Protective and therapeutic potentials of Dunaliella salina on aging-associated cardiac dysfunction in rats
Farouk K. El-Baz, Gehad A. Abdel Jaleel, Dalia O. Saleh, Rehab A. Hussein
1Plant Biochemistry Department, National Research Centre (NRC), 33 El Bohouth St. (Former El Tahrir St.), Dokki, Giza, P.O.12622, Egypt
2Pharmacology Department, National Research Centre (NRC), 33 El Bohouth St. (Former El Tahrir St.), Dokki, Giza, P.O.12622, Egypt
3Pharmacognosy Department, National Research Centre (NRC), 33 El Bohouth St. (Former El Tahrir St.), Dokki, Giza, P.O.12622, Egypt
Keywords:Aging Cardiac dysfunction Dunaliella salina Rats
ABSTRACT Objective: To investigate the possible protective and/or therapeutic potentials of Dunaliella salina (D. salina) biomass, its carotenoid and polar fractions on cardiac dysfunction associated with D-galactose (D-GAL) induced aging in rats. Methods: Aging associated cardiac dysfunction was induced in rats by injection of D-GAL (200 mg/kg; i.p) for 8 weeks. D-GAL injected rats were treated with two regimens; protective regimen where D. salina biomass(250 mg/kg), its carotenoid (250 μg/kg) and polar (250 μg/kg) fractions were given orally for two weeks concurrently with D-GAL injection as well as treatment regimen where the three treatments were given orally for 28 consecutive days after D-GAL injection. Results: D-GAL injection for 8 weeks was accompanied with dramatic electrocardiographic changes as well as profound elevation in serum levels of homocysteine, creatinine kinase isoenzyme and lactate dehydrogenase in addition to the reduction of the cardiac content of glucose trasporter 4. D-GAL also induced reduction in cardiac superoxide dismutase activity and elevation of inducible nitric oxide synthetase and interleukin-6. On the other hand, oral administration of D. salina carotenoid fraction as well as the total biomass significantly attenuated the D-GAL-induced disturbances in the above mentioned parameters where the protective regimen appeared more successful in controlling the manifestations of cardiac dysfunction. The histopathological examination further emphasized the promising results. Besides, the HPLC analysis of the carotenoid fraction of D. salina revealed the presence of 2.31% β-carotene. Conclusions:D. salina carotenoid fraction as well as the total biomass ameliorate D-GAL-induced aging associated cardiac dysfunction which is attributed to the potent antioxidant activity of β-carotene.
1. Introduction
Aging is associated with functional and structural decline of various body systems which leads to elevated morbidity and mortality. Increasing prevalence of cardiovascular disorders is clearly related to aging of the population[1]. Myocardial fibrosis,ventricular wall thickness, and valvular calcification are among the major changes observed in the human heart of elderly, leading to reduced ventricular compliance. Age-associated macroscopic alterations within major blood vessels in rats which are evidenced as dilation, medial thickening and formation of an intima[2]; are alike in various ways to those that happen in humans[3]. Moreover, damage of arterial elasticity leads to reduction of the diastolic pressure and elevation of arterial pulse pressure that regulates coronary artery perfusion. Similarly, aging forces a maximum on cardiac output that imitates both the age-related elongation of time needed for myocardial elasticity and decreased maximal heart rate (HR)[4].
Age-related cardiac remodeling mechanism is not totally clear,however, there are a number of postulations describing this process.The most significant assumption is that elevated production of reactive oxygen species causes major alterations in the cardiac structure as reported in a number of studies. Moreover, the altered secretions of nitric oxide and endothelin which are potent regulators for the arterial tone from the endothelium lead to endothelial dysfunction.Several studies performed on humans and animals have shown that aging is strongly linked with reduced nitric oxide bioavailability leading to a decline in the endothelial dependent vasodilatation[3,5].Endothelial dysfunction is considered to be the hallmark of a number of cardiovascular disorders, viz. hypercholesterolemia, hypertension and atherosclerosis.
Microalgae are unicellular microorganisms that drew increasing interest due to their multiple physiological and biochemical mechanisms to withstand stress in environmental conditions.These defensive strategies involve the synthesis of a wide array of phytochemical compounds as secondary metabolites. These metabolites exhibit various biological and pharmacological activities,including antioxidant, antiviral, antidiabetic and anticancer effects.One of the most important microalgae that withstand very high concentrations of salt is Dunaliella salina (D. salina). D. salina has been isolated and identified one hundred years ago. It is an unicellular marine phytoplankton that is related to the phylum Chlorophyta[6]. It is isolated from the salty lakes in El-Fayoum and Lake of Bardawil-Sinai in Egypt. It is rich in carotenoids which accounts for the red coloration of these salty lakes. It is considered to be the richest natural producer of carotenoids. However, β-carotene remains the major carotenoid accumulated by D. salina[7]. Its complex composition of the 9-cis and all-trans isomers supplies more potent antioxidant efficacy than the synthetic β-carotene. Therefore, D.salina is considered to be a powerful antioxidant due to elevation of free radical quenching capabilities and its ability to protect cells from oxidative destruction[8].
