Characterization and differential expression of three GnRH forms during reproductive development in cultured turbot Schophthalmus maximus*
ZHAO Chunyan (赵春彦) , XU Shihong (徐世宏) FENG Chengcheng (丰程程) ,LIU Yifan (柳意樊) , YANG Yang (杨阳) , WANG Yanfeng (王彦丰) ,XIAO Yongshuang (肖永双) , SONG Zongcheng (宋宗诚) ,LIU Qinghua (刘清华) , , LI Jun (李军) ,
1 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
4 Weihai Shenghang Aquatic Product Science and Technology Co. Ltd., Weihai 264200, China
Abstract Turbots ( Schophthalmus maximus), one of the most important economic marine flat fish species, fail to undergo final spawning and spermiation naturally under arti ficial farming conditions. In vertebrates, reproduction is regulated by the brain-pituitary-gonadal axis (BPG-axis), and gonadotropin releasing hormone (GnRH) is one of its key components. Therefore, to better understand the physiology of reproduction in the turbot, three of the genes encoding GnRH subtypes— sbGnRH, cGnRH- II and sGnRH—were cloned and sequenced by isolating the cDNA sequences. The localizations and patterns of expression of their mRNAs were also evaluated during seasonal gonadal development. All three mRNAs were expressed abundantly in the brain; sbGnRH and sGnRH mRNAs were also detected in the gonads and pituitary gland,and sbGnRH expression was much higher than that of sGnRH, indicating the critical role of sbGnRH in regulating the BPG-axis. Moreover, the brain expression patterns of sbGnRH and sGnRH mRNAs showed an increased trend during gonadal development, peaking in mature stages. This indicated the direct regulation of gonadal development by the GnRH system. In addition, cGnRH- II mRNA expression showed no signi ficant variations, suggesting that cGnRH-II is not critically involved in the control of reproduction.Further, the mRNA abundances of the three GnRH forms in the breeding season were signi ficantly higher than those in immature and post-breeding stages in all analyzed brain areas. Therefore, we propose that sbGnRH is the most important hormone for the regulation of reproduction in turbot via the BPG-axis. These results will help in better understanding the reproductive endocrine mechanisms of turbots and lay the groundwork for additional studies aimed at comparing the reproductive physiology of wild individuals with those raised under arti ficial conditions.
Keyword: turbot; GnRH; brain; gonad; reproductive development
1 INTRODUCTION
Fish comprise the largest paraphyletic group of vertebrates, and exhibit a wide range of reproductive strategies. Their reproductive rhythms are complex,regulated by the brain-pituitary-gonadal axis (BPG-axis) (Weltzien et al., 2004). This is involved in several neuroendocrine and endocrine regulators including gonadotropin-releasing hormone (GnRH),gonadotropins (GTHs) and other neurohormones(Okubo and Nagahama, 2008; Chang et al., 2009).Brie fly, GnRH stimulates the synthesis and release of pituitary GTHs, and the most notable of these are follicle stimulating hormone (FSH) and luteinizing hormone (LH). Further, FSH and LH regulate the synthesis of sex steroids that are important for gonadal development and maturation (Taranger et al., 2010;Hildahl et al., 2011). Therefore, it is generally accepted that GnRH in the brain acts as a major upstream signaling molecule that controls the BPG-axis (Millar, 2005). Currently, the GnRH family includes 15 different homologs in vertebrates, and eight homologs have been identi fied in fish (Kah et al., 2007; Soverchia et al., 2007; Zohar et al., 2010).Each homolog was originally named by the species in which it was first identi fied, and subsequently this name was suggested as a universal nomenclature.Based on phylogenetic analysis, all GnRH homologs could be classi fied into three groups: GnRH1, GnRH2 and GnRH3 (Dubois et al., 2002). GnRH1 is a speciesspeci fic type, including mammalian GnRH (mGnRH),seabream GnRH (sbGnRH), medaka GnRH(mdGnRH), white fish GnRH (wfGnRH), cat fish GnRH (cfGnRH) and herring GnRH (hrGnRH).GnRH2, first identi fied in chicken (cGnRH-II)(Miyamoto et al., 1982), is a conserved type in vertebrates, including fish and mammals; whereas GnRH3 is a fish-speci fic type that was originally identi fied in salmon (sGnRH) (Sherwood et al., 1983).To date, all studied fish possess two (GnRH2 and GnRH3) or three types in the central nervous system(Lethimonier et al., 2004).
GnRH-expressing neurons in fish are distributed in different brain areas, probably with distinct functions.GnRH1-secreting neurons are localized primarily in the ventral telencephalon and preoptic area (POA),with their axons projecting throughout various brain regions (González-Martinez et al., 2001; Dubois et al., 2002). Generally, GnRH1 has neuromodulatory activities. GnRH2-secreting neurons are primarily found in the midbrain tegmentum, with their axons distributed widely throughout the central nervous system (Gray et al., 2002). However, the distribution of GnRH3-secreting neuronal populations partially overlaps with those secreting GnRH1, mostly in the olfactory bulb, terminal nerve ganglion region and POA. Moreover, neurons in the ventral forebrain can stimulate and release GTHs. Therefore, GnRH1 is considered to be the main hypophysiotropic type of hormone in most teleosts (Carolsfeld et al., 2000;Chen and Fernald, 2008; Zohar et al., 2010).
The turbot ( Schophthalmus maximus) is one of the most important marine flat fish species economically(Chi et al., 2013; Lin et al., 2013), and is widely farmed in Europe and northern China (Zhao et al.,2017). However, in aquaculture environments, turbots fail to undergo final spawning and spermiation naturally during the breeding season. This reproductive defect could reduce or inhibit the gamete production that is critical to successful aquaculture. Generally,this phenomenon appears to be caused by an endocrinological dysfunction associated with a lack of LH stimulus from the pituitary. Ultimately, this weakens hypothalamic GnRH-stimulated LH secretion, which in turn results in the low serum LH levels (Okuzawa et al., 2016). Furthermore, in jack mackerel ( Trachurus japonicus) (Imanaga et al.,2014), endocrine factors in the BPG-axis are responsible for reproductive dysfunction, and in Atlantic cod ( Gadus morhua) (Hildahl et al., 2011),analogs of GnRH1 and GnRH2 can stimulate ovulation. Therefore, it is necessary to investigate function of GnRH analogs in controlling sexual maturation and in improving the quality of gamete production. In addition, GnRH regulates physiological effects through its levels of transcription, translation and secretion. Although a previous study showed that three forms of GnRH, sbGnRH, cGnRH-II and sGnRH, could be characterized immunologically by enzyme-linked immunosorbent assay (ELISA) in the brain and pituitary of the turbot (Andersson et al.,2001), quantitative gene expression data are poorly established in the turbot, especially during the reproductive cycle.
