大黄酚通过拮抗Aβ1-42诱导的氧化应激改善大鼠空间记忆能力
潘艳芳, 贾晓涛, 方 艳, 应小平
(1陕西中医药大学基础医学院病理教研室, 陕西 咸阳 712046;2西安交通大学附属西安市中心医院神经内科, 陕西 西安 710003)
Alzheimer disease (AD), the most common neurodegenerative disorder, is characterized by the progressive deterioration of memory and cognition[1]. One of the major pathological hallmarks of AD is the presence of a high density of senile plaques composed of amyloid β-protein (Aβ) in the hippocampus and cerebral cortex[2]. Amyloid core is composed of aggregated neurotoxic Aβ1-42peptide capable of causing synaptic dysfunction and neuronal degeneration[3-4]. Recent studies indicate that oxidative stress is involved in the mechanism of Aβ-induced neurotoxicity and AD pathogenesis[5-6]. Moreover, oxidative damage and formation of oxidized lipids and proteins have been observed in the brain of AD patients[7-8]. Accumulative lines of evidence have suggested that antioxidants and free radical scavengers prevent neurons against Aβ-induced oxidative stress and lipid peroxidationinvivoandinvitro[9-10]. Although a number of therapeutic interventions, including tacrine, donepezil, galantamine and so on, have been used to reverse cognitive deficits, these drugs were not always well accepted due to their severe side effects[11-12]. Therefore, it is urgently necessary for searching safe, better tolerated and powerful drugs for AD treatment.
Chrysophanol (CHR), a bioactive constituent from rhubarb, is originally extracted from plants ofRheumgenus. CHR has a broad range of pharmacological effects in mammals, including anti-inflammatory, anti-oxidative, antimicrobial, antifungal, anti-cancer, and neuroprotective effects[13-15]. However, it has not been elucidated whether CHR exerts neuroprotective effect by reducing oxidative stress in AD. Therefore, we used intracerebroventricular injection (i.c.v.) of Aβ1-42, representing a rodent model of AD, to investigate whether CHR protects against Aβ-induced impairment of cognition of rats by Y-maze test, open-field test (OFT) and Morris water maze (MWM) test, and whether CHR exerts these effects by reducing oxidative stress in the model rats.
MATERIALS AND METHODS
1 Reagents and drugs
Aβ1-42was purchased from Sigma-Aldrich. Prior to injection, Aβ1-42was dissolved in normal saline at a concentration of 5 g/L and incubated at 37 ℃ for 4 d to induce aggregation before surgery[16]. The CHR product (Nanjing Zelang Medical Technological Co., Ltd.) with the purity of more than 98% was first dissolved in 0.9% NaCl including 1% DMSO and 1% Tween-80 prior to the treatments. The assay kits for superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT) and malondialdehyde (MDA) were purchased from Nanjing Jian-Cheng Bioengineering Institute.
2 Animals and surgical procedure
With approval of the Shaanxi Animal Research Ethics Committee, the adult male Wistar rats (230~250 g) supplied by the Research Animal Center of Xi’an Jiaotong University were housed in a room maintained at 23 ℃ with a 12 h light-dark cycle. The certi-ficate number of the animals is SCXK (Chuan) 2015-030. The surgical preparation procedure was similar to our previous report[17]. Briefly, the Wistar rats were anesthetized (chloralhydrate, 0.3 g/kg, i.p.) and placed in a stereotaxic apparatus (Narishige). Then, the skulls of the rats were opened, and burr holes were drilled at the corresponding position to allow for the i.c.v. of Aβ1-42(anteroposterior:-0.8 mm from Bregma; medial/lateral: ±1.3 mm; dorsal/ventral: -4.0 mm). The experimental procedures for single and multiple CHR treatments were outlined in Figure 1.

Figure 1.The experimental protocol of the Morris water maze for single(A)and repeated(B)CHR treatments.