In the present study, the protective as well as therapeutic potentials of D. salina biomass, its carotenoid and polar fractions on cardiac dysfunction associated with D-galactose (D-GAL)-induced aging in rats were investigated. To achieve this aim, electrocardiographs were recorded and the serum cardiac function; cardiac oxidative stress biomarkers and cardiac inflammatory mediators were assessed for all treated and untreated groups. Moreover, histopathological changes in the cardiac tissue were also examined.
2. Materials and methods
2.1. Cultivation of D. salina
D. salina was isolated and identified through the algal innovation group, National Research Centre. Isolation was done from salt deposition basins of The Egyptian Salts and Minerals Company,EMISAL. The culture was grown on BG11 media containing NaCl(100 g/L) and NaNO3(0.3) g/L[9]. Cultivation was done in small ponds with 80 L in capacity containing 70 L microalgae culture.The culture was supplied with continuous aeration and constant fluorescent light at intensity ≈≈2 500 lux. The temperature was adjusted at (22±3) ℃. Culture media were added twice a week.After two weeks, microalgal biomass was collected using electroflocculation[10] and allowed to dry on a water bath at a temperature not exceeding 60 ℃. The dried biomass was ground thoroughly for cell wall disruption and subjected to successive extraction. The carotenoid fraction was prepared using hexane, ethyl acetate (80:20)till exhaustion[11]. The residue was allowed to dry and further extracted with 70% methanol till exhaustion to render the polar fraction. The two fractions were dried under reduced pressure in a rotary evaporator apparatus at temperature not exceeding 40 ℃ till complete dryness and the dried fractions were kept in dark bottles in the refrigerator at a temperature less than 4 ℃ for further analysis.
2.2. HPLC estimation of β-carotene in carotenoid fraction
2.2.1. Sample preparation
The carotenoid fraction was dissolved in 5 mL methanol/acetone(1:1), filtered through 0.45 μm membrane filter, and kept in the dark for HPLC analysis.
2.2.2. Preparation of standard curve
Standard solutions of β-carotene in the range of 0.02-0.1 mg of standard β-carotene (purchased from Sigma-Aldrich, Germany)were dissolved in 5 mL methanol/acetone (1:1). Calibration curve was constructed by plotting the amount of standard against the peak area. The regression equation and the correlation coefficient (r2) were calculated.
2.2.3. HPLC analysis
Analysis was done using an analytical HPLC system equiped with a Zorbax-C18 column (5 μm; 250 mm × 4.6 mm) on an Agilent 1200 series instrument. An isocratic elution was done using mobile solvent system: methanol/water/dichloromethane/acetonitrile (70:4:13:13, v/v/v/v). Solvents used (HPLC grade) were filtered through 0.45 μm membrane and degassed before use. Flow rate was adjusted at 1.0 mL/min and the analysis was done at room temperature. Detection was recorded at wavelength 280 and 480 nm using photodiode-array detection system[12].
2.2.4. Identification and quantification of β-carotene
β-carotene was identified through comparison of the retention time against that of the reference standard. The amount of β-carotene was calculated from the calibration curve as mg%[12].
2.3. Pharmacological study
2.3.1. Animals
The study was carried out on male Wistar albino rats, weighing 130-150 g which were supplied from the Animal House Colony of the National Research Centre. The animals were housed in plastic cages under conventional conditions. They were fed on basal diet and water ad libitum and allowed to adapt to the laboratory environment before the experiment was performed. The experimental study was performed according to the ethical procedures that was permitted by the National Research Centre (Dokki, Giza-Egypt)-Medical Research Ethics Committee for the use of animal subjects under the approval number (17/005).
2.3.2. Chemicals
D-GAL was purchased from Sigma–Aldrich (St. Louis, Missouri,USA). All other chemicals used were obtained from standard commercial suppliers and were of beat available quality.
2.3.3. Experimental design
Aging associated cardiac dysfunction was induced in rats by intraperitoneal injection with D-GAL (200 mg/kg/day) for eight consecutive weeks. Rats were allocated randomly into eight groups,each group includes six rats. Group Ⅰ was considered as negative control group, group Ⅱ as positive control which received D-GAL for 8 weeks. Groups Ⅲ, Ⅳ and Ⅴ were injected by D-GAL for 8 weeks then respectively received D. salina biomass (BDS; 250 mg/kg; p.o.), its carotenoid fraction (CDS; 250 μg/kg; p.o.) and polar fraction (PDS; 250 μg/kg; p.o.) orally concurrently with D-GAL injection for another two weeks, in a protection regimen.
