Effect of n-3 polyunsaturated fatty acids on accelerating bone regeneration in mice
Abstract: Objective To investigate the effect of n-3 polyunsaturated fatty acids (PUFAs) on bone healing in both Fat-1 transgenic mice and WT mice.Methods To measure the potential role of n-3 PUFAs in fracture regeneration, a femoral fracture model was established in both Fat-1 transgenic mice and WT mice. The bone regeneration of each mice was measured by X-ray,micro-CT and histological assessment at 2, 3, 4 weeks after fixation.Results Histological tests results showed that, compared with WT mice, Fat-1 mice presented significant acceleration of bone regeneration at each time point. Moreover, X-ray and micro-CT analysis exhibited that faster remodeling callus formation were shown in the Fat-1 mice when compared with WT mice. Additionally, histological observation demonstrated that the n-3 PUFAs promoted the endochondral ossification and accelerated the mineralization of the calcified callus in bone regeneration.Conclusion In this study, our results demonstrated that n-3 PUFAs accelerated bone regeneration and fracture healing in mice. These observations suggest that the supplementation of n-3 PUFAs may play a positive role in bone healing.
Keywords: n-3 polyunsaturated fatty acids; bone regeneration; Fat-1 mice; bone remodeling
INTRODUCTION
Although improvements of internal fixations have been developed rapidly in clinical area, the high rate(20%) of bone union of fracture is presented a severe clinical issue worldwide[1]. In past decades, numerous investigators focused their studies on vitamin D and calcium intake for the diet management of bone regeneration[2]. However, vast intake of either vitamin D or calcium did not accelerate bone regeneration in vivo due to the limited digestive as well as the whole body metabolic balance. Recently, the modulatory role of fatty acids (FAs) in bone regeneration process has been reported[3, 4, 5]. In addition, past clinical reports suggested that saturated fatty acids might elevate the risk of fracture[6, 7]. Recent investigations showed that the long-term supplementation of n-3 PUFAs promoted the long bone strength in mice[8], and, simultaneously,Fat-1 mice showed remarkable resistant to ovariectomy-induced bone loss through adipogenesis reduction in bone marrow[9]. Moreover, clinical data suggested that serum n-3 PUFAs levels were positively correlated with bone mass, as risk of fracture was supposed to be increased by saturated FAs intake[3, 6, 10-12].
Nevertheless, though the potential effects of n-3 PUFAs on bone regeneration are promising, few studies have revealed the role of n-3 PUFAs on fracture healing or bone regeneration.Fat-1transgenic mice, a novel metabolically gene-modified mice, can convertn-6 ton-3 PUFAs endogenously; therefore, this model provides an opportunity to distinguish the potential positive role ofn-3 PUFAs[13]. This study is established to measure the efficacy of n-3 PUFAs on bone regeneration in fracture.
METHODS
Animals
To obtain Fat-1 positive C57BL/6 mice (Fat-1)and Fat-1 negative C57BL/6 mice (WT), Fat-1 transgenic mice were crossed with C57BL/6 wild-type mice, then the genotypes were identified by the presence of the Fat-1 gene. Mice were housed under specific pathogen-free (SPF) conditions with day and night cycling of 12 h each. The temperature and humidity were maintained at 25±3 °C and (65±5)%and mice were fed diets containing 10% corn oil. After 3 months postnatal, 30 Fat-1 mice and 30 WT mice were selected randomly and were divided into two groups:WT (n=30) and Fat-1 (n=30). All animal experiments were conducted in accordance with the guidelines of ethical treatment and carried out with the approval of the Southern Medical University Animal Care and Use Committee.
Surgical Procedure Mice were anesthetized by intraperitoneal injection of xylazine (25 mg/kg) and ketamine (75 mg/kg), then the patella was dislocated through a 4-mm medial incision of right knee. After a 0.5 mm hole of intracondylar-notch was drilled, a needle was removed to stabilize the impending fracture.Then, a 0.5mm nail was implanted in the femur. The patella was repositioned, and the extending beyond of the needle was cut finally. Mice were anesthetized by intraperitoneal injection of pentobarbital sodium phosphate-buffered saline (PBS) solution to the dose of 30mg/kg body weight and were sacrificed for right femur harvesting on 2, 3, 4 week after surgery. The soft tissues of right femurs were cut and then the femurs were stored in 4% paraform (4°C) for further analyzing.X-ray Radiography and Micro-CT
Samples of each group at 2, 3, 4 weeks postfracture were analyzed respectively by a digital radiographic system (Kodak, DirectView DR 3500,Rochester, NY). Bone generation was measured by callus maturity by using Goldberg classification system(stage 1: nonunion, stage 2: possible union, and stage 3:complete union)[14]. Mean radiological scores of each time point were calculated for both groups. Micro-CT scanned in this study was measured using ZKKS-MCT-Ⅲ micro-CT system (Guangzhou Zhongke Kaisheng Medical Technology Company Ltd., Guangzhou,China).