3 The experimental procedures
3.1Y-maze test To assess working memory and exploration behavior, the rats in both single and repeated treatment groups were tested by Y-maze. The Y-maze consisted of 3 identical arms at a 120° angle from each other. Each arm was 30 cm long, 10 cm wide, and restricted by 20-cm-high walls. The animals were placed at the end of 1 arm facing the center and allowed to move freely through the maze during an 8 min period, and then the order of arm entry and the total number of the arm entries of rats were recorded manually. Accor-ding to Typlt et al[18], the percentage of spontaneous alternation, which is regarded as a measure of working memory, was calculated as (sequence of 3 consecutive arm entries)/(total arm entries-2). Total entries were scored as an indicator of locomotor activity in the Y maze, and the rats with scores below 12 were excluded.
3.2OFT To verify the effects of treatment with Aβ1-42on locomotor activity, OFT were performed as previously described[19]. The animals were placed in the middle of the open field and allowed to freely explore for 60 min. A black square arena (100 cm × 100 cm × 60 cm) was used for the test. During that time, locomotor activity of each animal was observed by recording total path and speed. The total distance of movements was evaluated using SMART video-tracking software (Panlab).
3.3MWM test To assess spatial reference learning and memory, a separate cohort of rats from both single and repeated treatment groups were tested by Morris water maze. The water maze apparatus consisted of a large circular pool (diameter, 150 cm; height, 50 cm) divided into 4 equally spaced quadrants and a small circular platform (diameter, 14 cm; height, 29 cm). The inner surface of the pool and the platform were painted black, and the platform was placed at a fixed position in the center of one quadrant and submerged approximately 1.0 cm below water surface at a temperature of (23±2) ℃. Prominent visual cues were fixed to the walls of the water maze room. Memory training was performed on the 7th day after Aβ1-42injection. The training was performed 4 times per day for 5 consecutive days before the probe task. Each rat was allowed to swim until it found the platform or until 120 s elapsed. The rat was then left on the platform for 10 s. In probe trials on the 6th day, the platform was removed from the pool, and the rats were allowed to swim for 120 s. The swimming escape latency, average swimming speed, time spent in the target quadrant, and times of the animal crossing the previous location of the platform were recorded. After the probe trials, visual, motor, and motivation skills were also tested using a visible platform located opposite the original position within the pool. The test comprised 4 trials with the visible platform elevated above the water level (approximately 2 cm), and the latency and swimming speed at which the animal arrived at the platform were recorded. These data were recorded and analyzed by a behavioral software system (EthoVision 3.0, Noldus Information Technology).
3.4Antioxidant assay After behavioral tests, the rats were sacrificed by decapitation, and the bilateral hippocampal tissues were collected. The hippocampus was weighed, and the tissue homogenate was prepared at 5% (W/V) in normal saline. The homogenate was centrifuged at 3 000×gfor 15 min at 4 ℃, and the supernatant was collected. The levels of GSH-Px, SOD, CAT and MDA were measured according to the manufacturer’s instructions.
4 Statistical analysis
All statistical analysis were performed by SPSS 17.0. All data were expressed as mean ± standard error of the mean (SEM). A statistical analysis was performed using one- or two-way ANOVA followed by Dunnett’s post-hoc test in which each group was compared with the Aβ1-42group. In MWM test, the latency and swimming distance in 4 trials for each rat on each day were averaged. A two-way repeated measures ANOVA with Tukey’s post-hoc analysis for multiple comparisons was used with day as the repeated measure of latency or distance. For probe trials, the 2 trials for each rat were averaged, and the means were compared between groups via one-way ANOVA. Statistical significance was accepted atP<0.05.
