Selective aerobic oxidation of p-cresol with co-catalysts between metalloporphyrins and metal salts☆
Yuanbin She*,Yao FanLei ZhangYing XuMin YuHaiyan Fu*
1State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology,College of Chemical Engineering,Zhejiang University of Technology,Hangzhou 310032,China
2The Modernization Engineering Technology Research Center of Ethnic Minority Medicine of Hubei Province,School of Pharmaceutical Sciences,South Central University for Nationalities,Wuhan 430074,China
Keywords:Aerobic Oxidation Selectivity Co-catalyst Metalloporphyrin Cresols
ABSTRACT A green and efficient method for the selective aerobic oxidation of p-cresol to p-hydroxybenzaldehyde catalyzed by co-catalysts between metalloporphyrins and metal salts was investigated and developed.The relationship between the synergistic catalytic effects and the composition as well as amount of co-catalysts was investigated.Moreover,the Influence of different reaction conditions was studied in details.A high p-cresol conversion(>99%)and p-hydroxybenzaldehyde selectivity(83%)were obtained using only 1.125 × 10−5mol T(p-CH3O)PPFeIIICl-Co(OAc)2used under mild,optimized reaction conditions.A possible mechanism for the reaction wasalso proposed.Thiswork wouldbemeaningfuland instructivefor the further researchesand applicationsofco-catalyst systemon oxidation of cresolsandcouldgivesomeenlightenmenton theselectively catalytic oxidation of the side-chain alkyls of aromatics.
1.Introduction
p-Hydroxybenzaldehyde(PHBA)is an important organic intermediate for preparing pharmaceuticals,perfumes and electroplating chemicals.Direct oxyfunctionalization of cresols at their benzylic positions provides an effective method for synthesizing hydroxy benzaldehydes[1–5].
A number of catalytic systems that employs cobalt salts as highly efficient catalysts have been used in synthesizing PHBA from p-cresol through liquid-phase oxidation.In addition,supported cobalt catalysts have been used in the heterogeneous oxidation of p-cresol since the 1980s[6].A 90%conversion and 59%selectivity can be obtained in the preparation of PHBA from p-cresol using cobalt chloride(CoCl2)as a homogeneous catalyst[7].However,the intricacies of catalyst separation and reuse restrict the application of CoCl2,because of its rapid conversion into black solid residues(which consist of a mixture of cobalt oxide and cobalt hydroxide)during oxidation reactions[8].Consequently,other kinds of metalsalts(suchas copper,nickel,cerium,and iron salts)have been introduced into the reaction as co-catalysts to achieve higher PHBA yield[9,10].Although the obtained results are better,the aforementioned systems are not applicable to industrial production because of their generous use of catalysts,complex postprocessing procedures,and related environmental pollutions.
Metalloporphyrins[11,12]have been efficiently used as biomimetic catalysts in hydrocarbon oxidation under mild conditions[13–16]because of their low dosage requirement and eco-benign properties.The application of metalloporphyrins in p-cresol oxidation has been proposed previously[17].Our group improved this reaction by using smaller amounts of single metalloporphyrin catalysts,thus achieving a conversion of 70%and a selectivity of 87%[18].
Transition-metal salts have been introduced into metalloporphyrincatalyzed systems as co-catalysts,thereby resulting in enhanced reaction activities.Ji et al.[19]and Guo et al.[20]reported the cocatalytic effect of cobalt acetate[Co(OAc)2]and metalloporphyrins on the aerobic oxidation of cyclohexene and p-xylene,respectively.
In the current study,metalloporphyrins and metal salt co-catalysts were introduced in the aerobic oxidation of p-cresol(Fig.1)to achieve high p-cresol conversion and PHBA selectivity.p-Cresol reactivity and PHBA yield were both remarkably enhanced by the iron tetraphenyl porphyrins and Co(OAc)2co-catalysts.
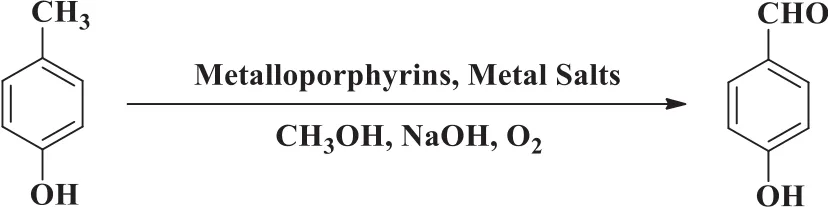
Fig 1.Catalytic oxidation of p-cresol to p-hydroxybenzaldehyde by the co-catalysts.