Here we measured GnRH mRNA subtype concentrations in the brain and gonads of turbots to gain insight into their roles, and to clarify the GnRH form predominantly involved in gonadal development and gamete maturation. For this, we characterized cDNAs encoding sbGnRH, cGnRH-II, and sGnRH,evaluated the localization of their mRNAs, and quanti fied their abundance patterns during the entire reproductive cycle. Further, the possible physiological roles of each GnRH form during gonadal maturation were identi fied.
2 MATERIAL AND METHOD
2.1 Animals and samples
Adult turbots were obtained from Oriental Ocean Sci-Tech Co., Ltd. (Shandong Province, China). Fishwere anesthetized with a 0.05% solution of MS-222(Merck KGaA, Darmstadt, Germany). Various tissues from immature fish were rapidly excised, snap-frozen in liquid nitrogen, and stored at -80°C until RNA extraction. Gonads, brains and pituitaries from male and female turbots at different stages of gonadal development were collected to investigate the expression pro files of the sbGnRH, cGnRH- II and sGnRH gene using quantitative reverse-transcription polymerase chain reaction (RT-qPCR) analysis.
2.2 Cloning full-length of sbGnRH, cGnRH-II and sGnRH cDNA sequences
Total RNA was extracted from the brain of a 1-year-old turbot using the Simple P total RNA Isolation Kit (BioFlux, Hangzhou, China). Firststrand cDNA was synthesized using a Transcript Firststrand cDNA synthesis kit (Transgen Biotech Inc., Beijing, China) with an oligo (dT) primer,following the manufacturer’s instructions. A 205-base pair (bp) cDNA fragment of the turbot sbGnRH gene was ampli fied with degenerate primers (Table 1)designed according to the highly conserved regions of sbGnRH in other fish species. Similarly, a 183-bp cGnRH- II fragment and a 181-bp sGnRH fragment were ampli fied with forward and reverse primers,respectively (Table1). PCR was performed using a PTC-100 thermal cycle (Bio-Rad, Hercules, CA,USA) with a PCR ampli fication procedure of denaturation at 94°C for 5 min, 30 cycles of ampli fication at 94°C for 30 s, 58°C for 45 s, 72°C for 30 s, and an additional extension at 72°C for 10 min.
Subsequently, to clone the full-length coding sequence, 5′-rapid ampli fication of cDNA ends(RACE) and 3′-RACE were carried out using a SMARTer RACE cDNA Ampli fication kit (Clontech,Mountain View, CA, USA). The 5′- and 3′- ends of GnRH genes were ampli fied using a PCR anchor primer paired with a gene-speci fic primer (GSP)(Table 1). The first round of PCR was performed on a PTC-100 thermal cycle (Bio-Rad) with a PCR ampli fication procedure of denaturation at 94°C for 5 min, followed by 35 cycles of ampli fication at 94°C for 1 min, 65°C for 1 min, and 72°C for 2 min, plus an additional extension at 72°C for 10 min after the last cycle. The diluted first-round PCR products served as templates for nested PCR with corresponding GSP-nest primers (Table 1). The second nested PCR program used the same cycle conditions as the first round of PCR.
2.3 Phylogenetic analysis
Homology searches of the deduced amino acid sequences of the obtained sbGnRH, cGnRH-II and sGnRH cDNAs were carried out using the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/). Multiple alignments of deduced amino acid (aa) sequences were conducted by BioEdit with the Clustal W alignment tool. A phylogenetic tree was constructed with Mega7 software by the neighbor-joining method, using bootstrap analysis of over 1 000 replicates.
2.4 RT-qPCR analysis
For each stage, three samples were used for RNA extraction. Reverse transcription was performed using 1 mg total RNA using PrimeScript RT reagent kits (TaKaRa Bio Inc., Dalian, China). All primers for RT-qPCR analysis are listed in Table 2 and were validated with PCR program and agarose gel electrophoresis. The qPCR was performed in an ABI 7300 real-time PCR instrument (Applied Biosystems,Foster City, CA, USA) with SYBR Premix Ex Taq kits (TaKaRa, Dalian), using the standard curve method with β- actin as the reference gene. According to the manufacturer’s protocol, samples were run in triplicates in a 25- μ L reaction volume containing 2 μ L of diluted cDNA (1:10), 0.4 μ mol/L of each primer and 12.5 μ L of 2 × buffer. PCR was performed using 95°C for 30 s, 40 cycles at 95°C for 5 s, and 68°C for 30 s. A dissociation curve was added at the end of each program to check ampli fication speci ficity.
2.5 Statistical analysis
All the results are expressed as the mean±standard deviation (SD). Statistics were performed using SPSS software, version 15.0 (IBM Corp., Armonk, NY,USA). The changes in GnRH mRNAs during gonadal stages were analyzed by one-way analysis of variance,followed by a Bonferroni multiple comparison test.P <0.05 was considered statistically signi ficant, and different letters in figures indicate signi ficant differences between gonadal stages.
