Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
Pan Sun,Kun Huang*,Xiaoqin Wang,Na Sui,Jieyuan Lin,Wenjuan Cao,Huizhou Liu*
1CAS Key Laboratory of Green Process and Engineering,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
2University of Chinese Academy of Sciences,Beijing 100049,China
Keywords:Three-liquid-phase extraction Vanadium Chromium Separation High-chromium vanadium–titanium magnetite
ABSTRACT A new method by liquid–liquid–liquid three phase system,consisting of acidified primary amine N1923(abbreviated as A-N1923),poly(ethylene glycol)(PEG)and(NH4)2SO4aqueous solution,was suggested for the separation and simultaneous extraction of V(V)and Cr(VI)from the acidic leach solutions of highchromium vanadium–titanium magnetite.Experimental results indicated that V(V)and Cr(VI)could be selectively enriched into the A-N1923 organic top phase and PEG-rich middle phase,respectively,while Al(III)and other co-existing impurity ions,such as Si(IV),Fe(III),Ti(IV),Mg(II)and Ca(II)in acidic leach solutions,could be enriched in the(NH4)2SO4bottom aqueous phase.During the process for extraction and separation of V(V)and Cr(VI),almost all of impurity ions could be removed.The separation factors between V(V)and Cr(VI)could reach 630 and 908,respectively in the organic top phase and PEG middle phase,and yields of recovered V(V)and Cr(VI)in the top phase and middle phase respectively were all above 90%.Various effects including aqueous pH,A-N1923 concentration,PEG added amount and(NH4)2SO4 concentration on three-phase partitioning of V(V)and Cr(VI)were discussed.It was found that the partition of Cr(VI)into the PEG-rich middle phase was driven by hydrophobic interaction,while extraction of V(V)by A-N1923 resulted of anion exchange between and .Stripping of V(V)and Cr(VI)from the top organic phase and the middle PEG-rich phase were achieved by mixing respectively with NaNO3aqueous solutions and NaOH-(NH4)2SO4solutions.The present work highlights a new approach for the extraction and purification of V and Cr from the complex multi-metal co-existing acidic leach solutions of high-chromium vanadium–titanium magnetite.
1.Introduction
Vanadium and chromium are extensively used in various industries,suchas alloy,stainless steel,battery materials and are recognized as import ant strategic resources[1–5].Panxi region in Sichuan of South China has reserves of 3.6 billion tons of high-chromium vanadium–titanium magnetite,which is one of the most important resources for vanadium and chromium in China[6,7].Hydrometallurgical leaching of the highchromium vanadium–titanium magnetite is the main process for extraction of vanadium and chromium.However,the acidic leach solutions of high-chromium vanadium–titanium magnetite are extremely complicated,containing various metal ions such as V(V),Cr(VI),Ti(IV),Fe(III),Al(III),Ca(II),Mg(II),Si(IV),etc.[8,9].Extraction and selective separation of vanadium and chromium from those multi-metal co-existing leach solutions are difficult.In the past decades,lots of techniques have been developed to treat the acidic leach solution,such as chemical precipitation[10],ion exchange[11],and adsorption[12].However,low recovery rate,poor separation efficiency,and complicated operation hinder their further industrial application.
Liquid/liquid extraction is a highly efficient technique used in hydro metallurgy for separation and purification of valuable metal ions in complex leach solutions[13–16].Considerable efforts have been made by using various solvent extraction systems to recover and concentrate vanadium and chromium in acidic leach solution of high-chromium vanadium–titanium magnetite[17–19].However,numerous impurity ions co-existing in the leach solutions are unfavorable to the separation and purification of vanadium and chromium.In order to obtain a pure product,repeating adjustment of initial aqueous acidities of the feed solution,and multi-step pre-treatment are always unavoidable due to different requirements for selective extraction.As a result,it is extremely complicated to extract and separate vanadium and chromium in high-chromium vanadium–titanium magnetite.
