基于铕配合物的荧光分子印迹的制备及其选择性检测痕量2,4,6-三氯苯酚
胡 博 高 林 乔 宇 车广波
(1吉林师范大学环境友好材料制备与应用教育部重点实验室,长春 130103)
(2吉林师范大学化学学院,四平 136000)
(3吉林师范大学环境科学与工程学院,四平 136000)
0 Introduction
Chlorophenols(CPs)are a kind ofsynthetic organic compounds which are proved to have adverse effects on humans.CPs are included as priority pollutants by the US Environmental Protection Agency in 2007[1].CPs are considered as endocrine disruptors;CPs and their derivatives would lead carcinogenicity,genotoxicity and mutagenicity to human body[2].Through mother to child transmission,CPs can cause prenatal CPs exposures in utero[3-4].CPs can be used in synthesis insecticides, wood preservatives,bactericides, fungicides, herbicides and process intermediates.They are distributed throughout the environmentand have been detected in sludge products,surface waters,wastewater,groundwater and various types of human biological samples[5-6].CPs include mono-,di-,tri-,tetra-and penta-chlorinated phenols,and 2,4,6-trichlorophenol(2,4,6-TCP)is a representative characteristic of CPs and have been studied extensively as a target substance for catalysis[7],detection[8]and adsorption[9].Existing detection technology for 2,4,6-TCP have some shortage such as complex operations,lacking of detection accuracy or anti-interference.Therefore,it′s necessary to find an affordable,highly sensitive and selective technique to detect the trace target in complex environment.
Fluorescence analysis is a usefuldetection technique,it can be used to detect inorganic matter,organic matter,ion and biomolecules based on fluorescence spectroscopy or fluorescence colorimetry[10-13].In recent years,various of materials have been used as fluorescence sensing such as quantum dots,organic dyes and lanthanides[14-16].Due to their unique 4f electrons,lanthanides relative to other fluorescent materials have high luminescence quantum yield,narrow bandwidth,long-lived emission and large Stokes shifts.As they have an excellent signal-tonoise ratio which improved detection sensitivity,low toxicity,high extinction and variety colors,lanthanides can be widely used in biological fluorescence related applications[17-18].Furthermore,exclusive near infrared radiation leads to less photodamage of the biological samples and more efficiently penetrate in tissues than those in the visible range[19].Based on these features,lanthanides have been designed as coordination compounds,chelate compounds and metal-organic frameworks for probe,energy storage,catalysis,magnetic,luminescent materials[18,20-21].Though lanthanides showed excellent luminescent properties for detection,their thermal and chemical stability still need to improve for adapting complex sample environment.
Molecular imprinted polymers (MIPs)are highly selective polymeric materialswith good thermal,chemistry and mechanical stability that have been widely applied to various fields[22].According to the different forms such as nanoparticles,nanocapsules,nanorods,nanotubes and membranes,MIPs have been employed in a variety of applications including food analysis,contamination detection,diagnosis and targeted treatment,membrane separation and proteomic analysis[23-24]. Traditional MIPs have low detection sensitivity,and the recognition process of target need simplification,so fluorescence analysishasbeen introduced to improve the performance of MIPs[25-26].
Fluorescence quenching between fluorophores and target molecule can realize the transformation of chemicalsignals to opticalsignals.Fluorescent molecularly imprinted polymers(FMIPs)are a kind of optical sensors with high detection sensitivity and rapid detection capability.In recent years,FMIPs are widely used in sample and biomarkers detection[27-29].
In the present study, FMIPs had been synthesized through precipitation polymerization with the specific recognition sites for 2,4,6-TCP molecules.The fluorescence was provided by highly luminescent Eu(MAA)3phen coordination compounds.The obtained composite materials were characterized by Fourier transform infrared spectroscopy (FTIR),scanning electron microscope (SEM),photoluminescence(PL)and thermos gravimetric analysis (TGA).The FMIPs were applied as sensors to detect 2,4,6-TCP based on their luminescence,and the results showed that this type of sensor is suitable for detecting 2,4,6-TCP;the emission intensity of Eu3+decreased with the increasing concentration of 2,4,6-TCP in the system,making the chlorophenols residues be easily tracked,identified and monitored by the change of luminescence.
