Infestation and morphological identi fication of the stalked epizoic barnacle Octolasmis on the blue crab Portunus pelagicus from the Red Sea
Rafat AFIFI KHATTAB
1 Taibah University, Faculty of Sciences, Department of Biology, Medina 344, Saudi Arabia
2 Suez Canal University, Faculty of Science, Department of Marine Science, Ismailia 41522, Egypt
Abstract The intensity of infestation of the epizoic stalked barnacle Octolasmis on the blue crab was calculated for a total of 180 individuals collected around Jeddah, the western Red Sea Saudi coast. More than 90% of the crabs were found bearing the Octolasmis at a prevalence rate of 92% and mean intensity of 18.5± 18.6 (0–127 epibiont per host). The intensity of infestation increased with host size in the range 90–135 mm. Generally, females were more infested (69%) than the males (31%). Octolasmis angulata occurred mostly on the gills, and the lower side of the gill chambers was more infested (84%) than the upper side (16%) and the left side of gills was also more infested than the right side in both males and females.Further studies are still needed to examine the commensal relationship and its impact on the fishery market of the Red Sea blue crab populations.
Keyword: Octolasmis; ecology; commensal infestation; Portunus pelagicus; Red Sea
1 INTRODUCTION
Some organisms in a process called epibiosis encrust sessile biota, an epibiont being an organism that live on the external surface of the host. Intensity and frequency of epibiosis results from limitation in substrate, thus this kind of association (commensal symbiosis) adds several advantages to the epibiont such as; gene flow and dispersal of the symbiont (Key Jr et al., 1996); anti predatory protection (Abelló et al., 1990); and improved access to food during host feeding as well as removal of waste residues produced by the symbiont (Wahl, 1989). The epibiont life cycle is highly dependent on successful adherence to the suitable substratum especially in soft bottom habitats of the coastal zones (Becker and Wahl, 1996).
The space available to an epibiont on exoskeletons of arthropod crustaceans and their carapaces renewal due to ecdysis are two important factors that govern epibiont colonization patterns in encrusting communities where the survival is dependent on occupation of new substrates (Connell and Keough,1985). Crustacean cirripeds comprise a large number of commensal symbiotic species especially the stalked pedunculated barnacles (Jeffries et al., 1982; Key et al., 1996).
Octolasmis is a genus of the gooseneck (or stalked)barnacles (Cirripedia: Thoracica) that attach themselves to the gills of decapod crustaceans as ectocommensals (Newman, 1967) or externally to the carapace and appendages (Voris and Jeffries, 1997).Adult stages of Octolasmis species have been found in the gill chamber of numerous brachyuran crabs(Humes, 1941; Walker, 1974; Jeffries et al., 1982; Yan et al., 2004; Cordeiro and Costa 2010). After internal fertilization and brooding by the female, the barnacle goes through six naupliar stages and one cyprid stage as a free-swimming planktivore larva before settling down on a crab host (Lang, 1976; Jeffries and Voris,1983). The adult barnacle, cemented to the gill lamellae of the host crab, filter feeds on particulate matter in the ventilatory stream. The barnacle gains no nutrients directly from the crab and is thought to harm its host only indirectly by occluding the ventilatory current when infestation levels are extreme(Walker, 1974). Overstreet (1982) assumed that the combination of heavy infestations and debris on the gills of the blue crab could impede gas exchange.
The barnacle has been reported to experience selective pressure to find optimal (i.e., better ventilated) sites within the host branchial chamber as well as optimal (non-molting adults) hosts because,during the host molt, it would be shed with the exoskeleton covering the gills and is not thought capable of survival outside the host (Walker, 1974).Because the barnacle is dependent on the host for the continued renewal of ventilatory water, selection pressure on the barnacle should minimize any detrimental impact on the host (Walker, 1974).
The blue crab Portunus pelagicus is abundant in Indo-Paci fic waters and is an important recreational and commercial fishery (Gaddes and Sumpton, 2004).Along the Gulf coast and the Saudi Arabian coast of the Red Sea, this species population levels are large enough to support commercial fisheries (Alsaqabi et al., 2010). By nature, Portunu s are very active crabs with a high rate of respiratory gas exchange that is vital to its metabolism and growth (Gannon and Wheatly, 1992). The crab is relatively long-lived (up to three years as an adult) (Van Engel, 1958), moves long distances (Oesterling and Adams, 1982) and provides a hard substratum in soft bottom estuaries(Williams, 1984). It serves as a host to numerous obligate and non-obligate commensals, such as the soft coral Leotoaoraia viraulata (Pearse, 1947), the bryozoan, Triticella eloncata (Maturo Jr., 1957), the leech Mvzobdella luaubris (Overstreet, 1982) and the sessile barnacle, Octolasmis spp. (Van Engel, 1958).