Three-liquid-phase system(TLPS)is a novel extraction and separation strategy.Generally,it is composed of an organic solvent-rich top phase,a polymer-rich middle phase,and a salt-rich bottom aqueous phase[20–23].The novel system provides a new medium with hydrophobic ity decreasing gradually from the top phase to the bottom phase,allowing a much higher selectivity than traditional oil/water or aqueous biphasic two-phase systems.In recent years,the application of TLPS for separation of various complex multi-metal co-existing systems has been reported.Our previous works explored the possibility for separation of Ti(IV),Fe(III)and Mg(II)in the acidic leach solutions of the high-chromium vanadium–titanium magnetite[24,25].The results demonstrated that Ti(IV)and Fe(III)could be concentrated into trialkyl phosphine oxide(TRPO)-rich organic top phase and poly(ethylene glycol)(PEG)-rich middle phase,respectively,while Mg(II)remained in the salt-rich bottom aqueous phase.In addition,separation of Cr(III)and Cr(VI)from aqueous solutions has also been studied using same TLPS[26].The results indicated that Cr(III)and Cr(VI)could be separated and enriched into organic phase and PEG-rich phase,respectively.Despite the possible application of TLPS for processing the acidic leach solutions produced from the high-chromium vanadium–titanium magnetite,challenges still exist.For in stance,the separation of V(V)and Cr(VI)is difficult,and co-extraction of various impurities ions with the partitioning of V(V)and Cr(VI)is unavoidable.
In present work,a novel three-liquid-phase system,consisting of acidified primary a mine N1923(abbreviated as A-N1923),poly(ethylene glycol)(PEG)and(NH4)2SO4aqueous solution,was suggested to simultaneously extract and separate V(V)and Cr(VI)from the acidic leach solutions of high-chromium vanadium–titanium magnetite.A simulated aqueous solution only containing Al(III)was firstly investigated to obtain the optimal operation parameters.Various effects including aqueous pH,A-N1923 concentration,PEG added amount and(NH4)2SO4concentration were discussed.For the purpose of future industrial application,the feasibility of this TLPS for processing the real acidic leach solutions of high-chromium vanadium–titanium magnetite containing Al(III),Si(IV),Fe(III),Ti(IV),Mg(II)and Ca(II)was also investigated.
2.Materials and Method
2.1.Chemicals and reagents
Primary amine N1923(chemical purity of 93%)was kindly supplied by Shanghai Rare-Earth Chemical Co.Ltd.Polyethylene glycol(PEG)with average molecular weight of 2000(denoted as PEG 2000)was purchased from Sinopharm Chemical Reagent Co.,Ltd.,China.The stock solutions respectively containing 50 mmol·L−1of V(V),Cr(VI),Al(III),Si(IV),Fe(III),Ti(IV),Mg(II)and Ca(II)were prepared by dissolving NaVO3,NaCrO4,NaAlO2,Na2SiO3,FeCl3,TiCl4,MgCl2and CaCl2(analytical grade,West long chemical Co.Ltd.China),respectively.Solutions of 1 mol·L−1H2SO4and 2 mol·L−1NaOH were used for pH adjustment as appropriate.The other chemicals are analytical grade reagents.All chemicals were used as received,without further purification.
Primary amine N1923 was pre-equilibrated with 0.5 mol·L−1HNO3solution according to a chemical stoichiometric ratio of 1.05:1 between HNO3molecules and–NH2groups in primary amine N1923 molecules,then the obtained acidified primary a mine N1923 was washed until neutral by deionized water.The acidified primary amine N1923 was dissolved into n-heptane and used as organic extract ant.
2.2.Three-liquid-phase extraction and separation
Experimental aqueous solutions containing various metal ions were prepared by diluting their corresponding stock solutions with deionized water and mixing together.A certain amount of(NH4)2SO4was then dissolved into above mixed aqueous solution,and the desired pH value of the aqueous solution was adjusted by adding either 1 mol·L−1H2SO4or 2 mol·L−1NaOH.A certain amount of polymer(PEG 2000)was then added into above mixed aqueous solution,with shaking thoroughly for 5 min followed by centrifugation for 10 min at a speed of 4000 r·min−1to obtain a stable aqueous two-phase system(ATPS).The organic phase composed of A-N1923 and n-heptane was added into the ATPS,with shaking thoroughly for 20 min and followed by 10 min of centrifugation at a speed of 4000 r·min−1.Then,a stable three liquid phases co-existing system could be obtained.After clear phase separation,the volume of each immiscible liquid layer in TLPS was recorded.All of above experiments were performed at room temperature(25°C±0.1°C).
2.3.Determination of the concentrations of metal ions and their partitioning in TLPS
After phase separation,the concentrations of metal ions in the polymer-rich middle phase and salt-rich bottom aqueous phase were analyzed by an OPTIMA 7000 DV inductively coupled plasma optical emission spectrometer(ICP-OES,Pekin Elmer,U.S.).The concentration of metal ions in the A-N1923 enriched top organic phase could be calculated from mass balance.The metal extraction experiments followed by analyses of the samples were conducted three times to check the repeatability and accuracy of measurements.The error bars were added in the following experimental results.