1 Experimental
1.1 Reagents and chemicals
Acetonitrile(≥99.0%),ethyl alcohol(≥99.7%)hydrochloric acid (HCl),and Eu2O3(≥99.99%)were purchased from Sinopharm Chemical Reagent Co(Shang Hai,China).Ethylene glycol dimethacrylate(EGDMA),2,2′-azobis(2-methylpropionitrile)(AIBN),methacrylic acid (MAA)were all obtained from Aladdin Chemistry Co.Ltd(Shanghai,China).Double distilled water was prepared and used for cleaning processes.All other chemicals used were analytical grade and obtained commercially.
1.2 Instruments
Infrared spectra(4 000~400 cm-1)were obtained on a Nicolet iS50 FTIR apparatus(Thermo Scientific).The morphologies and sizes of FMIPs and FNIPs were measured using a SEM (JEOL,JSM-7800F).Fluorescence intensity of the samples was observed by F-4600 FL-SPECTOROPHOTOMET (HITACHI).TGA was obtained on a STA 449 F3(NETZSCH).
1.3 Synthesis
1.3.1 Preparation of Eu(MAA)3phen
Eu(MAA)3phen were synthesized by the coprecipitation method[30].Solution of europium chloride(0.25 mol·L-1)was prepared from high purity Eu2O3by dissolving in concentrated hydrochloric acid(6 mol·L-1).MAA in anhydrous ethanol(15 mL)was added dropwise into the stirred solution of europium chloride in anhydrous ethanol(30 mL),and then the pH value of the mixture was adjusted to 8 using ammonium hydroxide.The mixture was stirred for 30 minutes,and then the solution of 1,10-phenanthroline monohydrate (phen)in anhydrous ethanol(0.1 mol·L-1,15 mL)was added dropwise.The resulting solution was stirred at room temperature for 3.0 h and standing overnight.The precipitate was filtered and washed with anhydrous ethanol by 3 times,and finally dried at 80℃to obtain Eu(MAA)3phen.
1.3.2 Preparation of FMIPs and FNIPs
FMIPsand FNIPswereboth prepared via precipitation polymerization according to the following procedure.Eu(MAA)3phen(50mg)were added to a mixture of MAA(40 μL),EGDMA(100 μL),a certain amount of 2,4,6-TCP and acetonitrile (60 mL).The mixture was ultrasonically degassed for 5 min.Selfassembly was performed in a water bath orbital shaker at 40℃for 3.0 h under nitrogen protection.The temperature subsequently rose to 60℃,and 30 mg of AIBN was added to initiate polymerization for 24 h.After polymerization,the mixture was centrifuged at 3 700 r·min-1for 5 min,and the suspension was removed.The solid particles were then washed with ethanol and water three times.Then the polymers were eluted with methanol/acetonitrile (4∶1,V/V)to remove the templates,and then dried in a vacuum chamber.FNIPs were used as a control to evaluate the molecular recognition properties of imprinted materials.The FNIPs were synthesized in the same manner as FMIPs but without the addition of 2,4,6-TCP.The synthesis routes of FMIPs were shown in Scheme 1.

Scheme 1 Schematic Illustration of the fabrication of FMIPs
1.3.3 Fluorescence detection of 2,4,6-TCP
The fluorescence of FMIPs and FNIPs was detected with the excitation wavelength of 335 nm.In brief,50 mg FMIPs were dispersed in 100 mL volumetric flask with ethyl alcohol solution,then the solution was preserved in the dark.Afterwards,5.0 mL FMIPs solution was mixed with 5.0 mL of 2,4,6-TCP solutions with different concentrations,respectively.In orderto adequately adsorb on target molecule,the mixed solution was dispersed with ultrasonic concussion for 20 min.Then,the fluorescence intensity of mixture was measured.Moreover,the fluorescence intensities of FNIPs with 2,4,6-TCP solutions with different concentrations were obtained in the similar procedure.