Groups Ⅵ, Ⅶ and Ⅷ were injected by D-GAL for 8 weeks then respectively received D. salina, biomass (BDS; 250 mg/kg; p.o.), its carotenoid fraction (CDS; 250 μg/kg; p.o.) and polar fraction (PDS;250 μg/kg; p.o.) orally for four weeks after D-GAL injection in the treatment regimen. The fractions doses were calculated according to their yield.
2.3.4. Electrocardiographic recordings
Twenty-four hours after the last dose of the drugs administration,the rats were kept under anesthesia using thiopental at dose of 5 mg/kg under warm conditions and heating lamp was used to prevent hypothermia. Electrocardiographic recording (ECG) (HPM 7100,Fukuda Denshi, Tokyo, Japan) was carried out via subcutaneous peripheral limb electrodes where HR, PR interval, RR interval, QT interval, ST height as well as QRS duration were monitored.
2.3.5. Serum collection and tissue preparation
Blood samples were collected and the sera were separated at the end of the experiment for further biochemical analysis. The heart was rapidly isolated after the rats were sacrificed by cervical dislocation, then homogenized and the resulting supernatants were stored for various biochemical assays.
2.3.6. Serum biochemical analysis
Estimation of serum cardiac and renal injury such as creatinine kinase isoenzyme (CK-MB), lactate dehydrogenase (LDH) was performed on stored sera. Serum CK-MB level was assessed using immune-inhibition method developed by Wurzburg et al.[13]. LDH level was determined enzymatically according to the method developed by Buhl and Jackson[14]. Serum levels of homocysteine(HS) was determined using enzyme-linked immunosorbent assay kit according to the user guide supplied by the manufacturer.
2.3.7. Cardiac biochemical analysis
The heart tissues were dissected out immediately, washed with ice cold saline, homogenated and the homogenate was used for the determination of cardiac levels of superoxide dismutase (SOD),interleukin-6 (IL-6), inducible nitric oxide synthetase (iNOS) and glucose trasporter 4 (GLUT-4) which were evaluated using enzymelinked immunosorbent assay kit according to the manufacture procedures.
2.4. Preparation of sections for histopathological examination
Cardiac tissues were dissected out after sacrifice, and fixed in 10%formalin. Tissues were then placed in paraffin afterwards; 5 μM sections were cut and examined microscopically for the evaluation of histopathological alterations.
2.5. Statistical analysis
Data was represented as mean ± SEM. Statistical analysis wasperformed by one-way analysis of variance (ANOVA) followed by Tukey-Kramer test for multiple comparisons. Statistical significance was acceptable at a level of P < 0.05. Data analysis was accomplished using the software program GraphPad Prism (version 5).
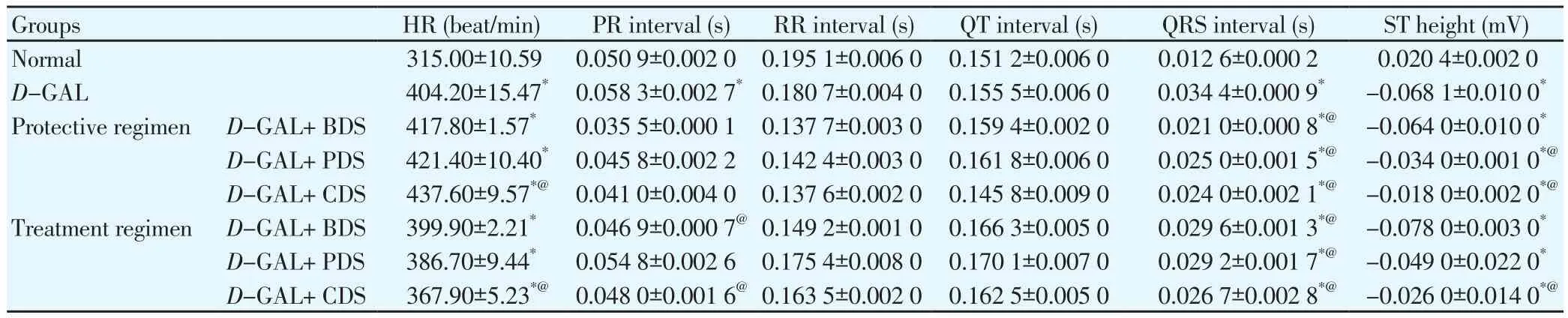
Table 1 Effect of D. salina and its fractions on HR, PR, RR, QT and QRS intervals and ST height in D-GAL induced cardiotoxicity in rats.