The mass fractions of metal ions in each phase in ATPS or TLPS can be calculated according to following equations.In ATPS,mass fractions of metal ions in the polymer-rich phase and salt-rich phase were expressed as follows:

In TLPS,mass fractions of metal ions in the top phase,polymer-rich middle phase,and salt-rich bottom phase were expressed as follows:

where M represents metal ions,and wM,p,wM,sare the mass fractions of metal ion M in the polymer-rich phase and salt-rich phase of ATPS.cM,pandcM,sare the concentrations of metal ion M in the polymer-rich phase and salt-rich phase.vpis the volume of the polymer-rich phase in ATPS.vsis the volume of the salt-rich phase in ATPS.In TLPS,wM,t,wM,m,and wM,bare the mass fractions of metal ion M in the top,middle,and bottom phases,respectively.cM,mand cM,bare the concentrations of metal ion M in the middle and bottom phases,respectively.vmand vbare the volumes of the polymer-rich middle phase and salt-rich bottom phase,respectively,in TLPS.m0is the total amount of metal ions added to the system.
2.4.Determination of the water content in PEG-rich phase
The aqueous two-phase system(ATPS)was prepared by adding 2 g PEG2000 into the aqueous solutions containing different(NH4)2SO4concentrations.After phase separation,3 ml samples were taken from the PEG-rich top phase in the different ATPS,and were weighed with a 0.1 mg precision.The samples were then dried for 24 h at 110°C under vacuum condition to remove the water in the samples.The dried samples were weighed.The water content inthe PEG-rich phase could be calculated according to following equations:

Table 1 Metal partition behaviors in two phase systems of PEG 2000-(NH4)2SO4-water

where m0represents the mass of PEG-rich phase,m1represent the mass of dried PEG-rich phase.
2.5.Other measurements and characterization
The pH measure ments were per for medusing HANNA pH211digital pH meter(Italy).The concentration of NO3−ions in aqueous phase was determined by ion chromatograph(ICS-1000,Dionex).FTIR spectra with a resolution of 4 cm−1were recorded on a Bruker TENSOR 27 FTIR spectrometer at room temperature(25°C).The OPUS spectroscopic software was used for data handling.
3.Results and Discussion
3.1.Partition behaviors of V(V),Cr(VI)and Al(III)in ATPS of PEG2000-(NH4)2SO4-water and TLPS of A-N1923-PEG2000-(NH4)2SO4-water
Compared with conventional liquid/liquid extraction systems composed of oil–water two phase,three liquid phase method could achieve extraction and separation of multiple target metal ions simultaneously in one process,while other co-existing impurity ions could not be extracted.To elucidate the partition mechanism during threeliquid-phase extraction processes,the investigations into the partition behaviors of metal ions in the polymer based aqueous two-phase systems are necessary.Herein,an aqueous two-phase system was prepared by mixing PEG 2000,(NH4)2SO4,and aqueous solutions containing V(V),Cr(VI)and Al(III).Experimental results are shown in Table 1.
As can be seen in Table 1,V(V)and Cr(VI)prefer to be enriched in the PEG-rich top phase.Besides,mass fraction of Cr(VI)in the PEG-rich top phase is higher than that of V(V),which indicate that the affinity of PEG-rich phase to Cr(VI)is stronger than that to V(V).On the contrary,most Al(III)remains in the(NH4)2SO4-rich bottom phase.Different partition behaviors of V(V),Cr(VI)and Al(III)in the ATPS provide potential for their further efficient separation.
A stable three-liquid-phase system is obtained when adding A-N1923/n-heptane organic solution into the above two phase system of PEG2000-(NH4)2SO4-water mixture.The partition behaviors of V(V),Cr(VI)and Al(III)in the three-liquid-phase system are shown in Table 2.
As shown in Table 2,V(V),Cr(VI)and Al(III)are selectively transferred into A-N1923-rich organic top phase,PEG-rich middle phase and(NH4)2SO4-rich bottom phase,respectively.Our previous researches demonstrated that anion species of V(V)could be effectively extracted into A-N1923-rich organic phase through anion exchange mechanism[27,28].According to the concentration-pH diagram of V(V),V(V)exist in the form ofin the solution at aqueous pH of 3.As a result,most of V(V)could be extracted into the A-N1923-rich organic phase.Most Cr(VI)exists in the form ofat aqueous pH of 3,which exhibits strong affinity for the PEG-rich phase[26].Compared to the ATPS,an increase of Cr(VI)concentration in the aqueous solutions was observed.It could be attributed to the increase of aqueous pH due to the protonation of N1923 by H+.Al(III)exist in the cation form of Al3+in the acid solutions,which exhibit weak affinity to both A-N1923-rich organic phase and PEG-rich middle phase.Therefore,it prefers to remain in the(NH4)2SO4-rich bottom phase.