P. pelagicus is considered one of the main economic species of marine organisms in the coastal region of the Red Sea and Arabian Gulf at the Kingdom of Saudi Arabia (Alsaqabi et al., 2012). It is widely distributed and contributes to the Gulf States and Saudi Arabia fisheries with a total of 3 248 and 4 472 tonnes (2007) in Bahrain and Saudi Arabia respectively (FAO, 2010). Despite its abundance in the Red Sea, no studies have been conducted regarding the infestation of P. pelagicus by epibionts or even parasites and its economic impacts on blue crab marketing.
Therefore, this study aims to describe the prevalence and mean intensity of infestation by the crustacean ectocommensal of the genus Octolasmis and identify to the species level as a first record on the Red Sea blue crabs.
2 MATERIAL AND METHOD
Crab specimens (180) were collected from the fishermen's catch at Jeddah fishing port on the west coast of Saudi Arabia in November, 2013. They were sexed and measured for their carapace width (CW) by means of a vernier caliper with an accuracy of 0.01 mm then divided into three size-based categories,small (90–120 mm), medium (120–136 mm) and large (136–155 mm). Thirty individuals representing each size class were selected for examination. For those, the carapace was lifted and gills were visually inspected for the presence of the ectocommensal barnacle. Attachment sites were recorded with respect to gill chamber (right or left), aspect (epibranchial or hypobranchial), gill number (#1–8, anterior to posterior), and the distance along the gill according to Gannon and Wheatly (1992). For the latter measurement, each gill was arbitrarily divided into thirds (basal, medial, and distal), which, because of the triangular nature of the phyllobranch gill, made up 56%, 33% and 11%, respectively, of the gill surface area Gannon and Wheatly (1992). The number of Octolasmis in each infested crab (intensity) and total number per sample/total number of infested crabs(mean intensity) were calculated. Finally, the prevalence of barnacles was calculated by dividing the number of infested hosts on the total number of examined individuals and expressed as a percentage.
Parametric analysis were used in this study on the assumption that the population data of Octolasmis were normally distributed. Moreover, parametric tests usually have more statistical power than their nonparametric equivalents to detect signi ficant differences when they truly exist (Campbell and Swinscow 2009).The relationships between crab size, sex and infestation rate were estimated using Pearson’s correlation test. Statistical analysis (ANOVA) were used to reveal the differences between host’s sizeclass infestation rates. The signi ficance criterion in all tests was set at P <0.05.
The barnacles were isolated and examined underneath a Wild stereo microscope for identi fication.Based on the presence of many variations within species, the whole body and all appendages were investigated and dissected in lactic acid using brightfield and differential interference microscopes (Nikon DM 6000). Drawings were carried out with a cameralucida attached to the microscope and an ocular micrometer. For scanning electron microscopy,specimens of barnacles were initially washed in filtered seawater, pure distilled water, and serially dehydrated through a 30%–100% ethanol and subsequently dried, then mounted on a stub, coated with gold palladium, and finally inspected with a Scanning Electron Microscope, SEM JSM-6360LV.