3. Results
3.1. HPLC analysis of β-carotene
The HPLC analysis of the carotenoid fraction of D. salina revealed the presence of 2.31% β-carotene which is equivalent to 77 mg/100 g D.salina biomass.
3.2. Effect of D. salina on the electrocardiographic measurements
D-GAL intraperitoneally treated rats for eight consecutive weeks showed dramatic changes in the ECG pattern in the form of irregular rhythm of heart beats, depressed ST height as well as negative T wave.Oral treatment of D-GAL-treated rats with CDS (250 μg/kg), BDS(250 mg/kg) and PDS (250 μg/kg) showed significant amelioration in the ECG pattern in ST height and T wave (Figure 1) in both protective and treatment regimens.
Moreover, D-GAL-treated rats reported marked elevation in PR interval and QRS interval by 14% and 173% respectively compared to normal group with decrease in the ST height by 433%,no significant changes in RR and QT intervals. In addition, HR significantly increased by 28%, in comparison with normal group.
Oral administration of BDS (250 mg/kg), CDS (250 μg/kg) in treatment regimen to D-GAL-treated rats showed a decrease in PR interval by 20% and 18% respectively. Similarly, treatment with PDS (250 μg/kg) and CDS (250 μg/kg) in protective regimen as well as CDS (250 μg/kg) in treatment regimen showed a decrease in ST height by about 50%, 74% and 62%, respectively.
Treatment with BDS (250 mg/kg), PDS (250 μg/kg) and CDS(250 μg/kg) in both protective and treatment regimens showed a restoration in the QRS interval by an average of about 26%. HR was also attenuated by treatment with CDS (250 μg/kg) in treatment regimen by about 9% (Table 1).
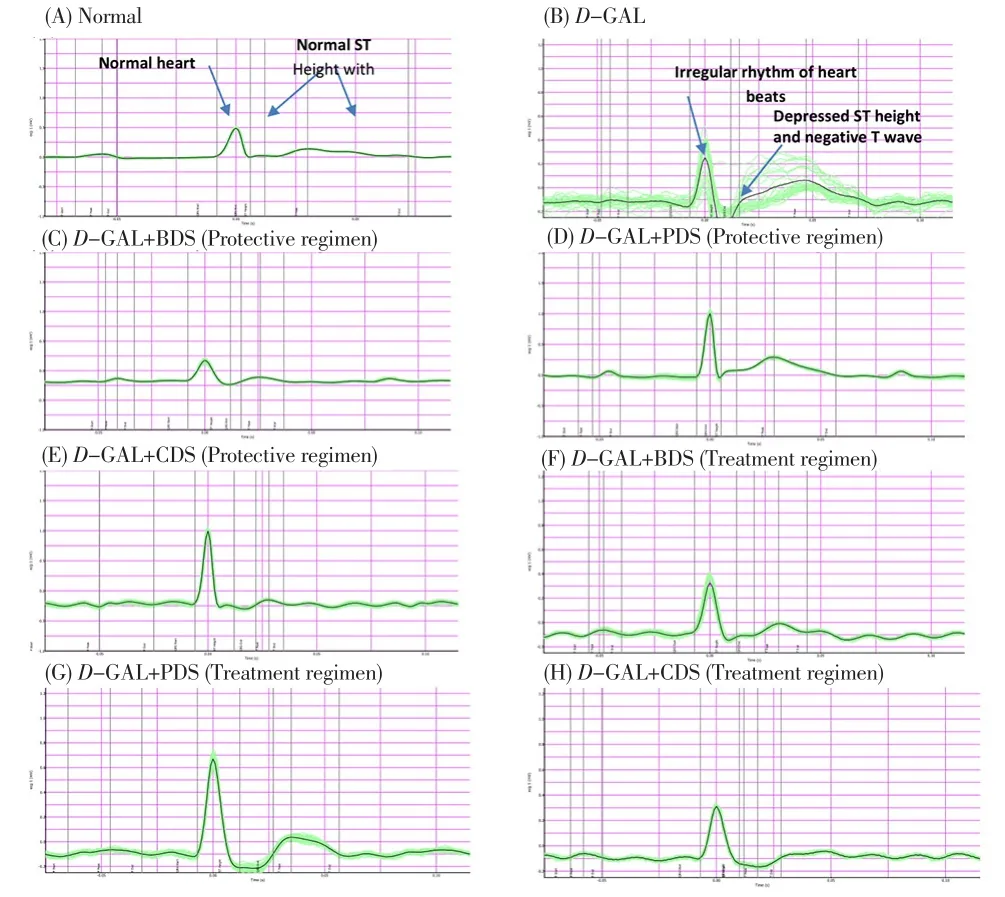
Figure 1. Effect of D. salina and its fractions on ECG patterns in D-GAL induced cardiotoxicity in rats.
