, 18]. In our study, the protein level of Bcl-2 was downregulated and Bax was upregulated by HG (75 mM)insult for 48 h, this effect was concentration-dependently reversed by BSHX extract (Figure.4C). All these results above suggested that BSHX extract protected PC12 cells against HG-induced mitochondrial depolarization by regulating the expression of Bax and Bcl-2.
Effect of BSHX extract on cell apoptosis via JNK/p38 MAPK signaling pathways
Some studies have shown that increased ROS production has been associated with the activation of MAPK pathways, to trigger cell apoptosis9 [19, 20]. So we investigated the effect of BSHX extract on Mitogen-Activated Protein Kinase (MAPK) signaling pathway to determine whether BSHX extract could attenuate HG-induced apoptosis. As shown in Figure 5,HG (75 mM) insult for 48 h increased the phosphorylations of c-Jun NH2-terminal kinase (JNK)and p38 in PC12 cells, which were significantly inhibited by BSHX extract in a concentration-dependent manner.However, similar result was not observed with Extracellular Regulated Protein Kinase (ERK) MAPK.The results suggest that BSHX extract could inhibit HG-induced cell apoptosis via JNK/p38 MAPK signaling pathways.
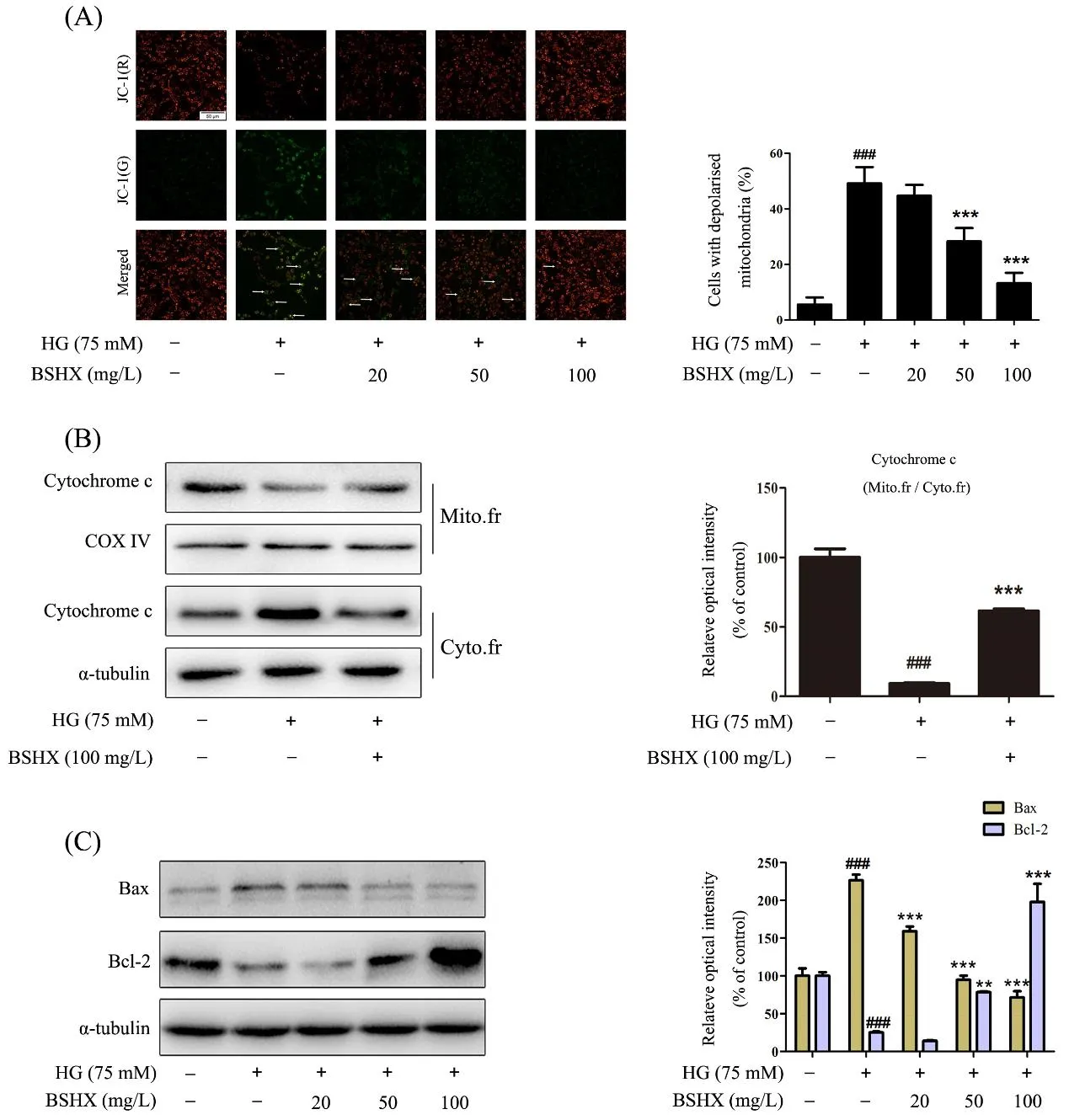
Figure.4 BSHX extract protects mitochondrial membrane integrity

Figure.5 BSHX extract inhibits JNK/p38MAPK pathways in HG-induced PC12 cells
Discussion
Diabetic encephalopathy (DE) is a common complication of type 2 diabetes mellitus (T2DM). It can cause cognitive impairment of the brains and is an important risk factor of mortality and disability in type 2 diabetes mellitus patients worldwide [3]. Thus, it is critical to study the mechanism of DE as well as its prevention and treatment strategies.
In China, traditional Chinese medicines (TCMs)have been used for the treatment of DM for many years and have shown a curative effect. Some clinical studies suggest that TCMs based on nourishing kidney and activing blood strategy can down-regulate blood glucose,promote circulation, and improve brain cognitive dysfunction. BSHX prescription is a representative of these TCMs. Our preliminary clinical study showed that BSHX prescription could effectively ameliorate blood glucose, homocysteine (HCY) and high-sensitivity C-reactive protein (hs-CPR) in serum, and improve the cognitive function of diabetic patients with mild cognitive impairment (MCI) [12]. However, there is a lack of evidence to prove the efficiency and pharmacological mechanism so far.