RESULTS
1 Single CHR treatment did not influence the cognitive function in an Aβ1-42-treated rat model of AD
To explore the effect of Aβ1-42and CHR on cognitive function, the rats were injected with a single dose of CHR (1, 10 or 100 mg/kg, i.p.) or saline (n=10) and underwent behavioral tests. The working memory of the rats was assessed by Y-maze test. As shown in Figure 2A, 5 nmol Aβ1-42(i.c.v.) resulted in a significantly decreased ratio of spontaneous alternation (P<0.01) compared with the control (saline, i. c. v.) group. We evaluated whether Aβ1-42affected motor function because almost all cognitive tests rely on motor behavior, and no significant difference of locomotor activity among the 5 groups was observed (Figure 2B,C). We then tested spatial learning and memory in MWM. As shown in Figure 2D, compared with the control group, 5 nmol Aβ1-42resulted in a significant decrease in spatial learning with the increase in training time, with increased latency searching for the underwater platform (P<0.01). In order to further investigate the spatial memory ability of rats in the MWM, probe trials without the platform were tested on day 6. As shown in Figure 2E, the percentage of total time in the target quadrant was significantly decreased in Aβ1-42group as compared with the control group (P<0.01). Twelve hours after the last water maze training, a single dose (1, 10 or 100 mg/kg) of CHR was administered (i.p.), and the rats were subjected to the MWM test to assess the spatial learning and memory abilities. Compared with the Aβ1-42group, no significant difference on any training day was observed in the model rats after single dose of CHR was given. In the probe trial, the percentages of total time spent in the target quadrant in CHR (1, 10 and 100 mg/kg) groups were (26.32±2.01)%, (26.96±1.38)% and (27.42±1.37)%, respectively, without any significant difference compared with the Aβ1-42group (Figure 2E). These results indicated that single CHR administration did not improve spatial reference memory.
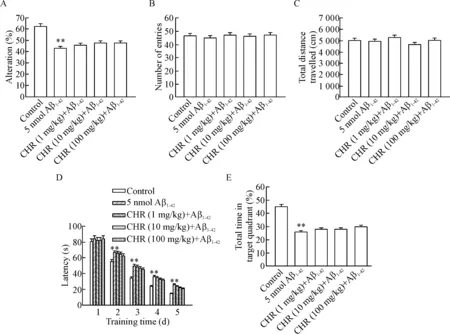
Figure 2.The effect of single chrysophanol(CHR)administration,on working memory,motor function and spatial lear-ning and memory in an Aβ1-42-treated rat model of Alzheimer disease. A: the spontaneous alteration in Y-maze; B: the total arm entries in Y-maze; C: the total distance travelled in open-field test; D: the latencies to search the hidden platform over 5 consecutive training days in Morris water maze training trials; E: the time spent in the target quadrant in probe trials. Mean±SEM.n=10.**P<0.01vscontrol group.
2 Multiple CHR treatment prevented Aβ1-42-induced impairment of cognitive function in a dose-dependent manner
The rats were treated with CHR (1, 10 or 100 mg/kg, i.p., once daily) or saline for 5 consecutive days. Each rat only participated in one of the behavioral tests, including the Y-maze test, OFT and MWM test. Pretreatment with CHR at 1, 10 and 100 mg/kg obviously prevented from Aβ1-42-induced impairment in the spatial cognition of the rats in a dose-dependent manner. In a Y-maze test, multiple CHR treatments significantly affected the spontaneous alternation (Figure 3A) but not total arm entries (Figure 3B). The spontaneous alternation in CHR (1 mg/kg)+Aβ1-42group was not different from that in Aβ1-42group. However, there was a significant increase in the spontaneous alternation in CHR (10 mg/kg)+Aβ1-42group and CHR (100 mg/kg)+Aβ1-42group (P<0.05), indicating an amelioration in working memory. The data from OFT suggested that there was no significant difference of locomotor activity among the 4 groups, indicating that general locomotor activity was not affected by Aβ1-42and CHR (Fi-gure 3C). As shown in Figure 3D and 3E, the impairment of learning was not significantly improved in CHR (1 mg/kg)+Aβ1-42group as compared with Aβ1-42group. In the probe trials, the percentages of total time spent in the target quadrant were not significantly improved. However, multiple administration of CHR at 10 and 100 mg/kg reversed the spatial learning and memory impairments induced by Aβ1-42(P<0.05). Compared with the Aβ1-42group, the average escape latency in searching for the hidden underwater platform significantly decreased after multiple administration of CHR at 10 and 100 mg/kg (P<0.05; Figure 3D). In contrast, the percentages of total time spent in the target quadrant after removing the platform significantly increased in the CHR (10 and 100 mg/kg)+Aβ1-42-treated rats as compared with the rats treated with Aβ1-42alone (P<0.05; Figure 3E). These results indicated that CHR dose-dependently attenuated Aβ1-42-induced cognitive function impairment. To rule out the possible effect of CHR per se on the spatial memory, we conducted the multiple CHR administration without Aβ injection to address this concern. No significant effect of CHR (1, 10 and 100 mg/kg) on the spatial learning and memory was found (Figure 4), indicating that the multiple CHR administration did not affect spatial me-mory.