3.3. Effect of D. salina on serum cardiac biomarkers
Induction of aging by D-GAL was associated with an elevation of serum levels of cardiac function markers especially HS, CKMB and LDH by about 2.6, 10, and 3 folds, respectively compared with normal group. Oral administration of BDS (250 mg/kg), PDS(250 μg/kg) and CDS (250 μg/kg) in both protective and treatment regimens to cardiac dysfunction-induced rats showed a decreased serum HS level by about 28%, 40%, 52%, 18%, 15% and 49%,respectively.
Similarly, serum CK-MB level was reduced by about 23%, 26%and 66%, respectively, in the protective regimen and by an average of about 90% in the treatment regimen. Regarding the serum LDH level, in the protective regimen CDS (250 μg/kg) reduced by 40%as well as PDS (250 μg/kg), CDS (250 μg/kg) in treatment regimen reduced by 36% and 38%, respectively (Table 2).
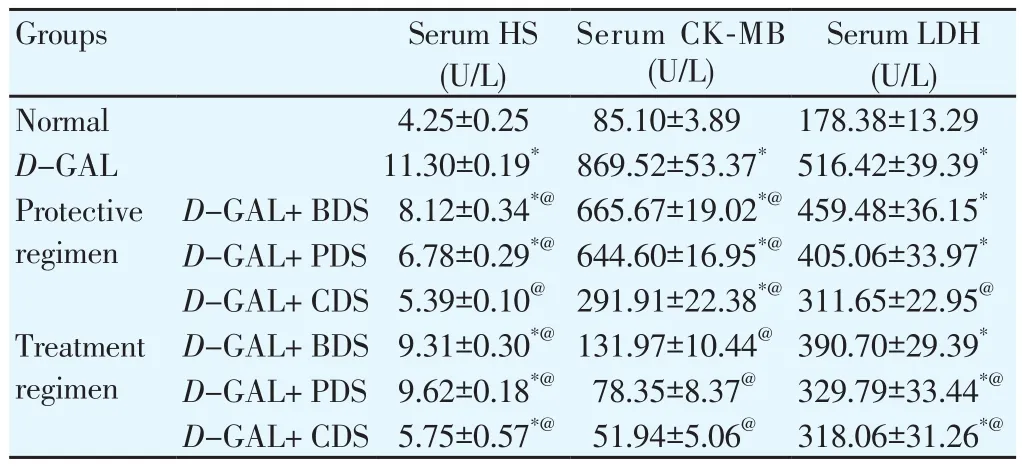
Table 2 Effect of D. salina and its fractions on serum levels of HS, CK-MB and LDH in D-GAL induced cardiotoxicity in rats.
3.4. Effect of D. salina on cardiac inflammatory mediators
Cardiac dysfunction was accompanied with a prominent increase in the cardiac levels of IL-6 and iNOS reaching about 5 and 22 folds of the normal value, respectively. Oral administration of BDS(250 mg/kg), PDS (250 μg/kg) and CDS (250 μg/kg) in protective regimen exhibited a reduction in the cardiac IL-6 and iNOS by an average of 55% and 60%, respectively. However, oral administration of PDS (250 μg/kg) and CDS (250 μg/kg) in treatment regimen decreased cardiac level of IL-6 by about 29% and 39%, respectively,and reduced the cardiac level of iNOS by about 35% and 64%,respectively (Figure 2).
3.5. Effect of D. salina on cardiac oxidative stress markers
Cardiac dysfunction showed a prominent reduction in cardiac SOD content by about 74%, as compared to control group. Oral administration of BDS (250 mg/kg), PDS (250 μg/kg) and CDS(250 μg/kg) in protective regimen showed an elevation of the cardiac level of SOD by 72%, 82% and 116%, respectively. Besides, CDS(250 μg/kg) in the treatment regimen showed an increase in the SOD content by about 173% (Figure 2).
3.6. Effect of D. salina on cardiac GLUT-4
Cardiac dysfunction was associated with a marked decrease in the cardiac GLUT-4 by about 64%. Treatment of cardiac dysfunction with PDS (250 μg/kg) and CDS (250 μg/kg) in protective regimen elevated the level of cardiac GLUT-4 by 60% and 70%, respectively.However, BDS (250 mg/kg), PDS (250 μg/kg), CDS (250 μg/kg) in the treatment regimen enhanced the level of cardiac GLUT-4 by an average of about 62% as compared to D-GAL treated group (Figure 2).