2.Experimental
2.1.Materials and equipment
All reagents(analytical grade)and standards(chromatographic grade)were purchased from local suppliers and used without further purification.Oxygen was supplied in a high-pressure cylinder.Metalloporphyrins were prepared according to our published procedures[21].The general formula of metalloporphyrins used in this study was shown in Fig.2.
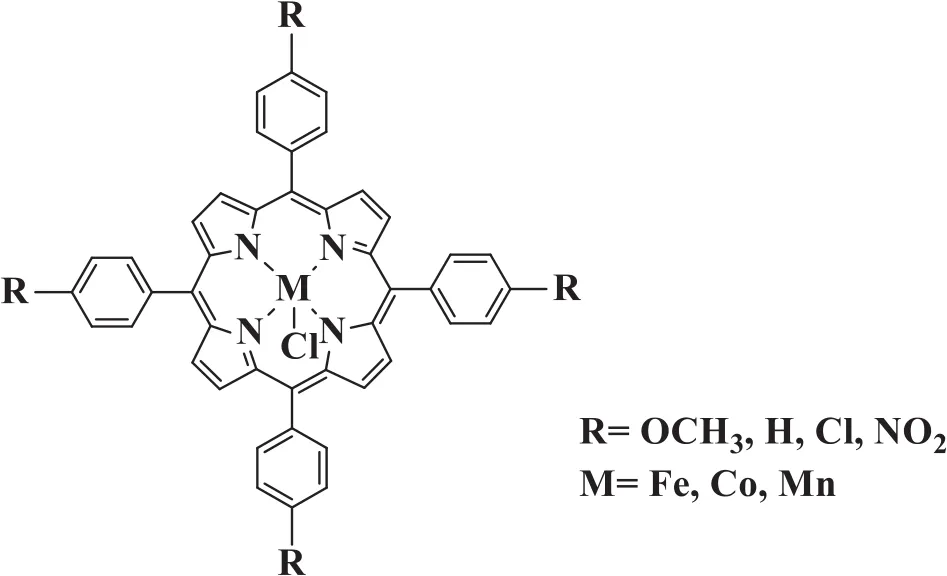
Fig.2.General formula for metalloporphyrin catalysts.
High-performance liquid chromatography(HPLC)was performed on an Agilent 1200 liquid chromatograph equipped with an ultraviolet detector.The mixture of oxidation products was analyzed in an HC-C18column(250mm×4.6mm,φ5μm)supplied by Agilent.Amixture of 30 vol%a cetonitrile and 70 vol%water was used as the mobile phase at a flow rate of 1.0 ml·min−1.
2.2.Oxidation reactions
Aerobic oxidation reactions of p-cresol were performed in a 100 ml autoclave provided with a gas inlet and vent,liquid sampler,safety rupture valve,and thermo well.In a typical reaction,methanol(40 ml)and NaOH(0.375mol)were placed into the preheated reactor and premixed through magnetic stirring until most of the NaOH was dissolved.Afterward,p-cresol(0.15 mol),metalloporphyrins(3.75×10−6mol),and metal salts(7.50×10−6mol)were added into the reaction mixture.The reactor was then pressurized with oxygen to 0.2 MPa.The reaction was initiated by agitating the reactor at 1800 r·min−1stirring speed.
After the reaction was stopped,the reaction mixture was cooled to room temperature,and a quantitative sample(0.2 ml)was taken.The sample was analyzed by HPLC after filtering through 0.45 μm organic membranes.
The oxidation products were identified by obtaining their retention times and comparing these with those of HPLC-grade standards.Yield was based on the added p-cresol,whereas selectivity was referred to the chemoselectivity of PHBA from p-cresol.
3.Results and Discussion
The catalytic performances of iron porphyrins,Co(OAc)2,and their complex in the aerobic oxidation of p-cresol to p-hydroxybenzaldehyde were listed in Table1.Data were taken under optimized reaction conditions,which would be further discussed in the subsequent sections.p-Cresol oxidation was indicated(Fig.3)according to the productsobserved in the reaction mixture,which included the desired product PHBA and three by-products,namely,p-hydroxybenzyl alcohol,p-hydroxybenzoic acid,and p-hydroxybenzyl methyl ether.