2 Results and discussion
2.1 Characterization of FMIPs and FNIPs
Fig.1(a,b)compares SEM images of FMIPs and FNIPs,whose size,morphology and dispersibility are clearly observed.As exhibited in Fig.1(a),the size of FMIPs is 1.5~2 μm and the monodispersity is excellent with a regular appearance.Moreover,the FNIPs in Fig.1(b)have shown that the range of size is 1.5~2 μm.Both of the two species have a smooth surface.In brief,it demonstrated that microspheres of FMIPs and FNIPs have been successfully prepared and the molecularly imprinted microspheres were better than FNIPs in term of size and monodispersity.
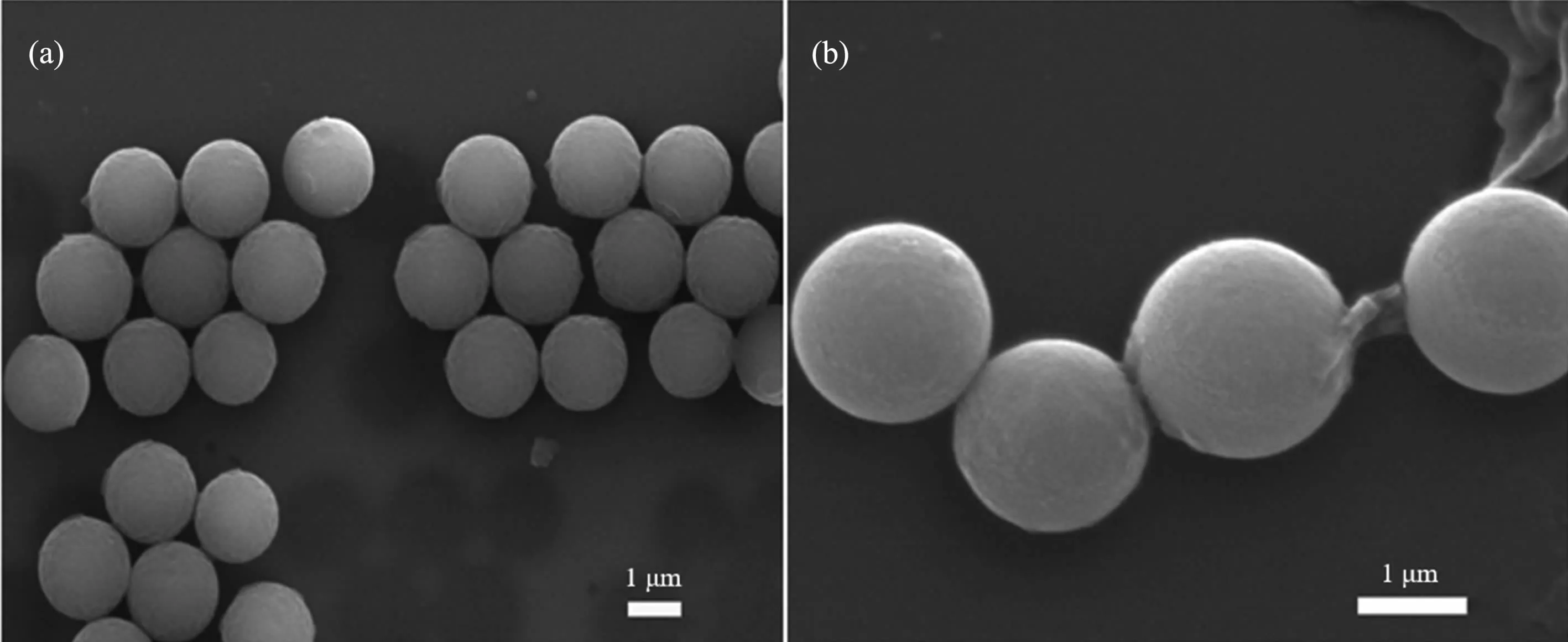
Fig.1 SEM images of FMIPs(a)and FNIPs(b)
The infrared spectra of Eu(MAA)3phen,FMIPs and FNIPs are shown in Fig.2.The feature peaks at 1 568 and 1 428 cm-1from Eu(MAA)3phen could be attributed to-COO-stretching vibration,which suggests that the-COOH from MAA is involved in the coordination,and the feature peaks at 1 646 cm-1conforms to the C=C stretching mode of MAA vinyl groups.Meanwhile,FMIPs and FNIPs have the same peaks at 1 729,1 254 and 1 155 cm-1,which indicated C=O stretching vibration of carboxyl,C-O asymmetric and symmetric stretching vibration of ester from EGDMA,respectively.All the results confirm that precipitation polymerization reaction was successfully initiated.