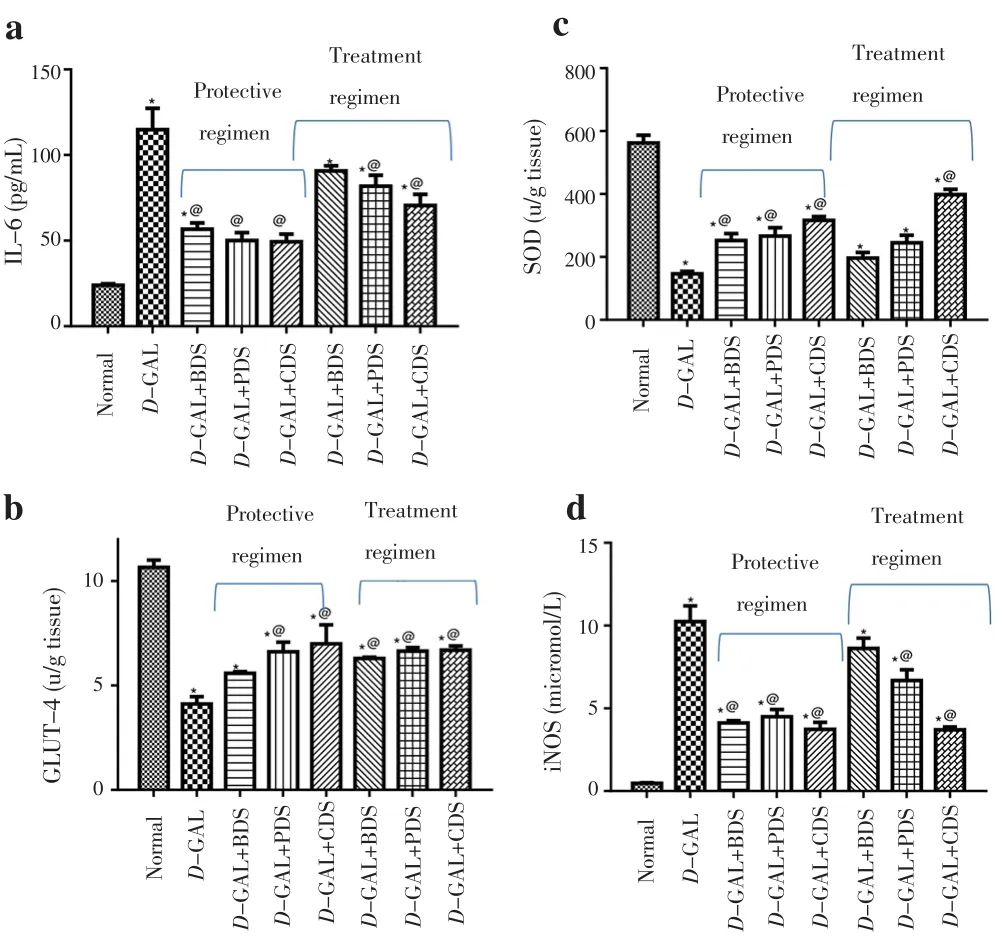
Figure 2. Effect of D. salina and its fractions on cardiac level of IL-6 (a),GLUT-4 (b), SOD (c) and iNOS (d) in D-GAL induced cardiac dysfunction in rats.
3.7. Effect of D. salina on the histopathological alterations
Photomicrography of the variable sections revealed that cardiac tissue isolated from D-GAL-treated rats showed abnormal myocardial architecture, decreased cellular volume with exhibition of spaces between the cells. Areas of calcification were also seen at variable sections (Figure 3B). Cardiac tissue isolated from rats in the protective regimen treated orally with BDS (250 mg/kg) exhibited areas of calcification, thrombosed vessel and inflammatory cellular infiltrate (Figure 3C) while PDS (250 μg/kg) revealed highest affection with the treatment and nearly normal appearing sections(Figure 3D) and CDS (250 μg/kg) showed aggressive insult with massive edema (brown arrow), inflammatory infiltrate (red arrow),calcification (black arrow), lipid deposition (yellow arrows) and areas of necrosis (blue arrow) (Figure 3E).
Moreover, cardiac tissue isolated from rats treated orally with BDS(250 mg/kg) in the treatment regimen showed preserved nuclei,average cell size with no abnormal spaces (Figure 3F), while PDS(250 μg/kg) revealed congested vessels with apparent cellular shrinkage evidenced by presence of intercellular spaces as well as cells showing vesicular nuclei along with pyknotic nuclei scattered all over the field as well as areas of fibrosis (Figure 3G). However,tissues isolated from rats treated with CDS (250 μg/kg) in treatment regimens revealed mostly regular architecture of cardiac muscle cells(Figure 3H).