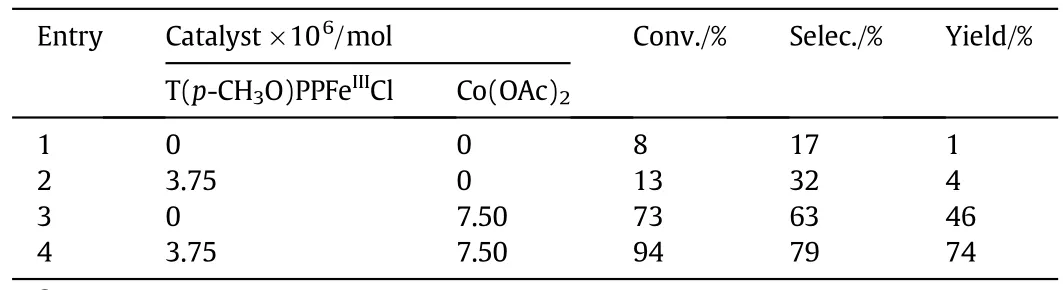
Table 1 Catalytic performances of iron porphyrins,Co(OAc)2and their complex in the oxidation of p-cresol to p-hydroxybenzaldehyde①

Fig.3.Catalytic oxidation of p-cresol.
Asshownin Table1,the oxidation of p-cresol proceeded slowly in the absence of catalysts(Table 1,entry 1).By contrast,T(p-CH3O)PPFeIIICl and Co(OAc)2co-catalyst provided a better result after reacting for 5 h(Table 1,entry 4)compared with the single-component catalyst.Moreover,the activity of this co-catalyst was higher than the combined activities of T(p-CH3O)PPFeIIICl and Co(OAc)2,thus illustrating the synergistic effect of the co-catalyst[22].
To prove the specificity of this co-catalyst,severalmetalloporphyrins and metal salts were studied in the experiments.The results showed that except for metalloporphyrins with substituted nitro group,the best results were generally observed when iron(III)porphyrins(Table 2,entries 1 to 4)were used.Cobalt(III)porphyrins(Table 2,entries 5 to 8)also showed a high activity in the co-catalysts.However,manganese(III)porphyrins yielded poor results under the sameconditions.A similar trend was observed when reaction time was increased from 5 h to 12 h.Therefore,iron(III)porphyrins were selected as the appropriate component of the co-catalysts.

Table 2 Effects of metalloporphyrin catalysts on oxidation of p-cresol①
Aside from the central metal ions of metalloporphyrins,substituting groups also affect the catalytic behaviors of co-catalysts.Early researches showed a positive linear correlation between the electron affinity(EA)of iron(III)porphyrins and Fe(III)/Fe(II)reduction potentials[23],which are directly related to the catalytic activities of iron(III)porphyrins[24].EA can be estimated by the energy of the lowest unoccupied molecular orbital(LUMO),as follows:EA≈−ELUMO[25].According to our earlier research on the substituent effects of geometric and electronic properties of iron(III)porphyrins using density functional theory,the calculated ELUMOfollowed the same sequence as the catalytic activities of iron(III)porphyrins,as follows:−NO2<−Cl<−H<−OCH3(Table 2,entries 1 to 4)[26].
To investigate the synergistic effect of metalloporphyrins and metal salts,several metal salts with different positive ions and negative ions were used in this study(Table 3).Co(II)salts exhibited significantly higher promotion and catalytic effects than Fe(II)and Mn(II)salts.When Mn(OAc)2was used,a relatively low conversion was observed(<15%)(Table 3,entry 4).