3 RESULT
3.1 Cloning and phylogenetic analysis of GnRH cDNAs
Complete coding sequences of the three GnRH forms were obtained successfully using 3′- and 5′-RACE. The full lengths of turbot sbGnRH, cGnRHII, and sGnRH cDNAs (GenBank Accession Nos.KY593267, KY593268, KY593269) were 456, 623,and 598 bp, respectively. These cDNAs containedopen reading frames of 285, 255, and 276 bp encoding precursor proteins of 94, 85, and 91 deduced aa,respectively (Fig.1). The three deduced GnRH proteins are composed of several conserved regions that include a 23–24 aa. signal peptide (SP), a 10-aa GnRH peptide, an enzymatic processing site (Gly-Lys-Arg [GKR]), and a 59- (sbGnRH), 49- (cGnRHII) and 54- (sGnRH) aa gonadotropin-releasing hormone associated peptide (GAP) (Fig.1). The deduced GnRH decapeptide sequences are“QHWSYGLSPG”, “QHWSHGWYPG” and“QHWSYGWLPG”, respectively. The alignment analysis of deduced aa sequences of turbot sbGnRH,cGnRH-II and sGnRH with other species is shown in Fig.2. The turbot sbGnRH showed 74% identity with sbGnRH from Paralichthys olivaceus, 69% with Epinephelus adscensionis, 54% with Oncorhynchus mykiss and 48% with mdGnRH from Oryzias latipes.Turbot cGnRH-II showed high identity with GnRH2 from other fish species (85%–96%); turbot sGnRH is 93% identical with sGnRH from Larimichthys crocea and 90% with Paralichthys olivaceus. Analysis showed that the turbot sbGnRH has high similarity with sbGnRH from Paralichthys olivaceus (90.4%)and Verasper variegatus (92.6%). The turbot cGnRHII and sGnRH sequences also showed high similarity with those