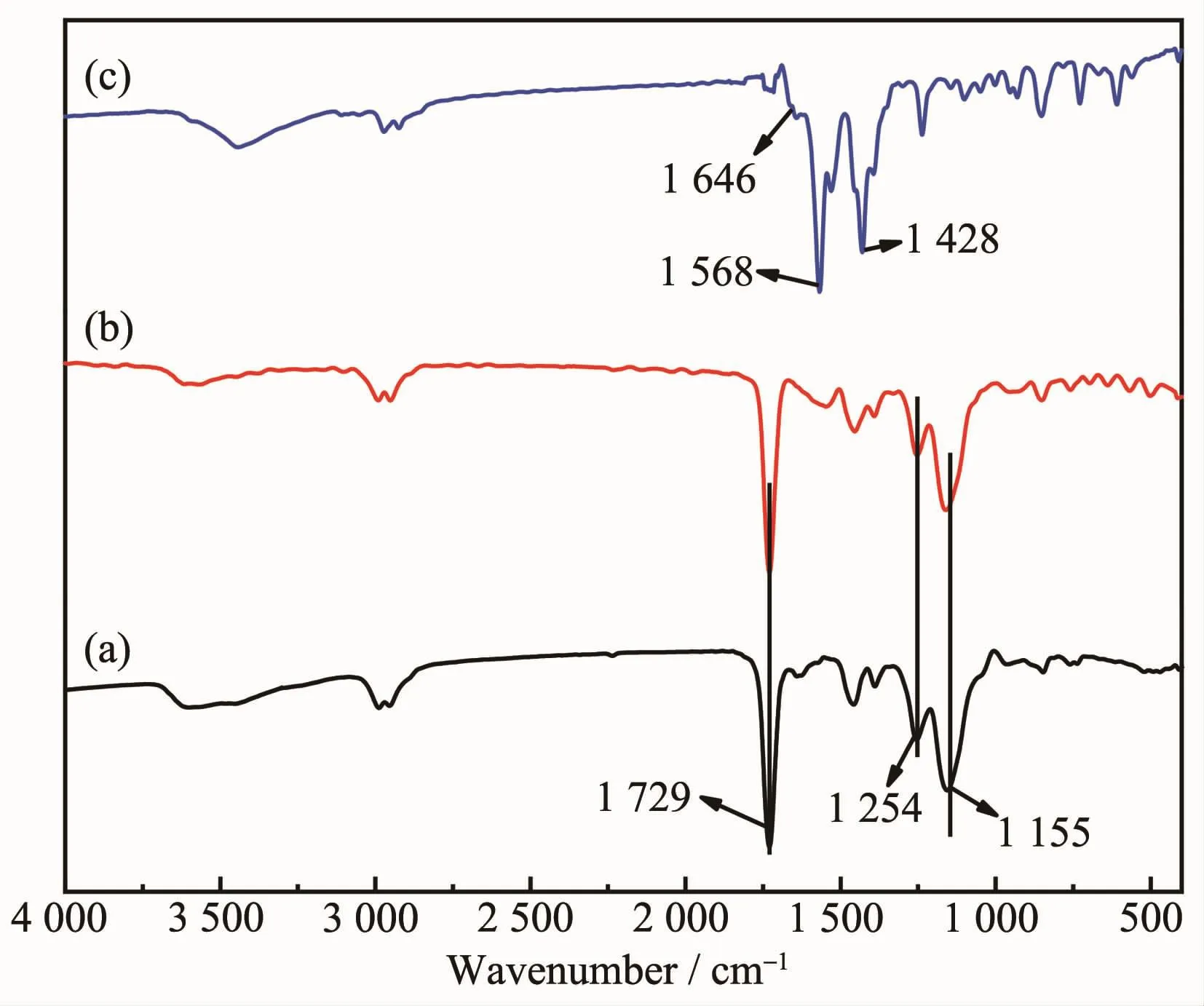
Fig.2 FTIR spectra of FMIPs(a),FNIPs(b)and Eu(MAA)3phen(c)
Thermal stability of Eu(MAA)3phen(black),FMIPs(blue)and FNIPs(red)were evaluated by TGA under N2atmosphere(Fig.3).As shown in Fig.3,the prepared nanospheres demonstrate excellent thermal stability at temperatures lower than 200℃.The TGA curve of FMIPs shows a high rate of weight loss about 90.53%at 600℃,as compared to 95.38%of FNIPs.The tinyweight loss differences of FMIPs and FNIPs are due to that the templates have combined more europium complexes.The TGA results suggest that FMIPs have excellent thermal stability in nature environment.