3.2.The effect of aqueous pH
Fig.1 gives the effect of aqueous pH on the partition of V(V),Cr(VI)and Al(III)in the TLPS.
As shown in Fig.1,the extraction of V(V)and Al(III)are highly pH-dependent.At low aqueous pH(pH<3),V(V)and Al(III)exist in the cation form ofand Al3+,respectively,which could not be extracted by both A-N1923-rich top phase and PEG-rich middle phase.As a result,most of V(V)and Al(III)would remain in the(NH4)2SO4-rich bottom phase.With the increase of aqueous pH,the exist forms of V(V)and Al(III)gradually convert to anions,such as,and,respectively,which could be extracted into the AN1923-rich top phase[27,28].Consequently,the mass fractions of V(V)and Al(III)in the A-N1923-rich top phase increase abruptly as increasing the aqueous pH.Cr(VI)mainly exists in the anion forms ofandwithin the pH range from 1 to 6,and the molar ratio oftodecreases with the increase of aqueous pH.It is well known that the high charge density of ions is in favor of the hydration of ions[29].As a result,the hydration ofis stronger than that of,due to the higher charge density ofcompared to that of.Hence,the hydrophilicity ofis stronger than that of.It has been reported that the hydrophobic interaction plays an important role in the extraction of PEG-rich phase toward metal ions[30,31].The strong hydrophilic nature ofhinders the interaction betweenand hydrophobic PEG-rich phase,which results in the decrease of extraction percentage of Cr(VI)in the PEG-rich phase during the increase of aqueous pH.
3.3.The effect of A-N1923 concentration
Fig.2 gives the effect of A-N1923 concentration on the partition of V(V),Cr(VI)and Al(III)in the TLPS.

Table 2 Three-liquid-phase partition behaviors of V(V),Cr(VI)and Al(III)in systems of A-N1923/n-heptane(20%A-N1923,v/v)-PEG2000-(NH4)2SO4-water
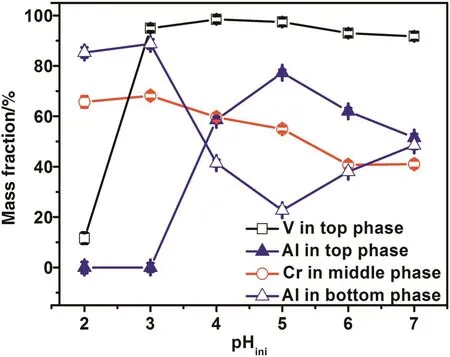
Fig.1.The effect of aqueous pH on the partition of V(V),Cr(VI)and Al(III)in the TLPS(Feed component:5.0 mmol·L−1V(V),5.0 mmol·L−1Cr(VI),2.5 mmo·L−1Al(III),the concentration of(NH4)2SO4was 1.5 mol·L−1,the added amount of PEG 2000 was 4 g,the organic phase consist of 0.20 ml A-N1923 and 0.80 ml n-heptane).
As depicted in Fig.2,the mass fraction of V(V)in the A-N1923-rich top phase increases abruptly to 93%as increasing A-N1923 concentration to 10%,then reaches equilibrium as further increasing AN1923 concentration up to 18%.The mass fraction of Cr(VI)in the PEG-rich middle phase increases firstly as increasing A-N1923 concentration to 10%,then decreases slowly as further increasing AN1923 concentration up to 18%.Most of Al(III)would be concentrated in the(NH4)2SO4-rich bottom phase and despite the increase of A-N1923 concentration.
Accordingto the results listed in Table 1,V(V)and Cr(VI)could both be extracted into the PEG-rich phase when A-N1923 concentration is low.As increasing A-N1923 concentration to 10%,V(V)extracted into the PEG-rich phase would then transfer into the A-N1923-rich top phase due to the special affinity of A-N1923 molecules toward V(V).Consequently,there are much more PEG that could be provided for the extraction of Cr(VI),which resulting the increase in mass fraction of Cr(VI)in the PEG-rich phase.While with further increasing the A-N1923 concentration up to 10%,most of V(V)would then be extracted in to the A-N1923-rich top phase.Hence,part of Cr(VI)extracted into the PEG-rich phase would then transfer into the A-N1923-rich top phase,which resulting the decrease of mass fraction of Cr(VI)in the PEG-rich phase when A-N1923 concentration increases from 10%to 18%.