Table 3 Effects of metal salts on p-cresol oxidation with the co-catalysts①
The previous results showed that iron(III)tetraphenylporphyrin-Co(OAc)2catalysts exhibited higher activities than the two single catalysts,thus indicating the occurrence of synergistic effects between iron(III)porphyrins and Co(OAc)2.Lyons et al.[24,27]proposed a hypothetical mechanism for the conversion of alkanes to alcohols using halogenated iron(III)porphyrins.In their research,iron(III)porphyrins lost axial ligands,whereas metal ions were converted into lowervalence forms that could combine with molar oxygen to form PFeIIIO2(P refers to porphyrin).This species then formed PFeIV=O through PFeIIIO2FeIIIP in alkane oxidation processes[28,29].High-valence species exhibited high activity in oxidations[30].In addition,PFeIV=O could transfer oxygen to alkanes.In molecular oxygen catalytic oxidation systems,dissolved oxygen can rapidly oxidize PFe(II)to PFe(III).The electron-transfer rates in this process can affect the catalytic activities of met all oporphyrins.To determine product distributions for the oxidation of p-cresol in different co-catalysts,the stirring speed was decreased to 900 r·min−1to reduce total reaction rate(Fig.4).When only iron(III)porphyrin was used,the amount of phydroxybenzyl alcohol obtained was higher than that of PHBA.After the addition of Co(OAc)2into the reaction,the formation of PHBA rapidly increased,whereas that of p-hydroxybenzyl alcohol decreased.These results could be explained by the lower reduction potential of PFe(III)/PFe(II)(−0.37 V)compared with that of Co(III)/Co(II)in Co(OAc)2(2.0 V)[31].Therefore,the oxidation of PFe(II)could have been promoted through the reaction,Co(III)+PFe(II)→Co(II)+PFe(III),thus enhancing the co-catalyst activity.Compared with positive ions,negative ion groups have negligible effects on catalytic activities.Therefore,the co-catalyst of T(p-CH3O)PPFeIIICl-Co(OAc)2exhibited better performance.
The effects of the composition and amount of the T(p-CH3O)PPFe-IIICl-Co(OAc)2co-catalyst were also investigated using different molar ratios of T(p-CH3O)PPFeIIICl to Co(OAc)2(Table 4).The 1:2 molar ratio was the most suitable and produced the highest PHBA selectivity and yield.The total amount of the co-catalysts was also determined(Fig.5).The selectivity of PHBA decreased when the co-catalyst amount exceeded 1.125× 10−5mol.Therefore,1.125× 10−5mol ofT(p-CH3O)PPFeIIICl-Co(OAc)2at a 1:2 molar ratio produced the highest activity,with p-cresol nearly consumed(>99%conversion)and PHBA selectivity reaching 83%.

Table 4 Effects of T(p-CH3O)PPFeIIICl-Co(OAc)2compositions on p-cresol oxidation①
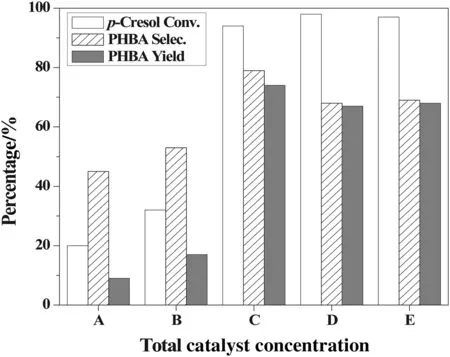
Fig.5.Effects of total catalyst amounts on p-cresol oxidation.Reaction conditions:pcresol(0.15 mol),NaOH(0.375 mol),molar ratio of T(p-CH3O)PPFeIIICl to Co(OAc)2(1:2),MeOH(40 ml),O2(0.2 MPa),343 K,1800 r·min−1stirring speed,5 h,total catalyst amount,A.2.25× 10−6mol,B.6.75× 10−6mol,C.1.125× 10−5mol,D.1.576× 10−5mol,E.2.250× 10−5mol.
Given the low concentrations of the co-catalysts and their solubility in MeOH,these species can be regarded as quasi-homogeneous systems in liquid-phase reaction mixtures.Thus,the catalytic oxidation of p-cresol only involved gas and liquid phases,with mass transfer as the possible rate-determining step.As shown in Fig.6,conversion and selectivity rapidly increased with stirring speed.A conversion of 94%and a selectivity of 79%were reached at a stirring speed of 1800 r·min−1.Increasing the stirring speed was considered helpful for the oxygen transfer from gas to liquid phase.In turn,the rate of contact of oxygen with the catalyst and the substrate as well as that between T(p-CH3O)PPFeIIICl and Co(OAc)2would increase to improve their synergistic effects.Moreover,increasing the stirring speed could help diffuse the developing heat and maintain the stability of system temperature.Therefore,1800 r·min−1was used as the stirring speed because of the experimental limitations of our equipment.

Fig.6.Effects of stirring speed on p-cresol oxidation.Reaction conditions:p-cresol(0.15mol),NaOH(0.375 mol),T(p-CH3O)PPFeIIICl(3.75× 10−6mol),Co(OAc)2(7.50×10−6mol),MeOH(40 ml),O2(0.2 MPa),343 K,5 h.