Fig.3 TGA curves of Eu(MAA)3phen,FMIPs and FNIPs
2.2 Fluorescence detection of 2,4,6-TCP@FMIPs
In this study,our aim is to demonstrate the recognition ability of FMIPs for 2,4,6-TCP and we are able to intuitively obverse the detection effect.Because 2,4,6-TCP molecular added into the FMIPs solvent could give rise to decline of fluorescence intensity,we concluded FMIPs could detect 2,4,6-TCP molecule.As shown in Fig.4,the fluorescence intensities of both FMIPs and FNIPs are gradually quenched with the increasing concentration of 2,4,6-TCP.Moreover,it was observed that fluorescence intensity of FMIPs declines more obviously than that of FNIPs,which indicates that FMIPs have a more excellent detection capacity with 2,4,6-TCP compared with FNIPs.
To obtain precisely the detection range of synthetic material,the quenching efficiency of microspheres was evaluated by the Stern-Volmer equation:

I0is the initial fluorescence intensity without analyte.I is the fluorescence intensity in the presence of analyte with the concentration of C(0~100 nmol·L-1),and Ksv(L·mol-1)is the quenching constant with 2,4,6-TCP.
To avoid the contingency of linear relation,the uniform experiment processes were repeated five times and acquired Fig.5 by their average.As manifestation in Fig.5(a),the excellent linear relationship of Stern-Volmer equation appears in the range of 0~70 nmol·L-1.The linear equation of FMIPs is I0/I-1=0.008 89C+0.012 77 and the