from other flounder species (>90%).Phylogenetic analysis indicated that the three turbot GnRHs along with GnRHs from other typical vertebrates clustered into three separate branches named GnRH1, GnRH2 and GnRH3, respectively(Fig.3). The GnRH1 and GnRH2 branches were further divided into two additional sub-branches,including a fish branch and a general tetrapod branch.However, the GnRH3 branch contained only one clade covering all fish species.
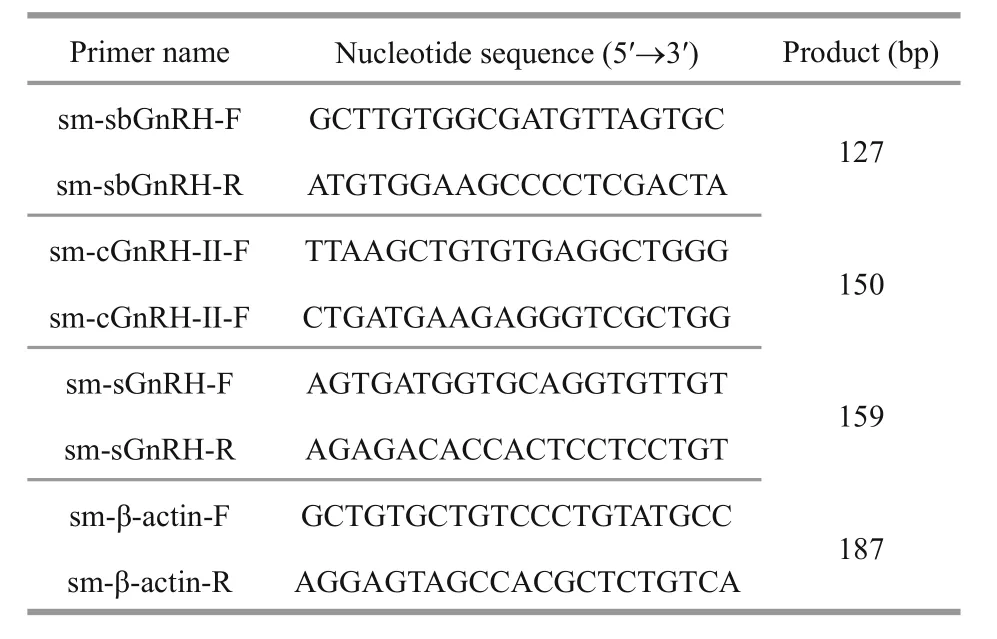
Table 2 List of primers used for RT-qPCR expressions analysis of GnRH mRNAs

Fig.1 cDNA sequence and deduced primary structures of sbGnRH (a), cGnRH-II (b) and sGnRH (c)
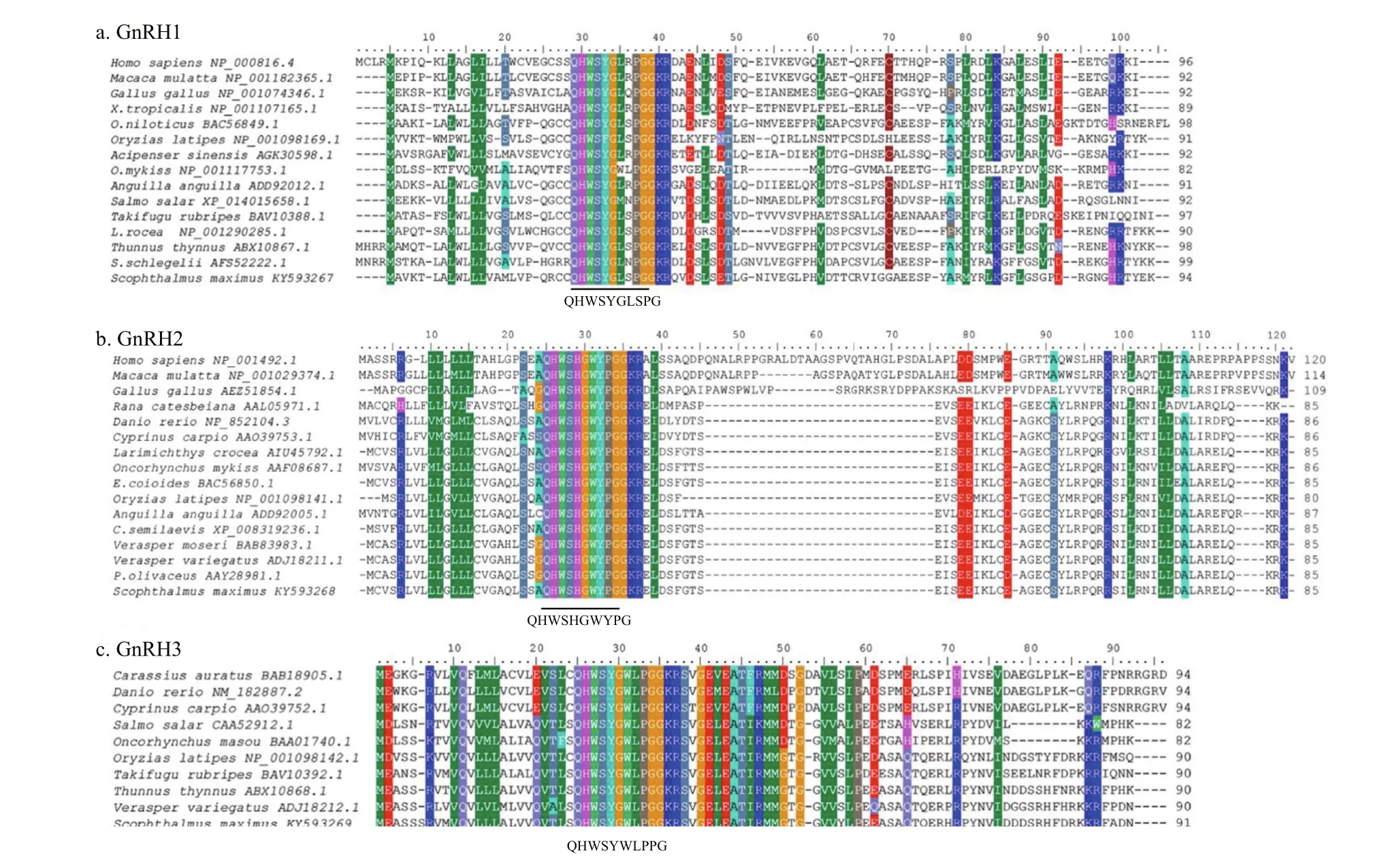
Fig.2 Sequence multiple alignments of prepro-GnRHs
3.2 Tissue distribution of GnRH mRNAs
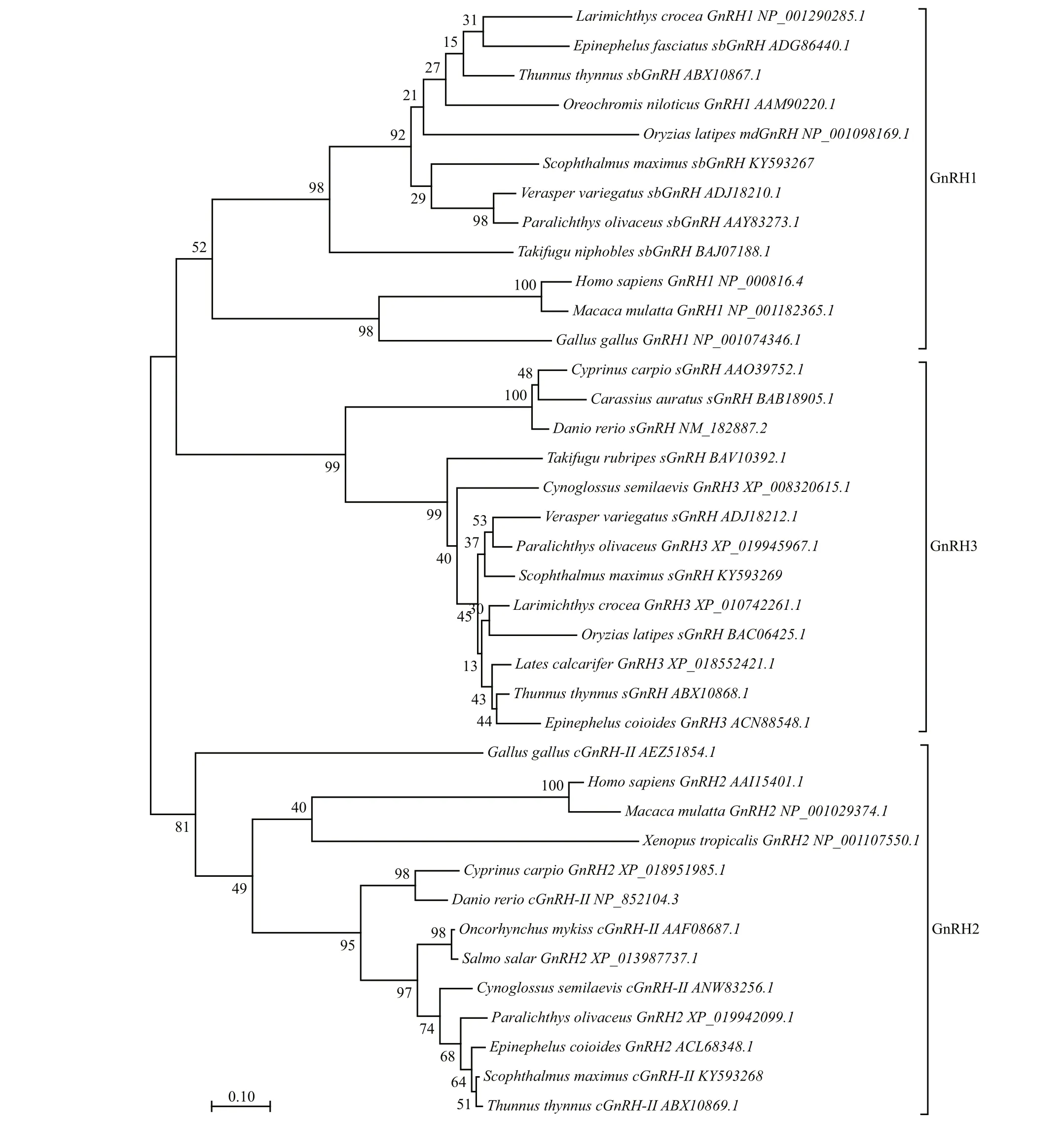
Fig.3 Phylogenetic tree of deduced aa sequences of GnRH1, GnRH2, and GnRH3, constructed using MEGA7 with the neighbor-joining method
Tissue distribution patterns of sbGnRH, c GnRH- II and sGnRH mRNAs were analyzed from a 1-year old male turbot by RT-qPCR (Fig.4). The three GnRH mRNAs were all highly expressed in the brain.However, the expressions were extremely different in other tissues examined. Speci fically, sbGnRH mRNA was expressed relatively strongly in the pituitary and gonads, with weak expression in skin, spleen and stomach. By contrast, cGnRH- II mRNA was expressed only in the brain. In addition, the highest sGnRH transcription value was also in the brain,whereas sGnRH mRNA levels were extremely low in the eye and skin samples.