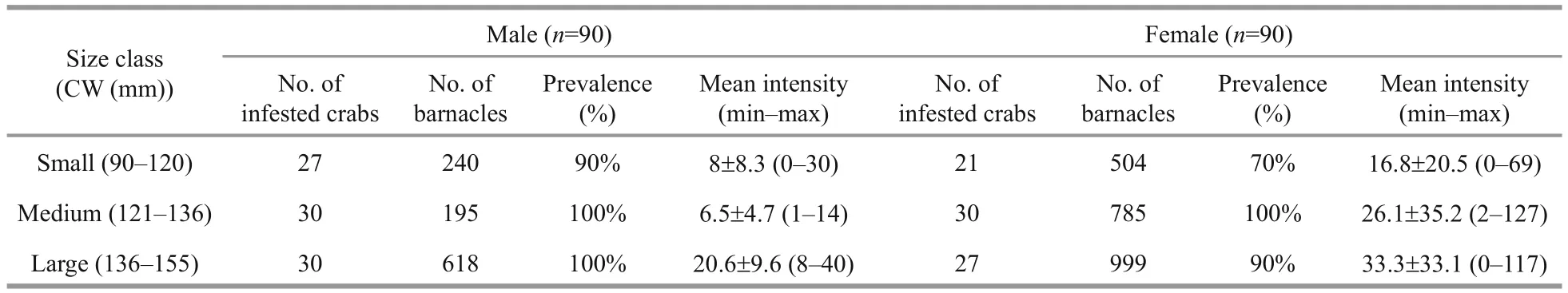
Table 1 Prevalence and mean intensity of infestation by O. angulata in the different size class categories of male and female P. pelagicus from the Red Sea, Saudi Arabia
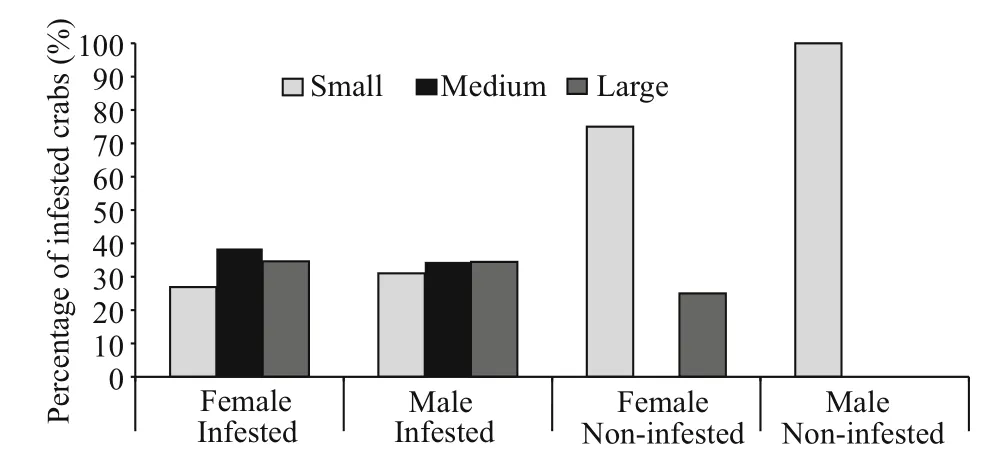
Fig.1 Percentage of different size class categories of male and female P. pelagicus infested with O. angulata compared with non-infested crabs from the Red Sea,Saudi Arabia
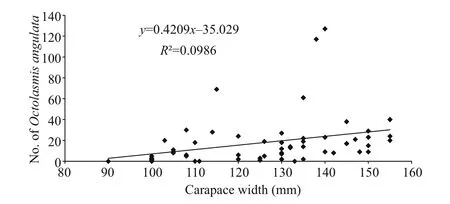
Fig.2 Intensity of infestation by the stalked barnacle ( O.angulata) according to host size (carapace width in mm) of the Red Sea blue crab P. pelagicus
3 RESULT
3.1 Distribution of O. angulata on the blue crab P. pelagicus
Of the 180 examined crabs, 165 (87 males and 78 females) were infested with a prevalence of 92% and mean intensity of 18.5± 18.6. A total of 3 472 epizooties were recorded (2 288 on female hosts and 1 053 on males). Individuals of both sexes within the middlesized class were all infested (Table 1) while small sized females were the least infested showing a percentage of 70% (Fig.1). Statistically, there was a signi ficant difference in the intensity of infestation between both sexes of infested crabs ( F=109.34, P <0.05).
The relationship between the number of the barnacle attached to the gills and the carapace width of the blue crab P. pelagicus is shown in Fig.2. The infested smallest-sized crab had 90 mm carapace width and the rate of infestation was found to increase with increasing the size of the male crabs. Pearson’s correlation coefficient showed that there were no signi ficant relationship between carapace width and length of the females ( R=0.272 and 0.304 respectively,n=90), and infestation rate by O. angulata. On the other hand, positive correlations were recorded in males ( R=0.599 and 0.589 for carapace width and length respectively, n=90).