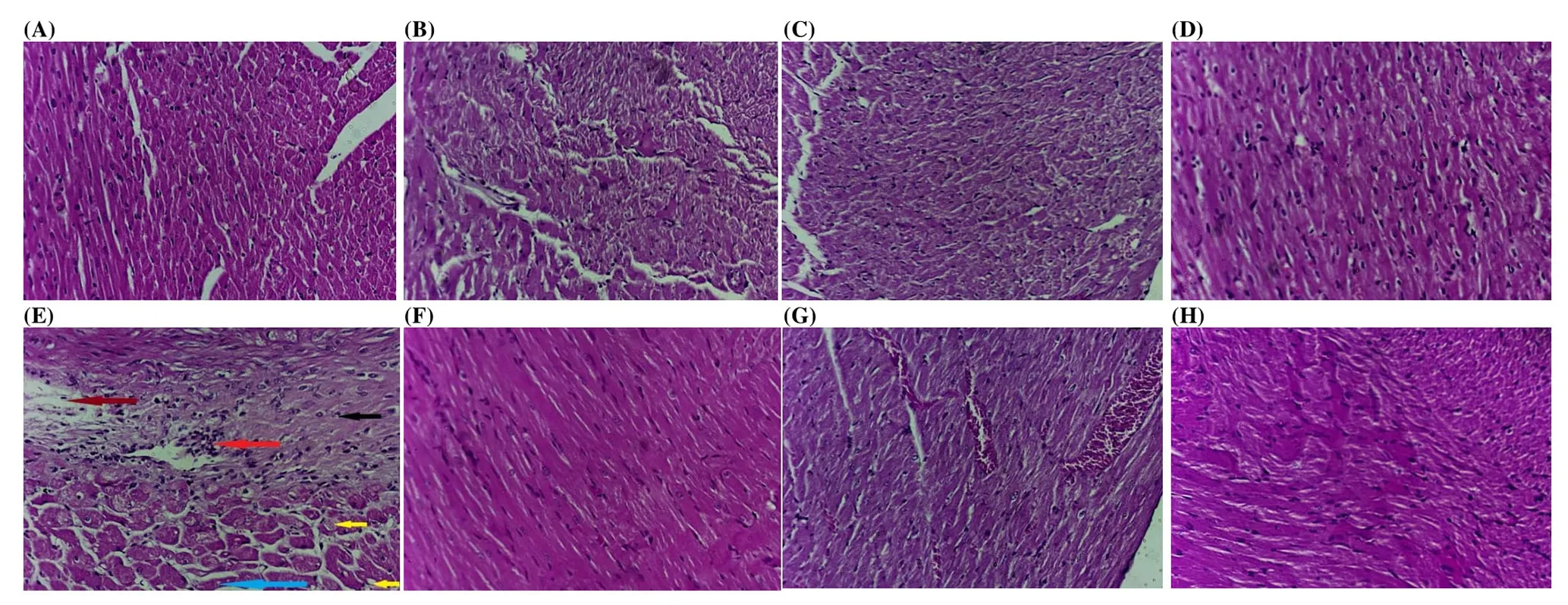
Figure 3. Photomicrograph of sections of heart tissue.
4. Discussion
Age related health conditions are drawing increasing interest with the expansion of the life span of human beings. As age advances,the heart’s ability to maintain the variations of the vascular system worsens causing reduced cardiac performance and dramatic myocardial deterioration[15]. Studies have reported an elevated prevalence of cardiovascular disease within elderly[16]. With the increasing evidence of the negative impact of oxidative stress on various human systems, the possible role of β-carotene rich D.salina on cardiac remodeling process in rats with hypertension and following myocardial infarction viz, cardiac aging was investigated. It has been shown that induction of aging with D-GAL was associated with dramatic changes in the ECG pattern and a prominent increase in the serum cardiac biomarkers levels as well as an elevation in cardiac oxidative stress and inflammation. Moreover,histopathological cardiac examination revealed prominent changes in the architecture.
In elderly with no signs of cardiovascular diseases, aging has been shown to be linked with multiple ECG changes which include mild prolongation of PR interval, QRS axis leftward shift, and augmented prevalence, density, and complexity of ectopic beats. Elevated QRS voltage, Q waves and QT interval prolongation are other abnormalities implicated with age related cardiovascular aliments[3].In the current study, aged rats were associated with irregular rhythm of heart beats, depressed ST height as well as negative T wave. The ECG pattern of D-GAL treated rats showed significant increase in PR interval and QRS interval with decrease in the ST height, and without significant change in RR and QT intervals.
Treatment of D-GAL induced cardiac dysfunction with BDS and CDS in treatment regimen showed a decrease in PR interval.Similarly, treatment with PDS and CDS in protective regimen as well as CDS showed decrease in ST height. Treatment with BDS,PDS and CDS in both protective and treatment regimens showed a restoration in the QRS interval. HR was also attenuated by treatment with CDS in treatment regimen.