The expression levels of three GnRH mRNAs in central brain areas were also investigated (Fig.5).Based on histology of sections, turbot brains including the pituitary gland were divided by scalpel into seven areas: the olfactory bulbs (Ob); the telencephalon (Te)including the POA; the mesencephalon and the thalamus (Me); the hypothalamus and saccus vasculosus (Hy); the cerebellum (Ce); the medulla oblongata (Mo); and the pituitary gland (Pi). The sbGnRH and s GnRH mRNAs were found in all analyzed brain areas, whereas cGnRH- II mRNA was only detectable in the Me. The sbGnRH mRNA showed strong expression in the Te, lower levels in the Me, Hy, and Pi, but was barely detected in other brain areas. For sGnRH, the mRNA transcription levels were signi ficantly high in the Te, low in Ob,and extremely low in the Ce and Pi. The GnRH mRNA expression patterns were similar in female and male turbot brain areas.
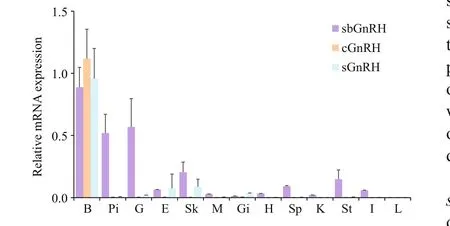
Fig.4 Quantitative gene expression of three GnRH variants in various tissues from a 1-year old male turbot
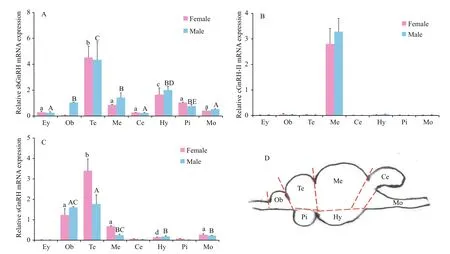
Fig.5 Quantitative gene expression of three GnRH variants in different brain areas and the eye
3.3 Expression pro files of GnRH mRNAs during gonadal development stages by RT-qPCR
In male turbots, we divided gonadal development stages into five stages: stage II containing mostly spermatogonia; stage III with primary spermatocytes;stage IV with secondary spermatocytes; stage V with spermatids or mature spermatozoa together with spermatogonia; and stage VI containing mostly spermatogonia. Correspondingly, in female turbots,the ovarian stages were classi fied as stage II with primary growth oocytes but no vitellogenic oocytes in ovaries; stage III with early vitellogenesis; stage IV with late vitellogenesis; stage V with ovulated oocytes; and stage VI with post-ovulatory follicles in degenerated ovaries.
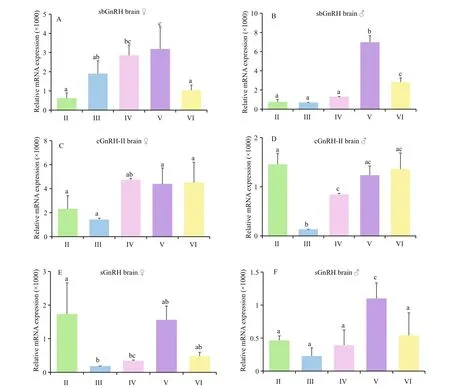
Fig.6 Quantitative gene expression of three GnRH mRNA variants in the brain throughout the reproductive cycle of male and female turbots
The expression levels of sbGnRH, cGnRH- II and sGnRH in whole brains during the various gonadal development stages are shown in Fig.6. In both male and female turbots, brain sbGnRH mRNA concentrations were lower at stage II and increased with gonadal development, exhibiting highest levels at mature stage V, followed by signi ficant decreases in the degeneration stage VI (Fig.6A, B). For female brain sGnRH mRNA, its highest expression levels were found at stage II, followed by reductions during gonadal stages III to VI (Fig.6E). Transcription of sGnRH in the male brains followed a similar pattern to that in female brains during the stages III to VI,peaking at the spawning phase V (Fig.6F). For the expression levels of cGnRH- II mRNA was not possible to discern a general trend (Fig.6C, D). Stage of III had the lowest cGnRH- II transcription, whereas the mRNA levels at stages of IV to VI were signi ficantly ( P <0.05) higher.
Given that sbGnRH mRNA was only expressed in gonads, its variations during gonadal development in both male and female gonads were quanti fied using RT-qPCR (Fig.7). The levels of sbGnRH mRNA increased gradually from stages II to V, and peaked at stage V of ovaries and stage IV of testes.
Finally, the expression levels of all three GnRH mRNAs in immature (stage II), the breeding season(stages IV and V) and post-breeding season (stage VI)of brain areas were investigated (Figs.8, 9). In all brain areas, including the Ob, Te, Me, Hy, Pi, Ce, and Mo, sbGnRH mRNA concentrations were signi ficantly higher during the breeding season, while they were lower at the immature stages and in the post-breeding season (Fig.8). The levels of cGnRH- II mRNA in Me and sGnRH mRNA in Ob, Te, Me were also analyzed(Fig.9). These were signi ficantly higher in the breeding season than in immature stages and the postbreeding season. The pattern of changes in male brain areas was identical to that in female brains.