3.2 Distribution and infestation rate of O. angulata in the different parts of the branchial chambers of the crab gills
In both sexes, the inner surface of the gills(hypobranchial) was the prevalent attachment sites over the outer one (epibranchial). The proximal and medial parts of the crab gills were the most infested followed by the distal parts. Octolasmids occurred with a percentage of 48.8% and 72.7% on the proximal and medial parts respectively, whereas only 8.5%harbored on the distal part. As shown in Table 2,statistical analysis (ANOVA) revealed insigni ficant difference in the distribution of O. angulata on the left and right sides of both sexes ( F=2.89, P >0.05).
3.3 Distribution of infestation by O. angulata among the eight gills
The study showed a variation in infestation levels among the eight gills of the crab, where the fourth gillfollowed by gill number five and six was the most preferred attachment sites in all crab sizes of males and females. The lowest number of barnacles were found on gill number one and two (Fig.3). The interaction of sex types and gills number under study conditions indicated that the intensity rate varied signi ficantly ( F=22.14, P <0.05) with gills number.The maximum value of intensity rate (3.07) appeared at the fourth gill, while the minimum value of 0.02 was observed at the first gill.
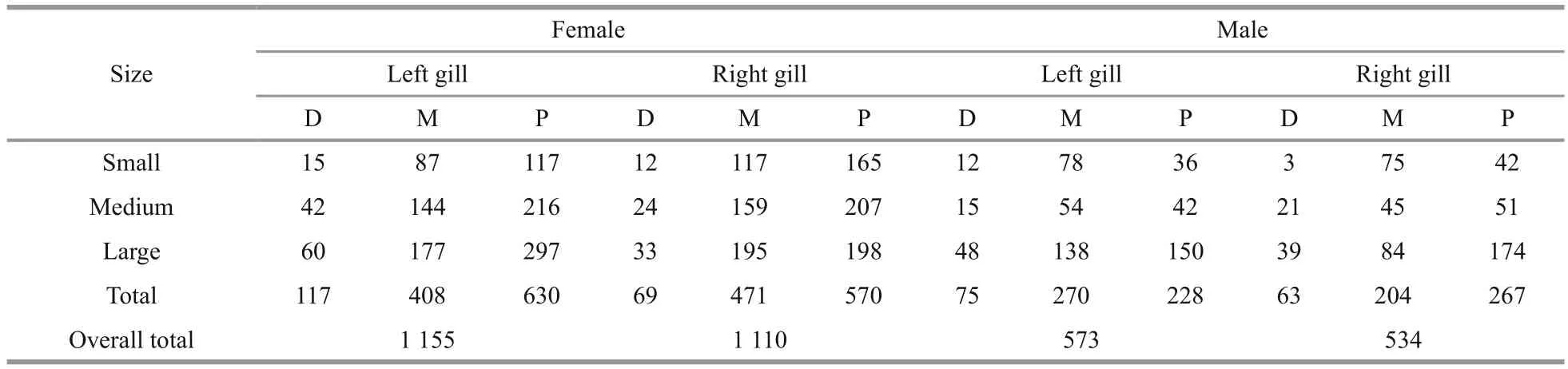