Fig.2.The effect of A-N1923 concentration on the partition of V(V),Cr(VI)and Al(III)in the TLPS(feed component:5.0 mmol·L−1V(V),5.0 mmol·L−1Cr(VI),2.5 mmol·L−1 Al(III),the concentration of(NH4)2SO4was 1.5 mol·L−1,the added amount of PEG 2000 was 4 g,aqueous pH was 3.0).

Fig.3.The effect of PEG added amount on the partition of V(V),Cr(VI)and Al(III)in the TLPS(Feed component:5.0 mmol·L−1V(V),5.0 mmol·L−1Cr(VI),2.5 mmol·L−1Al(III),the concentration of(NH4)2SO4was 1.5 mol·L−1,aqueous pH was 3.0,the organic phase consist of 0.10 ml A-N1923 and 0.90 ml n-heptane).
3.4.The effect of PEG added amount
Fig.3 gives the effect of PEG added amount on the partition of V(V),Cr(VI)and Al(III)in the TLPS.
As shown in Fig.3,most of V(V)would be extracted into the A-N1923-rich top phase despite the increase of PEG added amount.The mass fraction of Cr(VI)in the PEG-rich middle phase increases significantly with the increase of PEG added amount,due to the increase of interaction site between PEG molecules and Cr(VI).The mass fraction of Al(III)in the(NH4)2SO4-rich bottom phase increases slightly with the increase of PEG added amount.It is well known that the aqueous pH would decrease with the adding of PEG due to the acid property of PEG molecules.When 3 g PEG is added into the solutions,part of Al(III)existed in the form ofwhich could be extracted into the PEG-rich phase.With the increase of PEG added amount,the aqueous pH would then decrease,and thewould convert to Al3+which could not be extracted into the PEG-rich middle phase.As a result,the mass fraction of Al(III)in the(NH4)2SO4-rich bottom phase would increase with the increasing PEG added amount.
3.5.The partition mechanism of vanadium and chromium in the three liquid phase system
It has been reported that primary a mine N1923 pre-acidified with HNO3(A-N1923)could be used to extract V(V)from alkaline solutions,and the mechanism of extraction was con firmed to be anions exchange betweenand[27].In the present work,V(V)existed in the form of poly-anion in the acid solution according to the concentration pH diagram of V(V).Therefore,it was reason able to suppose that the extraction of V(V)by A-N1923 was the exchange between poly-anions of V(V)and.The reactions can be described as follows:

where Eq.(7)represents acidification process;Eq.(8)represents extraction process.To determine the extractable vanadium species Mn−in the Eq.(8).The extract ant A-N1923 was employed to extract V(V)from different volume of solutions containing same concentration of V(V).The amount of V(V)extracted by A-N1923 and the amount ofexchanged into aqueous phase were analyzed,and their ratios were also calculated.As shown in Table 3,the molar ratio of V(V)tois approximately 2.5,this means that ratio of vanadium atoms tocharge in the vanadium species which are extracted by A-N1923 was 2.5,andwas supposed to be the extractable vanadium species Mn−according to the concentration-pH diagram of V(V).The extraction reaction of V(V)by A-N1923 can be written as follows:

Table 3 The relative species contents at the extraction equilibrium
Fig.4 gives the effect of(NH4)2SO4concentration on the partition of Cr(VI)and water content in the PEG-rich phase.It can be seen that the mass fraction of Cr(VI)in the PEG-rich middle phase increases significantly with the increase of(NH4)2SO4concentration in the aqueous solution.In the TLPS,(NH4)2SO4is employed as salting-out agent which could peel off H2O from the hydration shell of PEG molecules.The increase of(NH4)2SO4concentration would lead to the increase of hydro phobic ity of PEG-rich middle phase.It has been reported that the hydrophobic interaction is crucial to the extraction of PEG-rich phase toward metal ions,the increase in hydrophobicity of PEG-rich phase would be in favor of the interaction between PEG-rich phase and Cr(VI)[31,32].To further investigate the role of hydrophobic interaction in the partitioning of Cr(VI)in the PEG-rich phase,FTIR has been employed to characterize the hydrophobicity of PEG-rich phase.