Fig.7 Quantitative gene expression of sbGnRH mRNA in the gonad throughout the reproductive cycle of male and female turbots
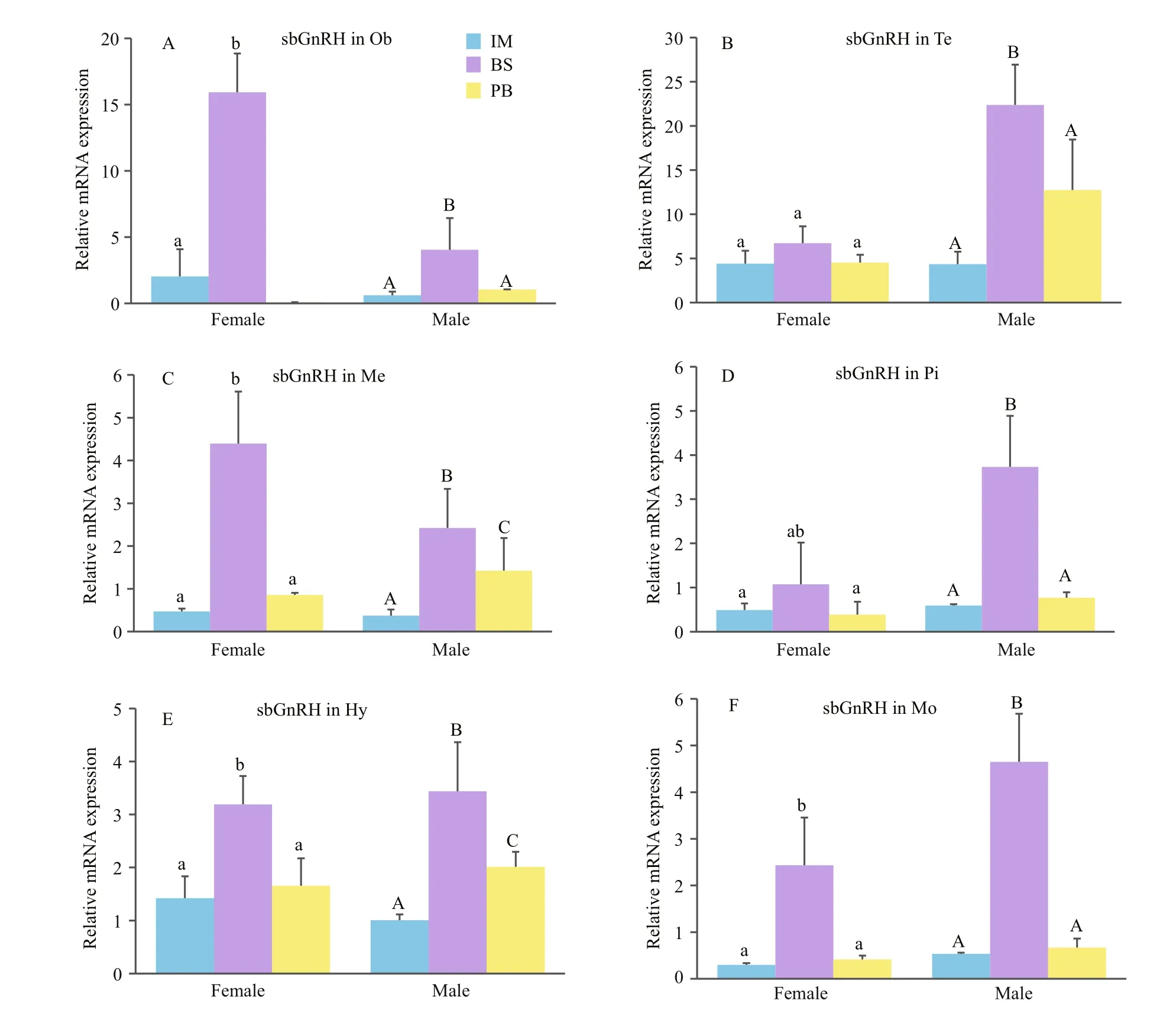
Fig.8 Quantitative gene expression of sbGnRH in Ob (A), Te (B), Me (C), Pi (D), Hy (E) and Mo (F) throughout the reproductive cycle of male and female turbots
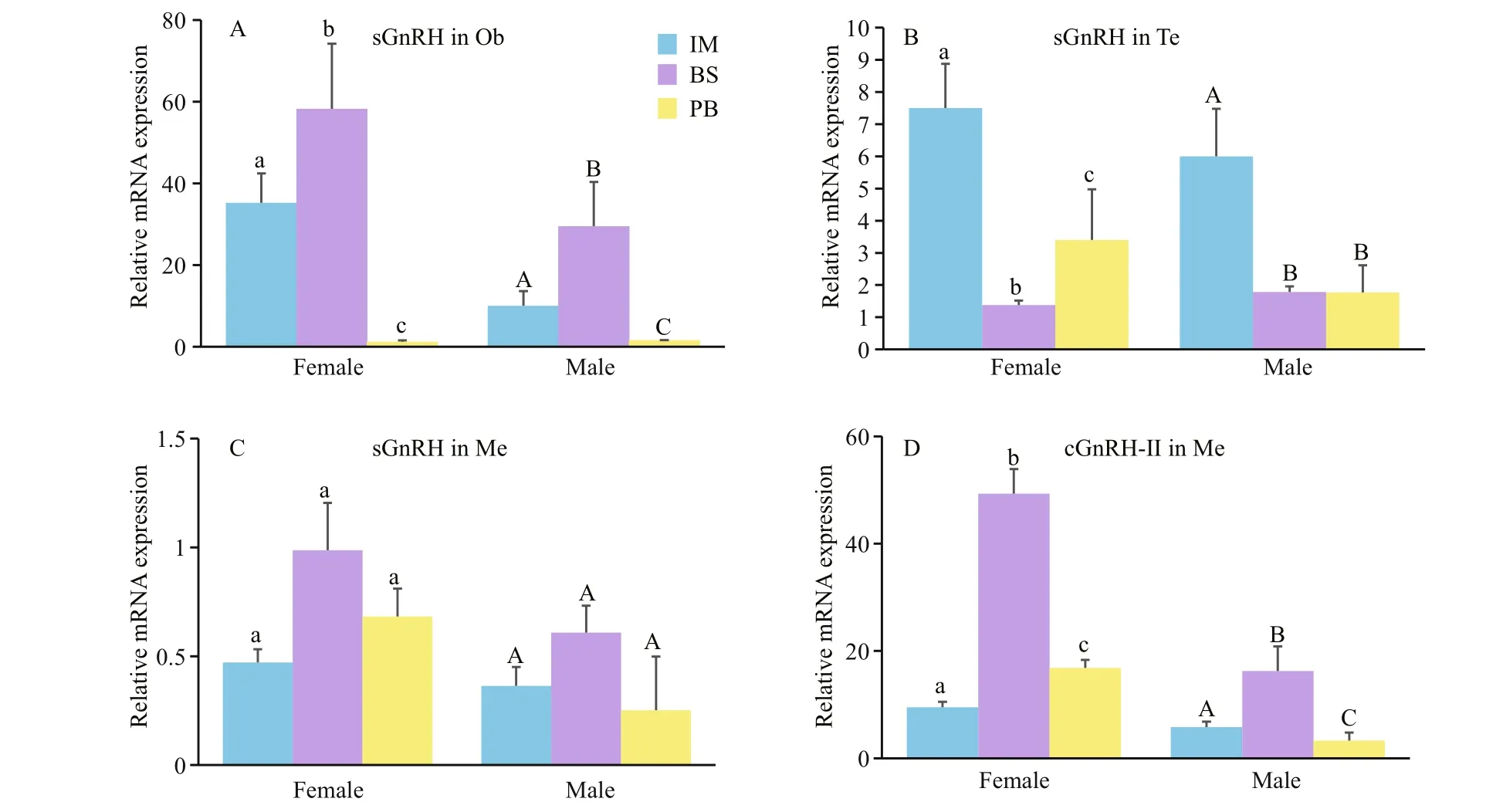
Fig.9 Quantitative gene expression of sGnRH in Ob (A), Te (B), Me (C) and cGnRH-II in Me (D) throughout the reproductive cycle of male and female turbots
4 DISCUSSION
Here, we characterized the three GnRH genes and analyzed their expression patterns during reproductive development in male and female turbots, and showed the functional diversi fication of the GnRH system.We first isolated three full-length cDNAs (sbGnRH,cGnRH-II and sGnRH), which con firmed that these three GnRH variants coexist in flat fish(Pleuronectiformes) (Andersson et al., 2001; Amano et al., 2004b; Pham et al., 2007). A primary typical conserved GnRH structure (Kah et al., 2007; Zohar et al., 2010) (Kah, Lethimonier et al., 2007)was con firmed by the deduced aa sequences. These consist of an SP, a GnRH decapeptide, an enzymatic processing site (GKR) and a GAP region (Fig.2). The function of conserved peptide regions might be to ensure the proper folding of precursor molecules and assist in the release of GTHs, as shown previously(Seeburg et al., 1987; Yu et al., 1988).
Studies of the alignments of GnRH protein precursor sequences revealed that these decapeptides are conserved in vertebrates, especially in teleosts(Dubois et al., 2002; Lethimonier et al., 2004). It is notable that the decapeptide sequences of cGnRH-II and sGnRH were the same as in other species.However, the sbGnRH decapeptide was the most divergent class, exhibiting dissimilar aa at position 8.This diversity might have been caused by differential evolutionary pressure (Miccoli et al., 2016), and sbGnRH might have undergone faster evolution because it is a species-speci fic gene (Zohar et al.,2010).