Table 2 Infestation rate (number of barnacles) found on surfaces of the right and left side of the gills of infested P. pelagicus
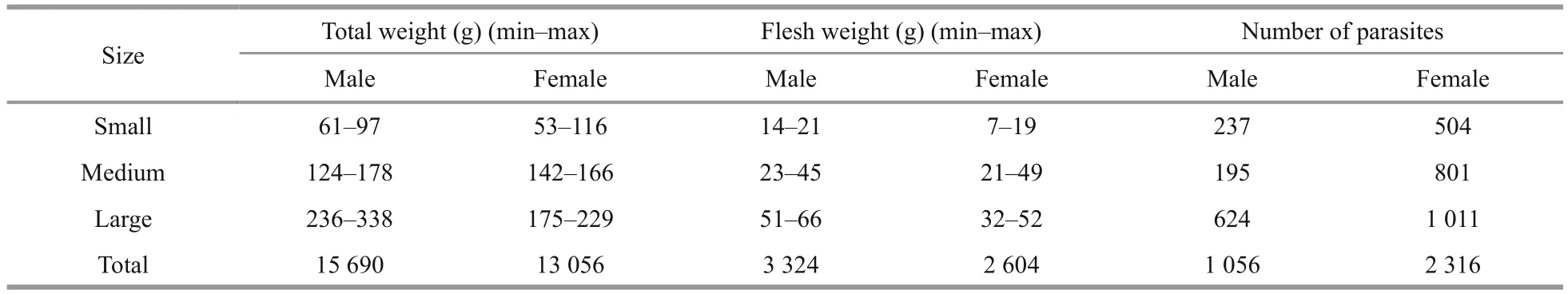
Table 3 Total and flesh weight (g) of the blue crab ( P. pelagicus) and the number of O. angulata according to size categories of the host
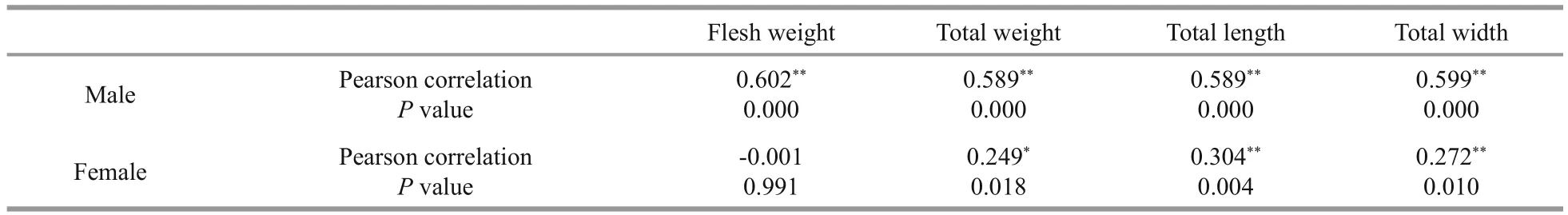
Table 4 Pearson’s correlation between host’s ( P. pelagicus) weight, length, width and number of O. angulata (3472) according to host’s sex
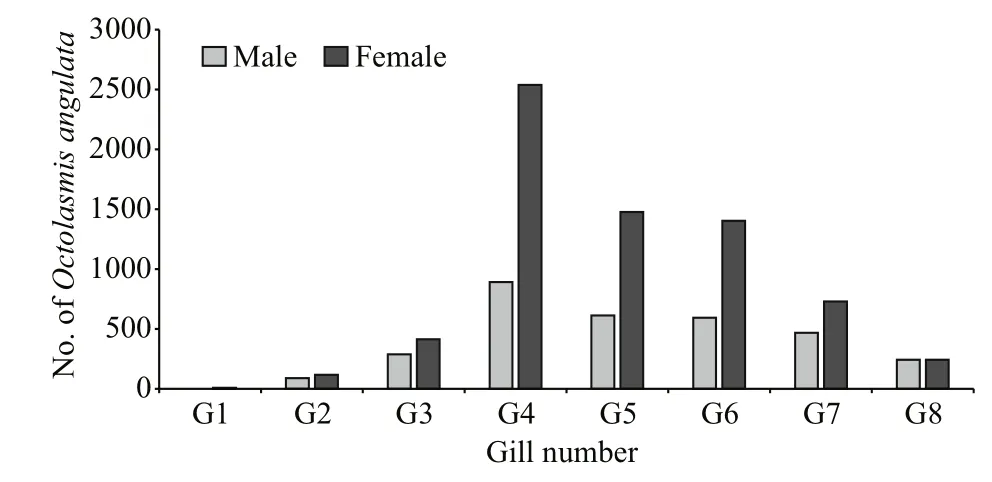
Fig.3 Distribution of the stalked barnacle O. angulata on the eight gills of males and females of the blue crab P. pelagicus
3.4 Relationship between the weight of edible portion of blue crab and degree of O. angulata infestation
The greater the infestation by O. angulata, the less edible portion mass (Table 3). The correlation coefficient revealed there were no relationship between infestation rate by O. angulata and both females total weight and flesh weight. On the other hand, results showed high signi ficance in males between number of parasites and the studied parameters (Table 4).
3.5 Identi fication of the epizoite
The epizoite was identi fied as Octolasmis angulata(Aurivillius, 1892) by means of several taxonomic features such as lack of tergal plates, structure of both carina and scuta, and shape of its mouth and thoracic appendages.