Induction of aging with D-GAL was accompanied with hyperhomocysteinemia and elevated levels of CK-MB and LDH;which are markers of cardiac dysfunction. HS, a cytotoxic and prooxidant sulfur-containing amino acid, requires folic acid, vitamin B12, and other vitamins to be transformed to another essential amino acid-methionine which is cardioprotective. It plays a major role in many degenerative diseases of aging leading to the inflammatory response and hence oxidative stress[17] by inducing DNA strand breakage, thus increasing the risk of heart disease, and causing various forms of vascular disease. Serum HS rises with age, perhaps contributing to the aging process[3]. In the present study, oral administration of D. salina biomass, its carotenoid and polar fractions in both protective and treatment regimens to cardiac dysfunctioninduced rats showed decreased serum levels of HS, CK-MB and LDH.
Oxidative stress theory of aging proposes aggravation of oxidative damage due to cellular metabolism which has been identified as one of the main etiologies of coronary heart disease, stroke, atherosclerosis,ischemia/reperfusion injury, and hypertension[16]. Since the heart is an organ that produces great levels of reactive oxygen species and aggravates oxidative stress by time due to low turnover of antioxidant molecules, elevated both oxygen consumption and cellular longevity,which in turn, makes it particularly sensitive to oxidative stress[18].In accordance with Marques et al.[18], cardiac SOD level decreased in D-GAL treated rats. Several studies have reported decreased cell’s capability to alleviate the oxygen radicals. These radicals affect the redox homeostasis leading to oxidative stress and/or oxidant sensitivity in the myocardium and heart failure is due to either insufficient activity and/or deficiency of antioxidants during aging[19,20]. Treatment with cardiac dysfunction with D. salina biomass, its carotenoid and polar fractions in both protective and treatment regimens showed an elevation of the cardiac level of SOD.Cardiac dysfunction associated with aging showed a marked elevation in the cardiac levels of IL-6 and iNOS due to elevated inflammatory process that occurs within the aged cardiac tissue[21].IL-6 is intensely released in the heart, particularly in the ageaugmented microvascular walls[21]. Oral administration of D. salina biomass, its carotenoid and polar fractions in both protective and treatment regimens exhibited a reduction in the cardiac IL-6 and iNOS. All of these findings were in accordance with a recent study that investigated the effect of carotenoids on cisplatin-induced cardiotoxicity in rats, demonstrating that it elevated the cardiac levels of SOD and catalase as well as depressed the levels of malondialdehyde, tumor necrosis factor alpha and IL-6[22].
In the present study, induction of cardiac dysfunction showed a reduction in cardiac GLUT4 level whose mediated glucose transport is a significant mechanism of glucose uptake by the cell[23].In chronic heart failure, it has been found that GLUT4 level is decreased with disease severity in patients[24] and is highest in the left ventricule in animal studies[25]. Treatment of cardiac dysfunction with polar and carotenoid fractions of D. salina in protective regimen elevated the level of cardiac GLUT-4. Furthermore, aging associated cardiac dysfunction showed abnormal myocardial architecture when examined histopathologically, decreased cellular volume with exhibition of spaces between the cells. However, treatment with D. salina and its fractions reversed these changes and showed few spaces in between the cardiac myocytes. These findings emphasize all the aforementioned parameters indicating an amelioration of the cardiac function.
According to the findings of this study, it can be concluded that β-carotene rich D. salina carotenoid fraction (250 g/kg) as well as the whole biomass (250 mg/kg) have protective and therapeutic potentials, although the protective effect showed to be more efficient, on the cardiac dysfunction associated with aging in rat as it succeeded to restore all the age-associated cardiac detrimental effects. This is attributed to its potent antioxidant activity and its significant HS reducing capacity.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements
The authors present their gratitude to Dr. Rofanda Bekir, Researcher of Pathology, National Research Centre, Egypt, for her dedication in the histopathological examinations.
 Asian Pacific Journal of Tropical Biomedicine2018年8期
Asian Pacific Journal of Tropical Biomedicine2018年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Biomolecular changes and DNA targeting effect of sesamol in human lung adenocarcinoma (SK-LU-1) cells by FTIR microscopy
- Effect of crocin carotenoid on BDNF and CREB gene expression in brain ventral tegmental area of morphine treated rats
- Anticancer potential of Alternanthera sessilis extract on HT-29 human colon cancer cells
- Anti-quorum sensing and anti-biofilm formation activities of plant extracts from South Korea
- Phytochemical investigation, antioxidant and wound healing activities of Citrullus colocynthis (bitter apple)