Figure 3.The effect of multiple chrysophanol(CHR)treatment on working memory,motor function and spatial learning and memory in an Aβ1-42-treated rat model of Alzheimer disease. A: the spontaneous alteration in Y-maze; B: the total arm entries in Y-maze test; C: the total distance travelled in open-field test; D: the latencies to find the hidden platform over 5 consecutive training days in Morris water maze training trials: the percentages of total time of rats spent in the previous target quadrant in the probe trials. Mean±SEM.n=10.*P<0.05vsAβ1-42group;△P<0.05vsCHR (1 mg/kg)+Aβ1-42group;▲P<0.05vsCHR (10 mg/kg)+Aβ1-42group.
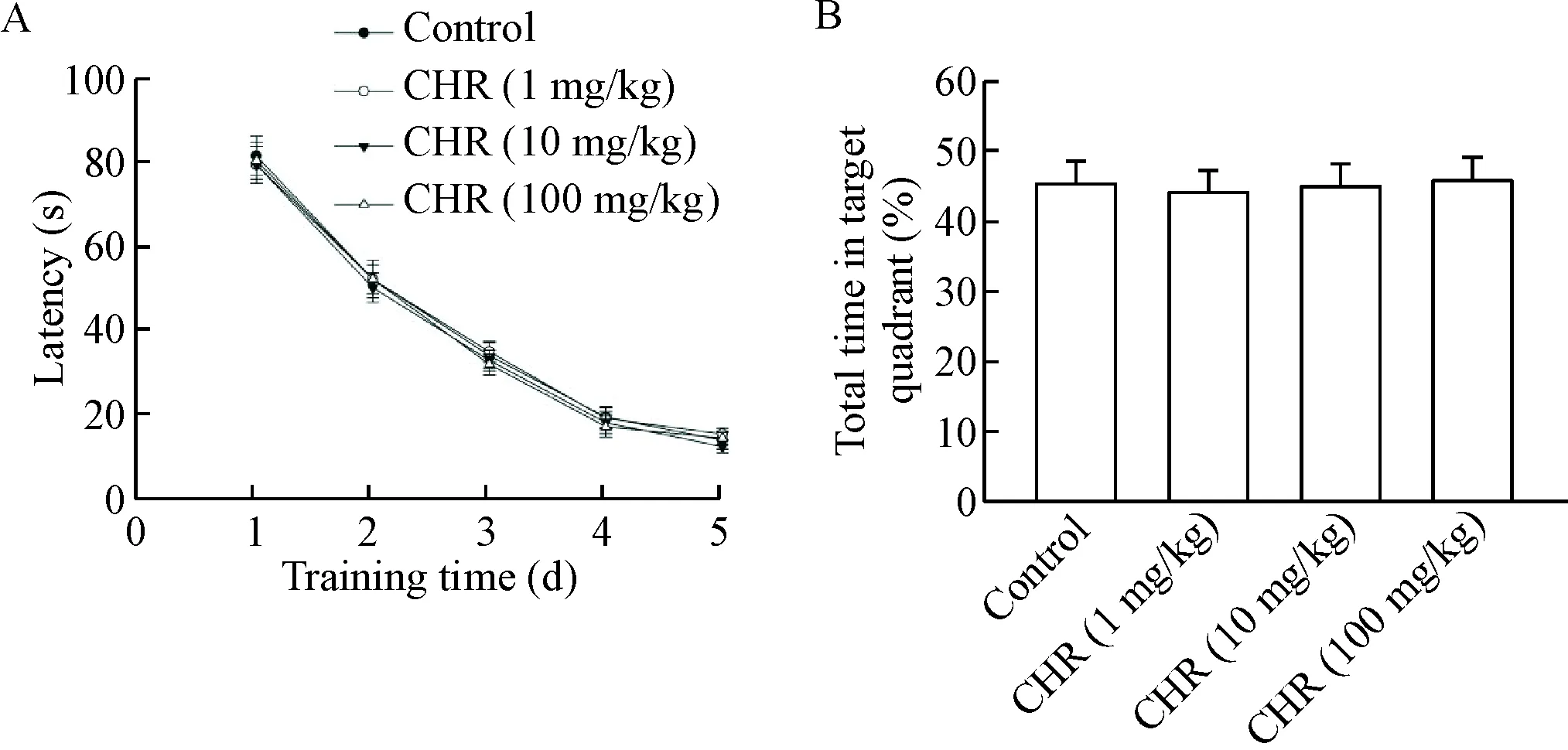
Figure 4.The effect of multiple chrysophanol(CHR)treatment alone on spatial learning and memory. CHR at 1, 10 and 200 mg/kg was administrated (i.p.) for 5 consecutive days in the rats without Aβ injection. A: the escape latency during water maze training trials over 5 consecutive training days; B: the time spent in the target quadrant in probe trials. Mean±SEM.n=6.
3 Both CHR and Aβ1-42 did not affect the vision and swimming speed of the rats
After the probe trials, the rat escape latencies were conducted with visible platform. The differences of escape time and swimming speed were not significant among all groups (Figure 5), indicating that none of the drugs used in the present study affected the vision and motor ability of rats.

Figure 5.Both Aβ1-42 and chrysophanol(CHR)did not affect the vision and swimming speed of the rats. A: the latency to find the visible platform; B: the swimming speed. Mean±SEM.n=10.
4 CHR effectively prevented Aβ1-42-induced changes in the levels of MDA,SOD,GSH-Px and CAT in the hippocampus of the rats