Our phylogenetic analysis showed that the deduced aa sequences of GnRH formed three branches:GnRH1, GnRH2 and GnRH3. All three branches supported expected vertebrate evolutionary relationships. Interestingly, the GnRH1 and GnRH3 branches formed mutual sister clades, both belonging to the forebrain lineage. The GnRH2 branch was also identi fied as belonging to the midbrain lineage (Kah et al., 2007; Shao et al., 2015). Recent evidence has shown that there is a third fish-speci fic genome duplication (Meyer and Van de Peer, 2005), and that the GnRH3 type is typical for fish (Kah et al., 2007).Thus, these sister clades infer a close evolutionary relationship of GnRH1 and GnRH3 (Chen and Fernald, 2008). In addition, the short length of the conserved primary GnRH peptide structures could prevent phylogenetic analyses being accomplished effectively (Guilgur et al., 2007). In that study,cGnRH-II and sGnRH were detected in the brain,while sbGnRH was detected in the pituitary. This indicated that sbGnRH could regulate turbot reproduction. Further, through immunohistochemistry and in situ hybridization, a general localization pattern of GnRH has been documented in other fish species(Chen and Fernald, 2008; Okubo and Nagahama,2008). The reproductively relevant sbGnRH neurons are predominantly distributed in the ventral forebrain,mainly in the pituitary-innervating POA and the hypothalamus. Conserved cGnRH-II neurons are located in the midbrain tegmentum, and the fishspeci fic sGnRH neurons that originate in the olfactory region are found in the terminal nerve ganglion, the ventral telencephalon and the hypothalamus (Gothilf et al., 1995; Abraham et al., 2008; Zohar et al., 2010).Other papers have also reported identical distributions of GnRH-secreting neurons in teleosts, in particular in the bar fin flounder ( Verasper moseri) (Amano et al., 2004a) and Japanese flounder ( Paralichthys olivaceus) (Pham et al., 2007). Therefore, we speculate that turbot GnRH secretion is similar to the distribution seen in other fish. However, our study was not aimed at describing the spatial localization of GnRH-secreting neurons, so more study is necessary to test this hypothesis.