In this study, we found BSHX extract could attenuate the cell viability reduction induced by HG, as measured by MTT, LDH assay. In order to further explore the protective effect of BSHX extract on cell viability, we studied apoptosis by Hoechst 33258 and AO/EB staining analysis. The results showed that BSHX extract could
significantly reduce apoptosis induced by HG.As the marker of apoptosis, caspase-3 and PARP were significantly increased after treatment with HG but that the activation of these pro-apoptotic factors was inhibited by BSHX extract in a concentration-dependent manner.These above results indicated that BSHX extract had protective effect on PC12 cell damage induced by HG.
ROS is formed during the process of oxidative phosphorylation of cells, and its increase in production or the damage of antioxidant systems can lead to oxidative stress in cells [21]. Studies have shown that the high glucose could give rise to the accumulation of intracellular ROS and eventually active apoptotic signaling pathway. And the common mechanism of diabetic complications is oxidative stress [22, 23]. Our results showed that high glucose could lead to the increase of ROS in PC12 cells, which was consistent with previous studies [24]. It is speculated that the toxicity of high glucose to cells may be achieved through ROS-induced apoptosis. Mitochondria are the major sites of intracellular ROS production and the major target organ for ROS attack as well [25]. Under pathological conditions, the rapid increase of ROS can result in mitochondrial dysfunction, mitochondrial membrane potential collapse and eventually lead to cell apoptosis[26]. So we detected the integrity of the mitochondria.Our results showed that high glucose significantly decreased the mitochondrial membrane potential,increased the ratio of Bax/Bcl-2 that controlled the switch of mitochondrial permeability transition pore (MPTP),and promoted the leakage of cytochrome c, while BSHX extract could reverse these changes. These suggest that the neuroprotective of BSHX extract against HG-induced PC12 cells may result from maintaining the integrity of mitochondria.