Description
Family: Poecilasmatidae Annandale, 1909
Genus: Octolasmis Gray, 1825
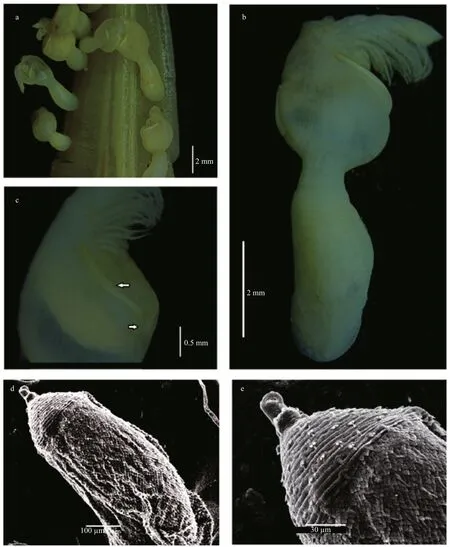
Fig.4 Octolasmis angulata from the Red Sea
Octolasmis angulata (Aurivillius, 1892)
Figs.4 & 5
Dichelaspis angulata Aurivillius, 1894, p. 22, pl.II, figs. 9–11; pl. VIII, figs. 18 24.
Dichelaspis bultata Aurrvillrus, 1894, p. 26, pl. II,figs. 12–13; pl. VI, figs. 10–11; pl. VIII, figs. 19.
Octolasmis angulata (Aurivillius): Nilsson–Cantell, 1934, p. 46, figs. 7–8.
Octolasmis angulata (Aurivillius): Hiro, 1937,p. 426, figs. 17–18.
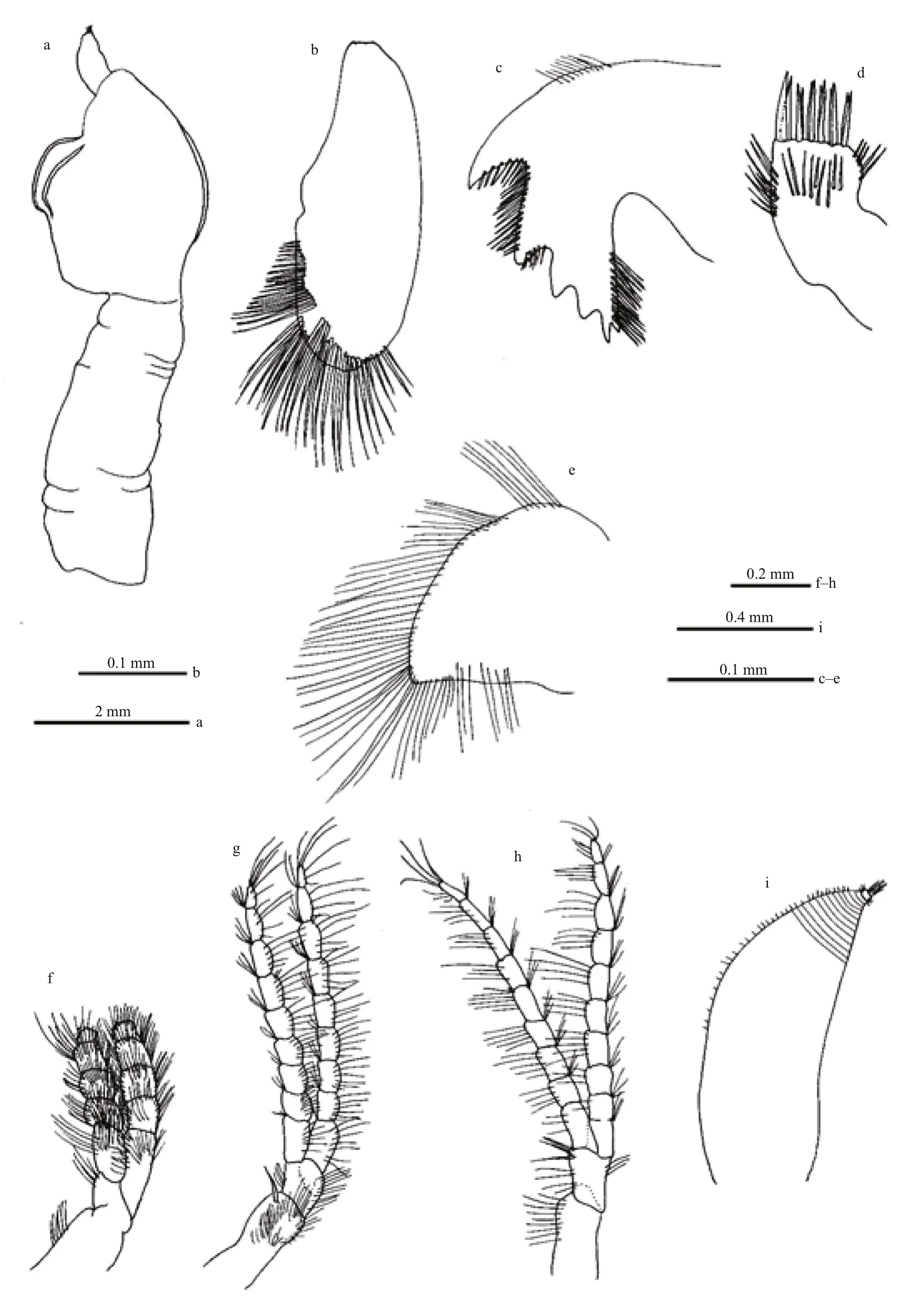
Fig.5 Octolasmis angulata from the Red Sea
Body slightly compressed and semi–transparent;capitulum robust, globular or nearly oval lateral view,slightly pointed apical and partially calci fied, carrying 3 plates (Fig.4a–c) Peduncle cylindrical, naked and longer than capitulum and marked with several folds(Fig.5a). Terga lacking; scuta paired, very narrow and sinuous, extended along nearly one-third length of the margin, with shorted basal arms tapering obliquely(Figs.4c, 5a); carina reduced (reaching base of scutum), tapered along proximal one-third to one-half of capitulum and not furcated at base (Figs.4b, 5a);surface structures of small specimen are smoother(Figs.4a, 5a). Labrum slightly bullated with eight acute teeth. Mandibulatory palp (Fig.5e) large,paddle-like, with several simple setae along its tip and medial margin. Mandible (Fig.5c) with 5 teeth,surface covered with numerous fine setae. Maxillule(maxilla I) narrow, without notch carrying 12 strong spines apically (Fig.5b); Maxilla (maxilla II)rectangular, anterior and medial margin covered with long setae (Fig.5d). Cirri densely setose; cirrus I(Fig.5f) 5-segmented; anterior ramus; cirrus II and III(Fig.5g, h) basically similar in structure with 9-segmented endopod and 10-segmented exopod; Cirri IV and cirri V with 9- and 10-segmented ramii respectively. Penis (Figs.4d, e, 5i) annulose, tapering gradually through its length, covered with triangular overlapping scales proximally, distal part with circular bars and terminated with languet or sheath-like cylinder with basal ring of setae, and with numerous long tuft of fine setae arising from ori fice of the sheath(Fig.5i).