The effect of reaction time on p-cresol oxidation at 0.1 MPa was investigated(Table 5).The conversion of p-cresol increased with reaction time and peaked at 94%after 10 h.However,a similar conversion was achieved after only 5 h at 0.2 MPa(Table 1,entry 4).These results clearly show that the reaction rate obtained at 0.2 MPa was higher than that at 0.1 MPa.This finding could be easily explained by Henry's law.In this study,the solubility of oxygen increased at 0.2 MPa.However,when oxygen pressure increased to 0.3 MPa(Table 6,entry 3),conversion and selectivity no longer improved.Therefore,the 0.2 MPa oxygen pressure was used.
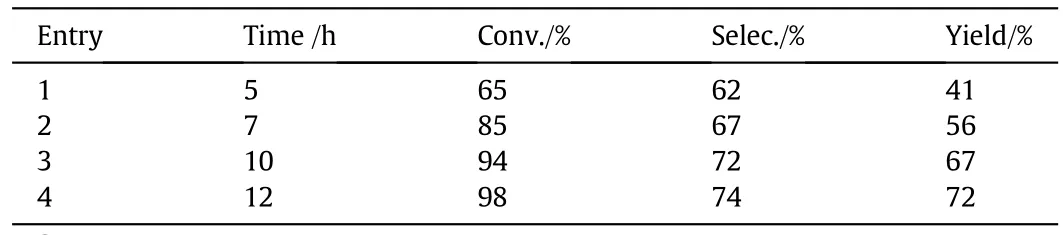
Table 5 Effects of reaction time on oxidation reaction of p-cresol at 0.1 MPa①

Table 6 Effects of oxygen pressure on p-cresol oxidation①
The effect of temperature(333 K to 353 K)on the reactions of p-cresol with T(p-CH3O)PPFeIIICl and T(p-CH3O)PPFeIIICl-Co(OAc)2co-catalyst were shown in Table 7.p-Cresol conversion and PHBA selectivity were both enhanced in the reactions with the cocatalyst compared with those with the single iron(III)porphyrin.The effect of temperature on the catalytic performance of the cocatalyst was also investigated(Fig.7).The conversion of p-cresol increased with reaction temperature,whereas the selectivity of PHBA peaked at 343K.As a result of the formation of p-hydroxybenzyl methyl ether,the selectivities of PHBA were lower at temperatures below343K but decreased at temperatures above 343 K.This result could be attributed to the deeper oxidation of PHBA to p-hydroxybenzoic acid.Therefore,the 343 K reaction temperature was chosen as optimumtemperature at which the synergistic effect of the co-catalyst was at its peak.
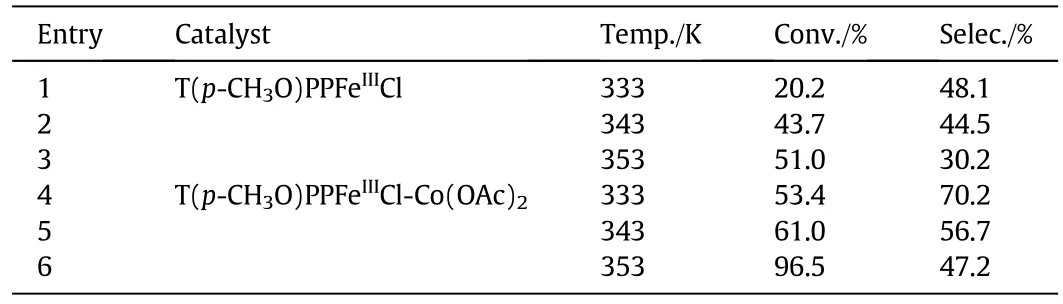
Table 7 Effects of temperature on p-cresol oxidation with different catalysts①

Fig.7.Effects of temperature on p-cresoloxidation.Reaction conditions:p-cresol(0.15 mol),NaOH(0.375 mol),T(p-CH3O)PPFeIIICl(3.75×10−6mol),Co(OAc)2(7.50×10−6mol),MeOH(40 ml),O2(0.2 MPa),1800 r·min−1stirring speed,5 h.