MAPK signaling pathway also plays an important role in cell apoptosis. JNK, ERK and p38 MAPK are the most studied members of MAPK family. Their main way of action is to phosphorylate their specific sites, thereby regulating cell apoptosis [8, 27, 28]. Moreover, studies have demonstrated that excessive production of ROS can trigger p38MAPK phosphorylation [29-31], and ROS can induce or mediate the activation of the MAPK pathways[32]. A number of cellular stimuli that induce ROS production also in parallel can activate MAPK pathways in multiple cell types [32, 33]. Potential mechanism for MAPK activation by ROS may include the inactivation and degradation of the MAPK phosphatases that maintain the pathway in an inactive state [19]. On the other hand ,to further confirm whether MAPK pathways were activated by ROS generation, cells were pretreated with antioxidants (e.g. NAC, a ROS scavenger) to prevent ROS accumulation. And the results showed that MAPK activation was almost inhibited after cell stimulation with cellular stimuli [32, 33], indicating the involvement of ROS in activation of MAPK pathways. In addition,Studies showed that when cells were treated with the specific JNK inhibitor SP600125, the apoptosis induced by Cardiotoxin III could be blocked [34]. Our previous study found that modified Wu-Zi-Yan-Zong prescription(MWP) could inhibit the activation of ERK/p38 MAPK in Aβ-activated BV2 microglia [10]. However, in this study, we found that BSHX extract could inhibit the phosphorylation of JNK and p38 MAPK and we did not achieve the same result with p-ERK. We speculate that leeches paly a different role in BSHX extract. The results suggest that another protective mechanism of BSHX extract against apoptosis in HG-induced PC12 cells may be through the regulation of JNK/p38 MAPK signaling pathways.
Conclusion
BSHX extract, a polyherbal formula, shows neuroprotective effects on PC12 cells against HG insult.BSHX extract effectively inhibited HG-induced PC12 cell apoptosis through decreasing intracellular ROS generation, maintaining the integrity of mitochondrial membrane, and blocking caspase-3 and JNK/p38 MAPK signaling pathways (Figure.6). Collectively, our findings suggest that BSHX extract is a promising agent for the treatment of DE in clinical applications.
Author contributions
Conceived and designed the experiments: Zeng KW,Wang XM. Carried out the experiment: Zhao SY.Analyzed the data: Zhao SY, Zeng KW and Wang XM.Contributed regents/materials/ analysis tools: Zhao SY,Dong X, Tu PF, Zeng KW and Wang XM. Wrote the pater:Zhao SY.
Acknowledgments
We are grateful to the State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, China for the assistance.
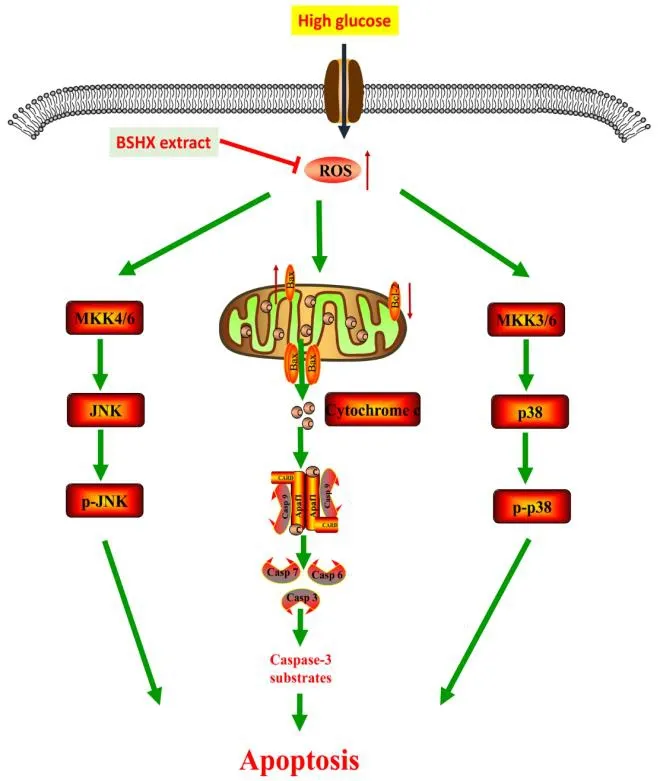
Figure.6 Mechanism of BSHX extract against HG-induced apoptosis in PC12 cells.