4 DISCUSSION
It is well documented that the pedunculate barnacles of the genus Octolasmis are frequently found on decapod crustaceans all over the world(Jeffries et al., 1982; Jeffries and Voris, 2004) causing infestation of their hosts and can therefore have a signi ficant impact on the population dynamics of infested species. The blue swimmer crab, P. pelagicus,collected from the Jeddah coastal waters displayed a markedly higher prevalence of Octolasmis (92%).Our results on the infestation rate are in agreement with the studies by Hashmi and Zaidi (1965) from the coastal waters of Karachi, Pakistan, Gaddes and Sumpton (2004) in Moreton Bay, Australia and Mushtaq and Mustaquim (2009) in Pakistani waters.However, it was markedly higher than other studies reported by Shields (1992) and Walker (2001) in Moreton Bay, Australia and by Kumaravel et al.(2009) in Parangipettai waters, Indian Ocean. These variations may be attributed to the differences in the size classes used in these studies. Small size crabs have been reported to not have high infestation rate of Octolasmis which could be related to the high molting frequency (Jeffries et al., 1992).
Many studies indicated that the length of intermoult period is the main factor facilitating epibiont establishment (Jeffries et al., 1992; Voris et al., 1994).However, the in fluence of other factors on infestation control (morphological, physiological or behavioral mechanisms) must be taken into consideration (Wahl,2008). Infestation prevalence and total mean intensity were higher in females than males. This could be related to the molting process, females stop molting upon maturation and can even spawn several times within the season which allows a better opportunity for the infestation by Octolasmis (Cordeiro and Costa 2010). On the other hand, Coker (1902) speculated that berried females of the blue crab Portunus pelagicus would be less vigorous and loaded by eggs in their movements, which corresponds to slow respiration and better chance for the cyprids attachment of Octolasmis.