Histological analysis
At 2, 3, 4 weeks post-surgery, right femurs were collected and were decalcified by a 19% EDTA solution to about 2-4 weeks, then dehydrated through successive grades in ethanol, rinsed with xylene, and embedded in paraffin. Sections of 4 μm were prepared sagittally for the fracture site. The sections were stained by Safranin O/Fast Green staining (SO/FG) and were observed under light microscope (Olympus BX51,Tokyo, Japan).
Statistical analysis
Data were expressed as means ± SD. Students’ t test was used to measure the statistical significance of each group. Representative results were presented in the result section of this study. Statistical significance was achieved whenp<0.05.
RESULTS
X-ray Measurements
The bone regeneration of each group was measured by X-ray scan at 2, 3, 4 weeks post-surgery.As shown in Figure 1, the fracture line was observed in the WT mice at 4 weeks post-surgery and was barely seen inFat-1mice. Meanwhile, results showed great callus formation inFat-1mice at 2 weeks post-surgery,which was significantly earlier than that in WT group(3 weeks). In addition, calcified calluses were observed in WT mice at 3 and 4 weeks post-surgery, but was accelerated inFat-1mice. Moreover, the remodeling of the calcified callus was initiated at 3 weeks postfracture inFat-1group when compared to WT group.

Fig. 1 X-ray images
Simultaneously, Goldberg classification was used to reveal the callus maturity of two groups at 4 weeks post-surgery. Our result exhibited that the score ofFat-1mice was significantly higher than that of WT mice(Figure. 2).
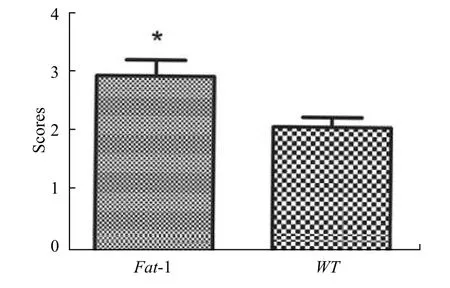
Fig. 2 Goldberg score
Micro-CT measurement
The 3D reconstruction of the femurs in each group was measured at 4 weeks after surgery, though all fracture healing process has completed at this time point, and the local bone remodeling and bone mass ofFat-1group showed significant elevation when compared to WT group (Figure. 3). These results suggested that in addition to bone regeneration, n-3 PUFAs intake may enhance the strength of new bone.Histological Analysis
To reveal the bone regeneration of each group post-fracture, SO/FG stain of each group was performed at 2, 3, 4 weeks after surgery. Classically,the bone regeneration process of mice underwent callus formation, endochondral ossification, and remodeling[15].As shown in Figure 4, mature woven bone and continuous callus presented in fracture area ofFat-1group at about 3 weeks post-surgery, earlier than that of WT mice, which suggested an acceleration of endochondral ossification during the fracture healing ofFat-1mice. In addition, clear remodeling was observed in fracture area ofFat-1mice at 3 weeks post-fracture,while remodeling was initiated in WT mice at 4 weeks post-fracture.

Fig. 4 RepresentedHistological Images
DISCUSSION
In the past decade, the positive effect of n-3 PUFAs in bone remodeling and the attenuation of bone loss have been reported[3, 16]. Meanwhile, the reducing serum n-6/n-3 PUFA ratio was correlated with bone mass and mechanical loading as well[17]. Omega-3 PUFAs are the essential fatty acids that cannot be synthesized endogenously; therefore, they are basic components in diet management[18, 19]. TheFat-1mice are accomplished by transgenic technique and can endogenously converse n-6 PUFAs to n-3 PUFAs,while the serum ratio of n-6/n-3 PUFAs is significantly lower inFat-1transgenic mice with sufficient n-6 PUFAs intake compared to WT mice[13]. In this present study, to eliminate the potential interference factors from diets,Fat-1mice were chosen to investigate the efficacy of n-3 PUFAs on fracture repair. Similar to recent report[8], our results showed a remarkable acceleration of healing time inFat-1mice when compared to WT mice. Past studies indicated that the classic n-6 PUFAs inhibited the endochondral ossification and bone mineralization through inflammation activating[20, 21]. Moreover, supplementation of n-3 PUFAs enhanced bone formation and reduced bone loss[22]. In addition, serum levels ofn-3 PUFAs were observed as positively associated with bone mass in multiple populations, and high levels ofn-3 PUFAs decreased the risk of osteoporosis in postmenopausal women[23, 24].
In this present study, our results suggested that n-3 PUFAs intake is believed to be a potential application for bone regeneration post-fracture. This study provides strong evidence to support the supplementation of n-3 PUFAs after fracture.