1. Riederer P, Korczyn AD, Ali SS, et al. The diabetic brain and cognition. J Neural Transm 2017, 124:1-24.
2. Hamed SA. Brain injury with diabetes mellitus:evidence, mechanisms and treatment implications.Expert Rev Clin Phar 2017, 10: 409-428.
3. Testa R, Bonfigli AR, Prattichizzo F, et al. The“Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutr 2017, 9: 437-445.
4. Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (Δψm) in apoptosis; an update.Apoptosis 2003, 8: 115-128.
5. Aouacheria A, Baghdiguian S, Lamb H M, Huska J D, Pineda F J, Harwick J M. Connecting mitochondrial dynamics and life-or-death events via Bcl-2 family proteins. Neurochem Int 2017, 109:141-161.
6. Bernardi P, Scorrano L, Colonna R, et al.Mitochondria and cell death. Mechanistic aspects and methodological issues. Febs J 1999, 264:687-701.
7. Yang L, Wu L, Du S, et al. 1,25-(OH)2D3inhibits high glucose-induced apoptosis and ROS production in human peritoneal mesothelial cells via the MAPK/P38 pathway. Mol Med Rep 2016, 14:839-844.
8. Tabakman R, Jiang H, Schaefer E, et al. Nerve growth factor pretreatment attenuates oxygen and glucose deprivation-induced c-Jun amino-terminal kinase 1 and stress-activated kinases p38α and p38β activation and confers neuroprotection in the pheochromocytoma PC12 model. J Mol Neurosci 2004, 22: 237-250.
9. Zou W, Zeng J, Zhuo M, Xu W, Sun L, Wang J, et al. Involvement of caspase-3 and p38 mitogen-activated protein kinase in cobalt chloride-induced apoptosis in PC12 cells. J Neurosci Res 2002, 67: 837–843.
10. Yu Q, Song FJ, Chen JF, et al.Antineuroinflammatory Effects of Modified Wu-Zi-Yan-Zong Prescription inβ-Amyloid-Stimulated BV2 Microglia via the NF-κB and ERK/p38 MAPK Signaling Pathways.Evid-Based Compl Alt 2017, 2017: 1-10.
11. Zeng KW, Zhang T, Fu H, et al. Modified Wu-Zi-Yan-Zong prescription, a traditional Chinese polyherbal formula, suppresses lipopolysaccharide-induced neuroinflammatory processes in rat astrocytes via NF-κB and JNK/p38 MAPK signaling pathways. Phytomed 2012, 19:122-129.
12. Fu H, Li WW, Wang H, et al. Effect of Bushen Huoxue Prescription on the Treatment of Mild Cognition Impairment of Type 2 Diabetes. Chin J Integr Trad West Med 2017, 37: 661-665.
13. Baskić D, Popović S, Ristić P, et al. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int 2006, 30: 924–932.
14. Galluzzi L, Lópezsoto A, Kumar S, et al. Caspases Connect Cell-Death Signaling to Organismal Homeostasis. Immunity 2016, 44: 221-231.
15. Hamed S, Brenner B, Roguin A. Nitric oxide: a key factor behind the dysfunctionality of endothelial progenitor cells in diabetes mellitus type-2.Cardiovas Res 2011, 91: 9-15.
16. Perelman A, Wachtel C, Cohen M, et al. JC-1:Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis 2012, 3: e430.
17. Perry S W, Norman J P, Barbieri J, et al.Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotech 2011, 50: 98-115.