corresponding correlation coefficient was R2=0.996 28.Hence,the limit of detection(LOD)was found to be 3.12 nmol·L-1,which was calculated with the equation:LOD=3σ/S(σ was the standard deviation of the blank signal and S was the slope of the linear calibration plot).In addition,the linear relationship of FNIPs were shown in Fig.5(b)and linear equation was I0/I-1=0.003 97C-0.008 59,with the concentration range of 0~80 nmol·L-1and R2=0.997 04.
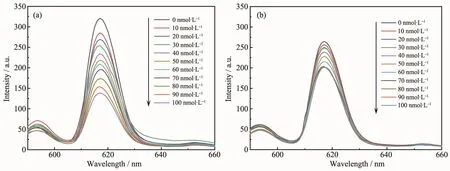
Fig.4 Response of(a)FMIPs and(b)FNIPs to 2,4,6-TCP in the concentration range from 0 to 100 nmol·L-1
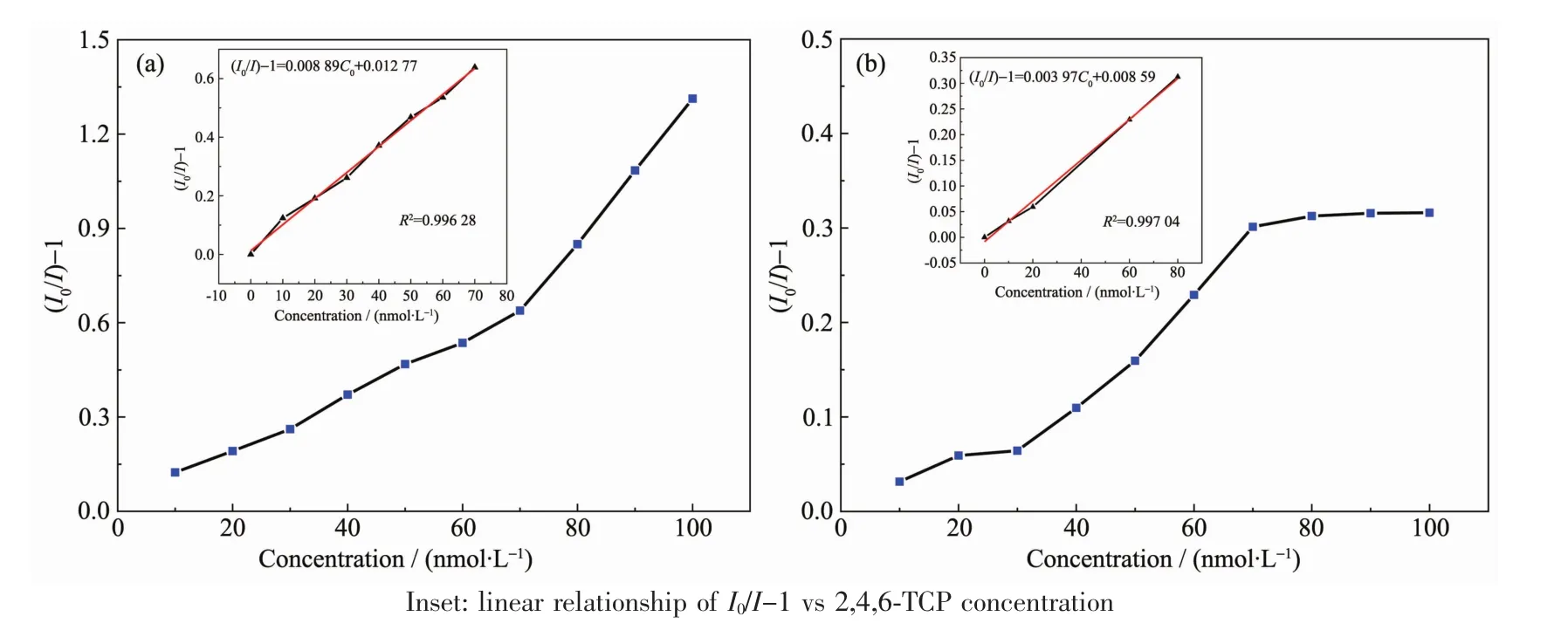
Fig.5 Dependence of fluorescence quenching efficiency on 2,4,6-TCP concentration
2.3 Selectivity determination of FMIPs and FNIPs
Several analogs with the similar structure including 2,4-DCP,2,5-DCP,2,6-DCP were chosen as chaff interferent to investigate the selectivity determination of FMIPs further.In brief,0.5 g·L-1of FMIPs (5 mL)were dispersed in 20 nmol·L-1of 2,4-DCP,2,5-DCP,2,6-DCP and 2,4,6-TCP(5 mL),respectively,which were vibrated with ultrasonic for 20 min.
In Fig.6,it is obviously observed that the quenching efficiency of FMIPs for 2,4,6-TCP is superior to that of FNIPs.Moreover,the quenching efficiency for 2,4,6-TCP is also much better than those for other chlorophenols.To sum up,a good selectivity determination of FMIPs for 2,4,6-TCP was well illustrated without interference of other chlorophenols.