To further elucidate the probable biochemical mechanism underlying the protection of CHR against Aβ1-42-mediated deficit in spatial cognition, we subsequently investigated whether CHR influenced oxidative stress in the hippocampus of AD-like rat brain by detecting the levels of MDA and antioxidant enzymes SOD, GSH-Px and CAT. As expected, Aβ1-42and multiple CHR treatments had significant effects on the levels of MDA and antioxidant enzymes. As shown in Table 1, Aβ1-42injection generated a dramatic increase in MDA content as well as a significant decrease in the activity of antioxidant enzymes SOD, GSH-Px and CAT in the rat hippocampus as compared with control group (P<0.05). Nevertheless, multiple administration of CHR at 10 and 100 mg/kg inhibited the decrease in the activity of antioxidant enzymes SOD, GSH-Px and CAT caused by Aβ1-42, and the increase in MDA production was also significantly attenuated by the treatment with CHR (P<0.05), indicating that CHR acted as a neuroprotective factor potentially by modulating the levels of MDA, SOD, GSH-Px and CAT in the rat hippocampus.

Table 1.The effects of CHR on the levels of SOD,GSH-Px,CAT and MDA in the hippocampus of the rats with Aβ1-42-induced memory impairment(Mean±SEM.n=10)
*P<0.05,**P<0.01vscontrol group;#P<0.05,##P<0.01vsAβ1-42group.
DISCUSSION
The major finding of this study was that Aβ1-42(i.c.v.) decreased the levels of antioxidant enzymes SOD, GSH-Px and CAT in the rat hippocampus, and the rats exhibited impaired performances in the Y-maze and MWM tests. Multiple CHR administration exerted an obvious protective effect on the cognitive functions of Aβ-treated rats, and this effect was associated with increased levels of antioxidant enzymes SOD, GSH-Px and CAT, and decreased level of MDA in the hippocampus.