18. Chipuk J E, Green D R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Bio 2008,18:157-164.
19. Son Y, Cheong Y K, Kim N H, et al.Mitogen-Activated protein kinases and reactive Oxygen species: how can ROS activate MAPK pathways? J Signal Transduct 2011: 792639.
20. Zhang L, Zhang Z, Chen F, et al. Aromatic heterocyclic esters of podophyllotoxin exert anti-MDR activity in human leukemia K562/ADR cells via ROS/MAPK signaling pathway. Eur J Med Chem 2016, 123:226-235.
21. Kanninen K, White AR, Koistinaho J, et al.Targeting Glycogen Synthase Kinase-3β for Therapeutic Benefit against Oxidative Stress in Alzheimer's Disease: Involvement of the Nrf2-ARE Pathway. Int J Alzheimers Dis 2011, 2011:985085.
22. Babizhayev MA, Strokov IA, Nosikov VV, et al.The Role of Oxidative Stress in Diabetic Neuropathy:Generation of Free Radical Species in the Glycation Reaction and Gene Polymorphisms Encoding Antioxidant Enzymes to Genetic Susceptibility to Diabetic Neuropathy in Population of Type I Diabetic Patients. Cell Biochem Biophys 2015, 71:1425-1443.
23. Volpe C, Villardelfino PH, Dos PA, et al. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis 2018, 9: 119-127.
24. Chen K, Zhang M, Chen BD, et al. Ginkgolide B inhibits apoptosis in high glucose-stimulated human umbilical vein endothelial cells. Chinese Pharmacological Bulletin 2017, 33: 378-383.
25. Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T, et al. Deleterious Role of Superoxide Dismutase in the Mitochondrial Intermembrane Space. J Bio Chem 2008, 283: 8446-8452.
26. Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Bio 2003, 206: 1107.
27. Wu N, Lin X, Zhao X, et al. MiR-125b acts as an oncogene in glioblastoma cells and inhibits cell apoptosis through p53 and p38MAPK-independent pathways. Brit J Cancer 2013, 109: 2853.
28. Shi L, Yu X, Yang H, et al. Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways. Plos One 2013, 8: e66781.
29. Chung H, Kim E, Lee DH, et al. Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology 2007,148: 148.
30. Palanivel K, Kanimozhi V, Kadalmani B. Verrucarin A alters cell-cycle regulatory proteins and induces apoptosis through reactive oxygen species-dependent p38MAPK activation in the human breast cancer cell line MCF-7. Tumor Biology 2014, 35: 10159.
31. Hua X, Chi W, Su L, et al. ROS-induced Oxidative Injury involved in Pathogenesis of Fungal Keratitis via p38 MAPK Activation. Sci Rep 2017, 7: 10421.
32. Mccubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 2006, 8: 1775-1789.
33. Torres M, Forman H J. Redox signaling and the MAP kinase pathways. Biofactors 2003, 17:287-296.
34. Chien CM, Yang SH, Yang CC, Chang LS, Lin SR.Cardiotoxin III induces c-jun N-terminal kinase-dependent apoptosis in HL-60 human leukaemia cells. Cell Biochem Funct 2008, 26:111-118.
 TMR Modern Herbal Medicine2018年3期
TMR Modern Herbal Medicine2018年3期
- TMR Modern Herbal Medicine的其它文章
- Antioxidative and antiapoptotic effects of (+)-clausenamide on acetaminophen-induced nephrotoxicity in mice
- A novel natural compound Shikonin inhibits YAP function by activating AMPK
- Pharmacological effects of Paeoniflorin and Albiflorin on IL-3, GM-CSF, IL-6 and TNF-α in the rats of syndrome of stagnation of liver qi and blood deficiency
- Effect of splitting combination of different components of Zhilong Huoxue Tongyu Capsule on vascular remodeling in hypertension
- Kangfuxin Fluid on the Treatment of Ulcerative Colitis with Retention Enema: a Systematic Review
- Evidence-based optimization of integrated traditional Chinese and Western medicine therapies for prevention and treatment of coronary heart disease: design and implementation