3.1.Effects of co-catalysts on oxidation of various substrates
To assess the generality of this method,the oxidation of various alkylaromatics were systematically investigated to evaluate the effects of co-catalysts(Table 8).It was shown that the co-catalysts served as effective catalysts in the oxidative reaction of toluene derivatives compared with single metalloporphyrin as a catalyst.The synergistic effects of T(p-CH3O)PPFeIIICl and Co(OAc)2resulted in a higher conversion of alkylaromatics anda better selectivity for carbonyl compound.
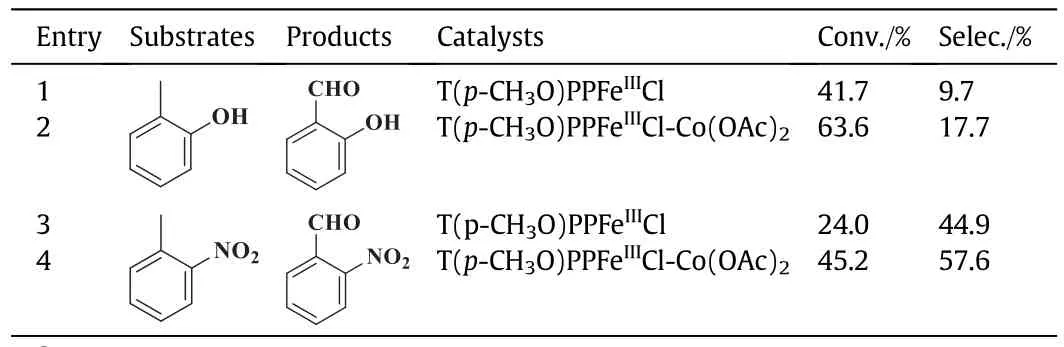
Table 8 Effects of co-catalysts on oxidation of various toluene derivatives①
Metalloporphyrins and its co-catalysis system not only exhibited good performances in the oxidation of substitute toluene,but also served as effective catalysts to oxidize ethyl group to acetyl.As shown in Table 9,this novel co-catalytic system was applicable to the direct oxidation of ethylbenzene to corresponding ketones with molecular oxygen.This method provided a more economical and environmentfriendly way for the preparation of acetophenone compared with traditional Friedel–Crafts acylation.It achieved to synthesis the valuable products under atmospheric pressure which were difficult to obtain in mild condition.The selective oxidation of 1,4-diethylbenzene was also tried to investigate the application on substrates with double ethyl(Table 9,entries 3–6).It can be seen that the selectivities of acetyl compounds could be controlled by thevariation of catalyts and reaction temperature.These findings would be meaningful and instructive forthe application of co-catalyst system on the selectively catalytic oxidation of the side-chain alkyls of aromatics.

Table 9 Effects of co-catalysts on oxidation of various ethylbenzene derivatives①
The initiation of thereaction obeys Lyons classical metalloporphyrin recycle mechanism(Fig.8)[24].The generation of benzyl free radical activated by metalloporphyrin was the first step of the reaction.Quinonoid compoundobtained from benzylfreeradicalinthePresence of PFeIIIOH[path(a)],followed by the further reaction with H2O and methanol to p-hydroxybenzyl alcohol and p-hydroxybenzyl methyl ether[path(c)and(d)].Meanwhile,A typical autoxidation process was also conducted[path(b)]with maintain the flux of oxygen.Peroxide intermediates decomposed into p-hydroxybenzyl alcohol by CoIIfor Haber-Weiss recycle mechanism[32][path(f)].A redox process proceeded simultaneously with the decomposition which speeds up the reaction[path(g)].P-hydroxybenzoic acid was detected as a further oxidation product in the oxidation mixture of p-cresol.No oxidation products were detected upon the addition of PPh3that verifies the free radical mechanism of the co-catalysts system.
4.Conclusions
Metalloporphyrins and metal salts co-catalysts for the selective aerobic oxidation of p-cresol with molecular oxygen were reported.Although the molar ratio of the total co-catalyst to p-cresol was only approximately 1:130000,an excellent yield(83%)and high selectivity(83%)for p-hydroxybenzaldehyde could be reached under mild reaction conditions.Given that the conversion of p-cresol could reach 99%,downstream processes could be simplified to save energy and reduce the cost of post-processing.A possible reaction mechanism for aerobic oxidation of p-cresol was also proposed.