As the primary constituent of senile plaques in AD, the neurotoxicity of Aβ has been widely studied ininvivoandinvitroexperiments[16, 20]. The direct Aβ1-42injection into the brains of rodents was shown to induce obvious memory impairment, mimicking the cognitive decline in AD[21]. Some studies showed that infusion of Aβ1-42induced progressive brain dysfunction even 80 days after the treatment[22]. Some behavioral tasks were used for assessing cognitive deficits, for example, spontaneous alternations in the Y-maze test, novel object recognition tasks and MWM test[23]. In the present study, the results demonstrated that the injection of Aβ1-42resulted in significant impairment of working memory (Y-maze test) and a deficit of short-term spatial learning and memory (MWM test).
The antioxidant and anti-inflammatory effects of CHR were reported in cerebral ischemic injury and in BV2 microglia[14-15].However, its role in the dementia model is unclear. Therefore, we used Aβ1-42-induced rat model in this research to explore the role of CHR. The administration doses of CHR were chosen according to the preliminary study (data were not shown here).
In the present study, we measured spontaneous alternation behaviors in the Y-maze test to appraise wor-king memory. Our results demonstrated that total number of arm entries in Y-maze test was not significantly different between Aβ1-42group and single CHR (10 and 100 mg/kg) treatment groups. However, the spontaneous alternation behaviors were significantly improved by multiple CHR (10 and 100 mg/kg) treatment. Spatial memory was assessed using water maze task. In all groups, training had a significant effect on spatial learning performance. This effect was more evident than the treatment effect. In the present study, we first confirmed the neuroprotective effect of CHR in the cognitive behavior experiment. Interestingly, CHR alone did not affect the mean escape latency and the percentage of total time spent in the target quadrant, but multiple CHR administration (10 and 100 mg/kg), not 1 mg/kg, dose-dependently prevented the Aβ1-42-induced deficits in spatial learning and memory of the rats. The mean escape latencies of rats in CHR (10 and 100 mg/kg)+Aβ1-42groups were significantly decreased in searching for the hidden platform of the MWM, while the swimming time spent in the target quadrant after removing the platform significantly increased in the probe trials compared with the rats treated with Aβ1-42alone.
The etiology of AD has not been revealed clearly. Nevertheless, lots of clinical studies have reported that oxidative stress plays an important role in the development and progression of AD[24-25]. So we performed an antioxidant assay in the rat hippocampus to elucidate the mechanism of cognitive-enhancing activity. In the pre-sent study, we found that CHR reversed Aβ-induced oxidative injuries. MDA is the end product of oxygen-derived free radicals and lipid oxidation, which reflects the damage caused by reactive oxygen species[26-27]. The present study showed that the injection of Aβ1-42enhanced MDA production in rat hippocampus and CHR (10 and 100 mg/kg) significantly decreased the level of MDA induced by Aβ1-42. SOD, GSH-Px and CAT are important antioxidant enzymes involved in cellular protection against damage caused by oxygen-derived free radicals through removing harmful peroxide metabolites and blocking lipid peroxidation chain reaction[28-29]. Our study showed that injection of Aβ1-42in the rats decreased the activity of SOD, GSH-Px and CAT. How-ever, CHR (10 and 100 mg/kg) significantly ameliorate these abnormalities. Therefore, CHR appeared to be an effective antioxidant, and its neuroprotective effect might be due to its antioxidant capacity.
In conclusion, the present study showed that CHR attenuates Aβ1-42-induced impairments of cognitive behaviors in a dose-dependent manner in rats. The neuroprotective action of CHR against Aβ1-42may be, at least in part, mediated by enhancing the activity of the antioxidative defense system. Further studies are necessary to figure out the more detailed mechanism of CHR in the treatment of AD bothinvitroandinvivo.