Older blue crabs specially females were found to be infested with the cyprids of Octolasmis in Lousiana and Texas (Humes, 1941; More, 1969). On the other hand, Yan et al. (2004) indicated that prevalence rate was high for males than females of the crab Charybdis feriatus from Day Bay, China. Host attractiveness to cyprid larvae is one of the factors that could in fluence the differences in the characteristics of infestation between sexes. Chemical cues were known to play a role is specifying substrate for many of cyprid larvae(Pawlik, 1992; Clare, 1995).
The present study corroborated what was found by other authors that Octolasmis seems to settle in ventilated locations, since higher infestation rates were observed on gills number 3, 4 and 5. This finding agrees with similar studies (Hashim and Zaidi, 1965;Jeffries et al., 1992; Mushtaq and Mustaquim, 2009).The proximal surfaces of the hypobranchial side of each gill were found to exhibit the highest infection rate while the distal surfaces on the hyperbranchial side showed the lowest. Similar pattern has been reported in previous studies Gaddes and Sumpton(2004) in the eastern Australia and Mushtaq and Mustaquim (2009) in Pakistani waters.
The distribution of Octolasmis angulata through the branchial chamber of the blue crab P. pelagicus is mostly linked to ventilatory flow which generally takes a U shaped route. According to Barnes (1974),the route starts at the inhalant aperture, moves posteriorly into the hypobranchial part of the chamber,then dorsally between the gill lamellae. The exhalent current then move forwardly in the dorsal area of the branchial chamber to the exhalent aperture (Barnes,1974). Distribution of O. angulata in the current study is similar to that found in the branchial chambers of the blue crab Callinectes sapidus (Walker, 1974) and Yan et al. (2004) in the blue crab Charybdis feriatus.As stated by Walker (1974), settlement on the hyperbranchial side of the gill can occur only when the respiratory flow is reversed and the cypris larvae will not be able to pass through the lamellae of the gills to the hyperbranchial side due to the close apposition of gills. Burrowing is usually followed by reversal of flow as a means of cleaning the branchial chambers and gills (Barnes, 1974).
Gannon and Wheatly (1992) investigated the effects of infestation by O. angulata on gas exchange in the blue crab Callinectes sapidus and found that high infection rates caused physiological stress on the host. In a comparable study by Gannon (1990), crabs with massive infestation did not survive the stress of experimental mortality when subjected to aerial exposure and high temperatures.
Compared to earlier studies, our specimens showed differences in the curvature and length of the scuta and carina, which varied in length and degree of calci fication or were sometimes missed (Aurivillius,1894; Annandale, 1909; Ihwan et al., 2014). The most important character in our Red Sea specimens is that the carina is not forked at the base as described in Annandale (1909), Aurivillius (1894), Walker (2001),Chan et al. (2011) and Ihwan et al. (2014).
5 CONCLUSION
The current study reported for the first time the infestation of the blue swimmer crab Portunus pelagicus by the ectocommensal stalked barnacle O. angulata in the Red Sea. This study showed that more than 6% of the studied crabs are heavy infested(61–127 barnacles per crab). Additional studies will be needed to elucidate the impact of heavy infestation on the fishery market of the Red Sea blue crab populations.
6 ACKNOWLEDGMENT
The author appreciates the help of Prof. Mohsen M. El-Sherbiny, Department of Marine Biology,Faculty of Marine Sciences, King Abdulaziz University, Saudi Arabia for great help in identi fication and also Abdullah Modkhaly for helping in collection and barnacles’ isolation. The author wish to thank Professor Andrew Lawrence (Head of biology Dept,Chester University, UK) for editing the manuscript.
 Journal of Oceanology and Limnology2018年4期
Journal of Oceanology and Limnology2018年4期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Recent insights into physiological responses to nutrients by the cylindrospermopsin producing cyanobacterium,Cylindrospermopsis raciborskii*
- Response of Microcystis aeruginosa FACHB-905 to different nutrient ratios and changes in phosphorus chemistry*
- In fluence of light availability on the speci fic density, size and sinking loss of Anabaena flos- aquae and Scenedesmus obliquus*
- Application of first order rate kinetics to explain changes in bloom toxicity—the importance of understanding cell toxin quotas*
- Regime shift in Lake Dianchi (China) during the last 50 years*