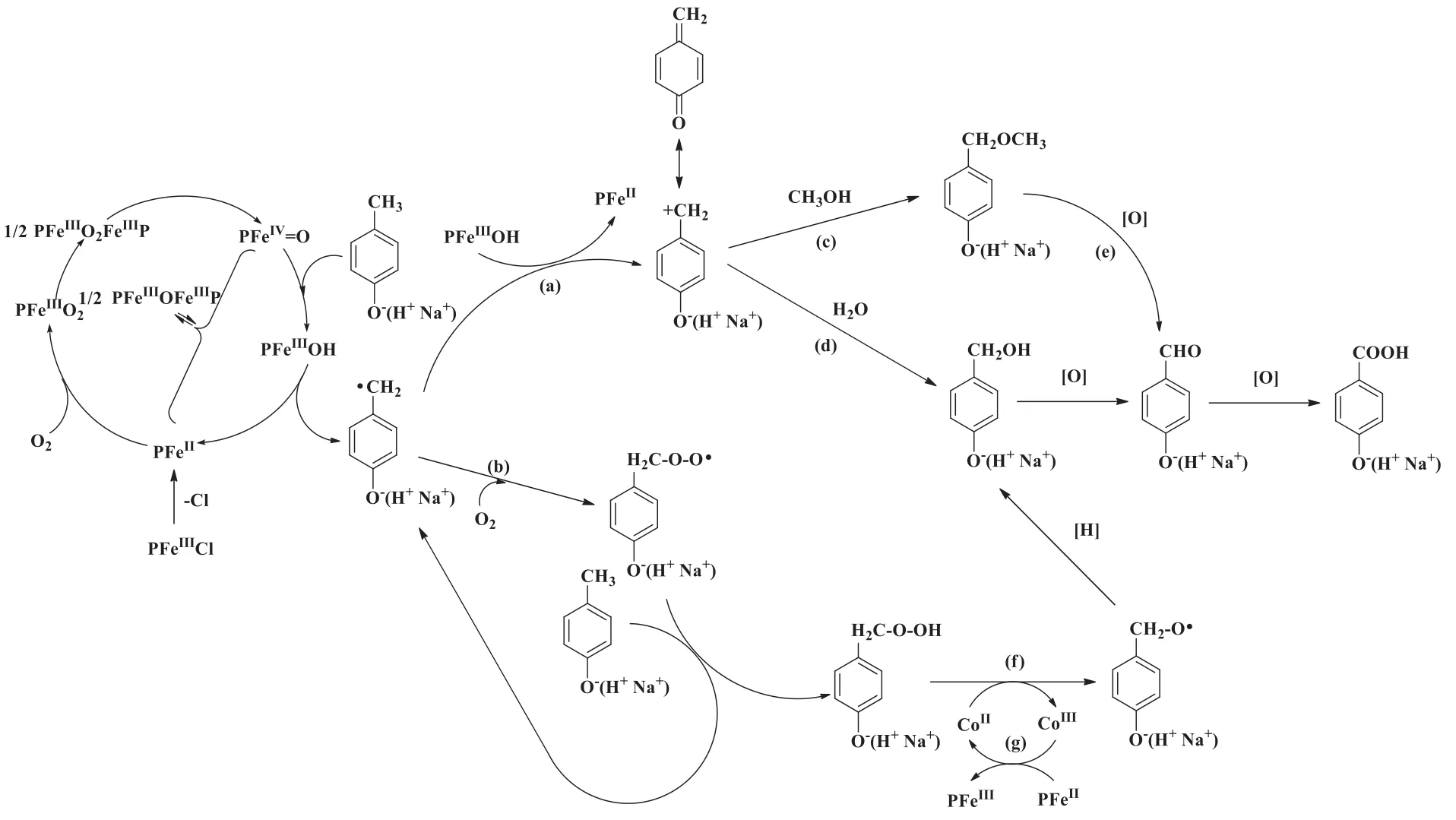
Fig.8.Predicted mechanism of p-cresol oxidation catalyzed by co-catalysts.
 Chinese Journal of Chemical Engineering2018年7期
Chinese Journal of Chemical Engineering2018年7期
- Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Understanding the Influence of microwave on the relative volatility used in the pyrolysis of Indonesia oil sands☆
- Degradation and mineralization of aniline by O3/Fenton process enhanced using high-gravity technology☆
- Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
- Insight into the degradation mechanism of cefixime under crystallization condition☆
- Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis☆