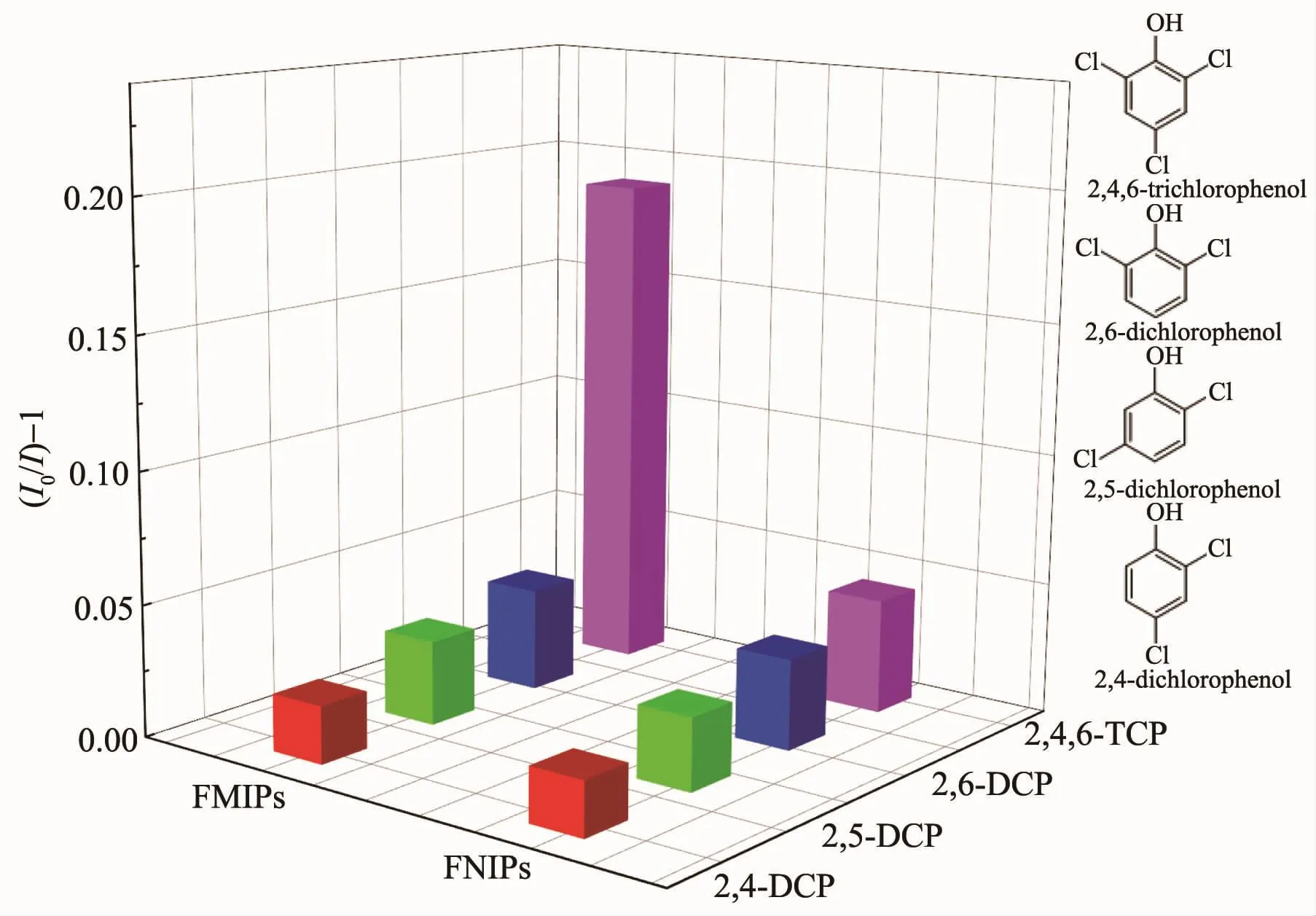
Fig.6 Quenching amount of FMIPs and FNIPs by different kind of chlorophenols
3 Conclusions
In summary,we presented a new application for the use ofeuropium complexes as fluorescent functional monomer in the synthesis of FMIPs.The newly prepared FMIPs are an efficient and selective detection method for trace level 2,4,6-TCP,which was successfully discriminated from other chlorophenols.The FMIPs have a linear fluorescent response in 0~70 nmol·L-1concentration range,and the correlation coefficient is 0.996 28.The FMIPs show excellent sensitivity and the limit of detection is 3.12 nmol·L-1.In addition,FMIPs show good thermal stability and appreciable selectivity over several analogs.Further studies are expected to design a multifunctional sensor based on europium complexes.In addition,our study have provided a new way to fabricate FMIPs with potential application in the recognition and sensitive sensing ofanalytes.In principle,this technique offers a general approach to fluorescence detection of chlorophenols which quench fluorescence in complex systems.