Analyzing the precise gene expression of GnRHs could broaden our knowledge of how various GnRH forms might function in fish reproduction. First, the tissue distribution studies con firmed that all the three GnRH mRNAs were highly expressed in brain.However, in the pituitary and gonads, only sbGnRH and sGnRH mRNAs were detectable: the level of sbGnRH mRNA was 61-fold higher than sGnRH mRNA in the pituitary and 28-fold higher in gonads.These results were consistent with a previous study in the Paci fic herring ( Clupea harengus pallasi)(Carolsfeld et al., 1996). These results indicate a critical role for GnRH1 in the regulation of GTH secretion (Mohamed et al., 2005; Zohar et al., 2010),and showed that GnRH3 functionally compensates for GnRH1 and provides rapid autocrine or paracrine regulation of pituitary cells (Kim et al., 1995; Morin et al., 2015). In addition, the presence of the GnRH pathway in gonads indicated that the GnRH system might activate the gonads, control gonadal development (Powell et al., 1996), and modulate androgen levels (Soverchia et al., 2007). On the other hand, the expression of the three GnRH mRNAs in bony fish have been considered previously to be restricted to speci fic brain areas (Guilgur et al., 2007;Shao et al., 2015). The present results showed high levels of sbGnRH and sGnRH mRNAs in the anterior brain including the olfactory bulbs, telencephalon and mesencephalon, suggesting an overlapping neuronal distribution of these two forms. This distribution was similar to that seen in other fish species such as white fish ( Coregonus clupeaformis) (Vickers et al.,2004), Atlantic croaker (Mohamed et al., 2005) and pejerrey fish (Guilgur et al., 2007). The sbGnRH mRNA has an abundant expression in the hypothalamus, where neurons converge towards the pituitary stalk (Kah et al., 1991). Therefore, sbGnRH should be the physiologically relevant form with respect to GTH release in the turbot. In addition,sbGnRH mRNA was observed in the eye, skin, spine and stomach. This wide distribution pattern of sbGnRH suggests that GnRH in vertebrates probably participates in additional functions, including chemosensory processes, osmoregulation, and the immune and digestive systems, which are associated with reproduction (Nabissi et al., 2000; Guilgur et al.,2007; Xu et al., 2012). Further, this distribution is also involved in the detection of environmental stimuli,such as photoperiods, lunar cycles and temperature.Such environmental stimuli are considered to trigger reproductive processes to occur in the most favorable season (Miccoli et al., 2016).
Here, the expression of cGnRH- II was restricted to the midbrain tegmentum and absent from the pituitary.This expression pattern was the same as in several other bony fish species (González-Martinez et al.,2001; Amano et al., 2002). Moreover, the brain cGnRH- II mRNA levels showed no signi ficant changes during the breeding period in turbots of both genders. These results indicated that cGnRH-II is not involved critically in the control of reproduction (Xu et al., 2012).
Interestingly, the relative abundance of cGnRH- II in the mesencephalon during the breeding season was much higher than in the immature gonads and during the post-breeding season. This result supported previous conclusions that cGnRH-II might act as a neurotransmitter and/or neuromodulator in teleosts(Millar, 2005; Shahjahan et al., 2010). Moreover,there have been several speculations that cGnRH-II might be involved in sexual behavior (Muske et al.,1994) and is sensitive to sexual steroids (González-Martinez et al., 2001). However, the precise functions of cGnRH-II are still enigmatic.
Here we de fined the expression patterns of GnRH mRNAs throughout the reproductive cycle of both genders of turbots. The expression levels of sbGnRH and sGnRH mRNAs increased during the breeding period and decreased at the gonadal degradation stage in the brain. Therefore, we speculate sbGnRH and sGnRH are likely to be involved directly in reproduction and evolutionary convergence (Imanaga et al., 2014; Miccoli et al., 2016).
The expression pattern of sbGnRH in the gonads was similar to that in the brain, and this indicates that there might be a common messaging component among brain and gonad mRNA trends. Thus, we have con firmed that GnRH could be involved in triggering reproduction and in the regulation of gonadal maturation and spawning in the turbot (Gray et al.,2002). In other fish species, such as gilthead seabream and chub mackerel (Gothilf et al., 1997; Selvaraj et al., 2012), GnRH peptides are crucial to the regulation of reproduction. Therefore, for more detailed analyses,research at the protein level (Millar, 2005;Ramakrishnappa et al., 2005) might be investigated further to test our speculations.
In turbots (Wang et al., 2010; Li et al., 2013), the biggest challenge is that the fish fail to undergo spawning and mature spermiation under arti ficial farming conditions. In the jack mackerel ( Trachurus japonicus) (Imanaga et al., 2014), captivity-induced reproductive dysfunction results from inhibition of the BPG-axis involving sbGnRH transcripts in the brain and GTH synthesis in the pituitary. As in the bar fin flounder (Amano et al., 2008), a high sbGnRH level might also be necessary for final ovarian maturation and ovulation. GnRH analogs are also considered to regulate LH release and induce spawning in the jack mackerel (Nyuji et al., 2013).Therefore, further studies will need to focus on comparing the expression levels of sbGnRH of wild with captive turbots during the reproductive cycle.This will be necessary to determine the reasons for impaired spawning and spermiation.
5 CONCLUSION
Here we present key information about the potential role of three forms of GnRH in the BPG-axis of turbots reared under arti ficial conditions. The three forms were identi fied and characterized by bioinformatics and phylogenetic analyses. To assess the reproductively relevant forms, we have described their expression patterns and propose that sbGnRH is the most important for the regulation of reproduction in turbots. These results will help in better understanding the reproductive endocrinology of turbots and lay the groundwork for additional studies aimed at further comparing the reproductive physiology of wild individuals with turbots in aquaculture conditions.
 Journal of Oceanology and Limnology2018年4期
Journal of Oceanology and Limnology2018年4期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Effects of seawater acidi fication on the early development of sea urchin Glyptocidaris crenularis*
- Dietary effects of A zolla pinnata combined with exogenous digestive enzyme (Digestin™) on growth and nutrients utilization of freshwater prawn, Macrobrachium rosenbergii(de Man 1879)
- Preliminarily study on the maximum handling size, prey size and species selectivity of growth hormone transgenic and non-transgenic common carp Cyprinus carpio when foraging on gastropods*
- Hydrodynamic characteristics of the double-winged otter board in the deep waters of the Mauritanian Sea*
- De novo transcriptome sequencing reveals candidate genes involved in orange shell coloration of bay scallop Argopecten irradians*