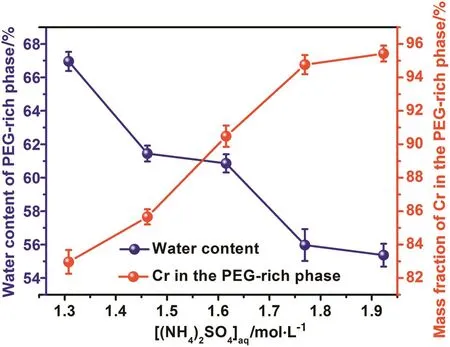
Fig.4.The effect of(NH4)2SO4concentration on the water content and mass fraction of Cr(VI)in the PEG-rich phase(Feed component:5.0 mmol·L−1V(V),5.0 mmol·L−1 Cr(VI),2.5 mmol·L−1Al(III),the added amount of PEG 2000 was 4 g,the organic phase consist of 0.10 ml A-N1923 and 0.90 ml n-heptane).
As depicted in Fig.5,the peak about 1110 cm−1is attributed to the stretching vibration of ether oxygen bond in PEG molecules,and is sensitive to change of environment and interaction.It can be clearly seen that the peak at 1116 cm−1in the PEG solid red shifted to 1100 cm−1when PEG solid was dissolved in the water.According to previous reporting,the red shift of stretching vibration of ether oxygen bond could be interpreted as the hydration of ether oxygen bond in the PEG molecules by H2O,which represent the in crease in hydrophilicity of PEG phase[32].In the case of PEG-rich phase of ATPS,the stretching vibration peak of ether oxygen bond is blue shifted from 1100 cm−1to 1105 cm−1in comparison with the PEG aqueous solution,and which could be attributed to the dehydration of PEG molecules resulting from the salting-out of(NH4)2SO4,which leading to an increase in hydrophobicity of PEG phase.In addition,the stretching vibration peak of ether oxygen bond is blue shifted from 1105 cm−1to 1106 cm−1as increasing the(NH4)2SO4from 10%to 25%,which indicates that the hydrophobicity of PEG phase increases during the increase of(NH4)2SO4concentration.Combining the experiment results above that the mass fraction of Cr(VI)in the PEG-rich phase would increase as increasing the concentration of(NH4)2SO4,we suppose that the increase of mass fraction of Cr(VI)in the PEG-rich phase during the increase of(NH4)2SO4concentration might result from the increase of hydrophobicity of PEG-rich phase.It can be then concluded that hydrophobic interaction between PEG-rich phase and HCrO4−accounts for the partitioning of Cr(VI)in the PEG-rich phase.

Fig.5.FTIR spectra of solid PEG 2000,15%PEG 2000,PEG-rich phase of ATPS with salt concentrations of 10%and 25%.
3.6.The separation of Vand Crfrom the real acidic leach solution containing Al,Si,Fe,Ti,Mg and Ca
It is well known that the composition of lixivium is always complex,due to the multi-metallic characteristics of ore.In order to demonstrate the feasibility of our suggested three-liquid-phase system for the application in processing of high-chromium vanadium–titanium magnetite,herein,various co-existing impurity ions were considered in the acidic leach solution.The chemical analysis of the acidic leach solution employed in present work is shown in Table 4.Fig.6 gives the partition behavior of those ions in the TLPS.
As depicted in Fig.6,most of V(V)and Cr(VI)could be selectively separated and concentrated into top phase and middle phase,respectively.The impurity ions including Al(III),Si(IV),Fe(III),Ti(IV),Mg(II),and Ca(II)could be intercepted in the bottom phase.Part of Si(IV)would be extracted into the PEG-rich phase.Obviously,the co-extraction of Si(IV)in the PEG-rich phase could not be avoided in the present study.There aretwo ways to deal with this issue.On the one hand,we could devise new middle phase in the TLPS,which exhibit excellent separation selectivity of Cr(VI)to Si(IV).On the other hand,after stripping of Cr(VI)and Si(IV)from the PEG-rich phase,further separation strategy could be employed to selectively extract Cr(VI)from Si(VI).

Table 4The chemical analysis of the acidic leach solution

Fig.6.The partition behaviors of V(V),Cr(VI),Al(III),Si(IV),Fe(III),Ti(IV),Mg(II),and Ca(II)in the three liquid phase extraction system(the organic phase consist of 0.10 ml A-N1923 and 0.90 ml n-heptane,the volume of aqueous solution was 20 ml).
3.7.The stripping of V and Cr from loaded organic rich phase and PEG rich phase
According to the results in Fig.1,the mass fraction of Cr(VI)in the PEG rich phase decreased with the increase of aqueous pH.Herein,the stripping of Cr(VI)from the PEG rich phase has been performed using the aqueous solution composed of NaOH and(NH4)2SO4.NaOH is employed tostrip Cr(VI)from the PEG rich phase into aqueous solution,while(NH4)2SO4is used to salt out PEG from the aqueous solution.
As shown in Fig.7,Cr(VI)could be stripped effectively from the PEG-rich phase by increasing the concentration of NaOH.More than98%of Cr(VI)would be stripped from the PEG-rich phase with the NaOH concentration of 0.1 mol·L−1.
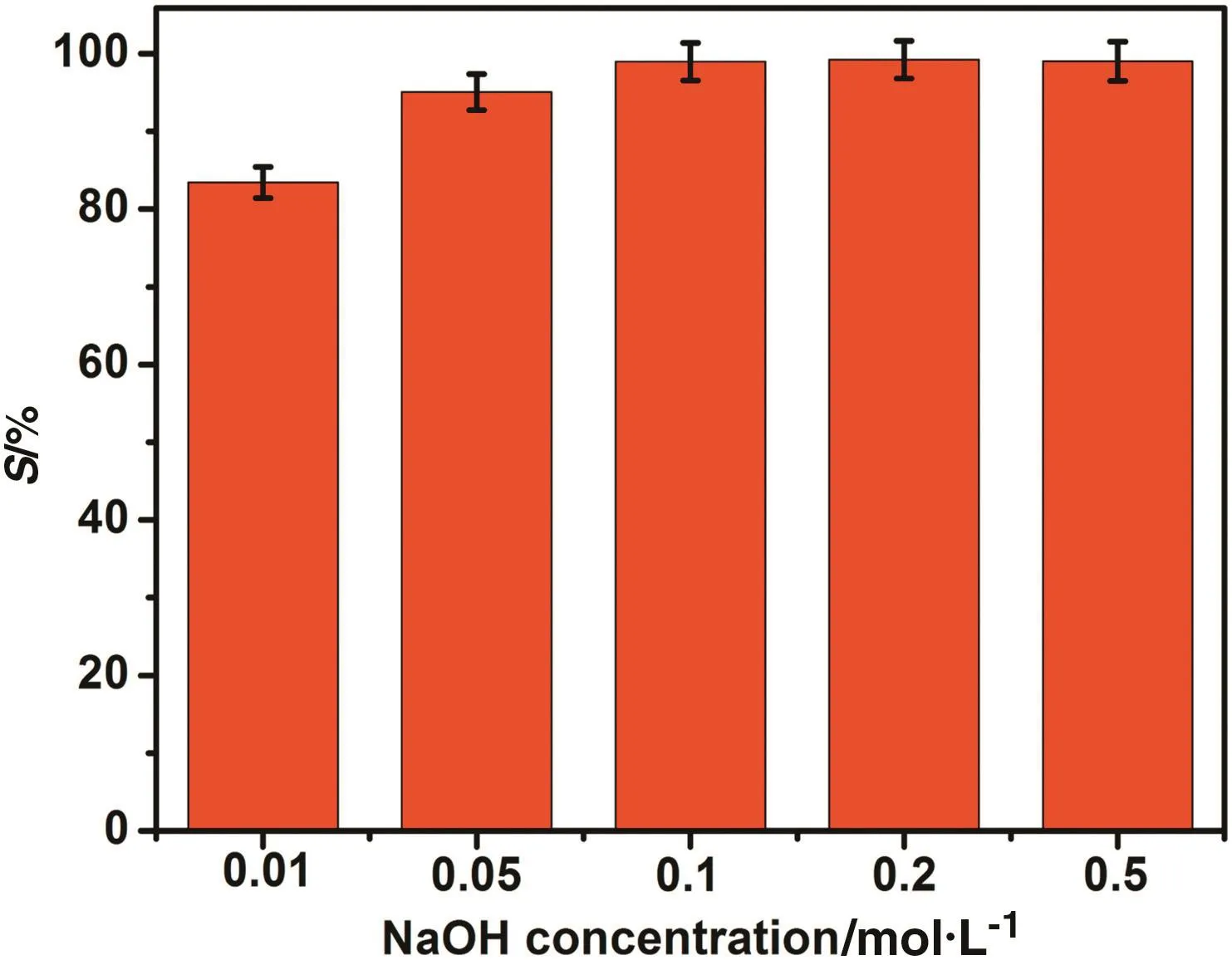
Fig.7.The effect of Na OH con centration on the strip percentage of Cr(VI)from loadedPEG middle phase(the strip solutions were 2 mol·L−1(NH4)2SO4aqueous solutions with different concentration of NaOH).
According to the previous reports,NaNO3aqueous solutions could be used to strip V(V)from the loaded organic phase[27,28].Thus,we select NaNO3as the strippant of V(V)in the present study.Fig.8 gives the effect of NaNO3concentration on the strip percentage of V(V)from loaded organic top phase.

Fig.8.The effect of NaNO3concentration on the strip percentage of V(V)from loaded organic top phase(the phase ratio of A/O are 10).
As depicted in Fig.8,the strip percentage of V(V)increases with the increase of NaNO3concentration.More than 99%of V(V)could be stripped by 1.5 mol·L−1NaNO3solution.
Based on the results above,we propose a process flow-sheet for extraction and separation of V(V),Cr(VI),Al(III),Si(IV),Fe(III),Ti(IV),Mg(II),and Ca(II)from the acid solutions produced from the highchromium vanadium–titanium magnetite,as shown in Fig.9.The recovery and purity of V(V),Cr(VI)listed in the Fig.9 were obtained from the experimental results.
In this process,V(V)and Cr(VI)are concentrated from the acid lixivium into the A-N1923-rich phase and PEG-rich phase,respectively.The impurity ions such as Al(III),Si(IV),Fe(III),Ti(IV),Mg(II),and Ca(II)are remained in the(NH4)2SO4-rich bottom phase.The V(V)loaded in the A-N1923-rich phase could be stripped by 1.0 mol·L−1NaNO3solutions,the obtained organic phase would then be used in the next extraction.The Cr(VI)loaded in the PEG-rich phase could be stripped by NaOH-(NH4)2SO4mixture solutions,and the obtained PEG-rich phase could also be used in the next extraction.
4.Conclusions
The present works demonstrate that the suggested three-liquid phase system,consisting of acidified primary amine N1923(abbreviated as A-N1923),poly(ethylene glycol)(PEG)and(NH4)2SO4aqueous solution,could indeed be employed for separation and simultaneous extraction of V(V)and Cr(VI)from the acidic leach solutions of high-chromium vanadium–titanium magnetite.Experimental results indicated that the A-N1923 organic top phase and the PEG-rich polymer middle phase in the TLPS of A-N1923/PEG/(NH4)2SO4exhibited a high separation selectivity for V(V)and Cr(VI),respectively,while Al(III)and other co-existing impurity ions,such as Si(IV),Fe(III),Ti(IV),Mg(II)and Ca(II)in the acidic leach solutions,could be enriched in the(NH4)2SO4bot to maqueous phase.During the process for extraction and separation of V(V)and Cr(VI),almost all of impurity ions could be removed.The separation factors between V(V)and Cr(VI)could reach 630 and 908,respectively in the organic top phase and PEG middle phase,and yields of recovered V(V)and Cr(VI)in the top phase and middle phase were all above90%.The hydrophobic interaction betweenand PEG determines the partition of Cr(VI)into the PEG-rich phase,and extraction of V(V)by A-N1923 resulted of anion exchange betweenand.It is feasible to strip V(V)and Cr(VI)from the top organic phase and the middle PEG-rich phase by mixing respectively with NaNO3aqueous solutions and NaOH-(NH4)2SO4solutions.

Fig.9.The process flow-sheet of three liquid phase extraction and separation of V(V),Cr(VI),Al(III),Si(IV),Fe(III),Ti(IV),Mg(II),and Ca(II)in acidic leach solution.
The present work highlights an efficient approach to simultaneously extract and separate V(V),Cr(VI)with removal of Al(III)and other impurities in multi-metal co-existing acidic leach solutions produced from the high-chromium vanadium–titanium magnetite.It might exhibit huge potential for future's exploitation of high-chromium vanadium–titanium magnetite in Panxi region,Sichuan,China.
 Chinese Journal of Chemical Engineering2018年7期
Chinese Journal of Chemical Engineering2018年7期
- Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Preparation of vapreotide-templated silver nanocages and their photothermal therapy efficacy☆
- DNA-assisted rational design of BaF2linear and erythrocyteshaped nanocrystals☆
- On the database-based strategy of candidate extractant generation for de-phenol process in coking wastewater treatment☆
- Hierarchical porous MgBO2(OH)microspheres:Hydrothermal synthesis,thermal decomposition,and application as adsorbents for Congo red removal☆
- Degradation analysis of A2/O combined with AgNO3+K2FeO4on coking wastewater
