Consequences of unilateral cryptorchidism on semen and sperm characteristics in West African Dwarf Goats
Chike F. Oguejiofor, Izuchukwu S. Ochiogu, Okechi L. Okoro, Vitalis U. Ogbu
Department of Veterinary Obstetrics and Reproductive Diseases, Faculty of Veterinary Medicine, University of Nigeria, Nsukka 410001, Nigeria
Keywords:Cryptorchidism Goat Semen Spermatozoa
ABSTRACT Objective: To evaluate the influence of unilateral cryptorchidism on semen and sperm characteristics in West African Dwarf (WAD) bucks. Methods: Semen was collected using electroejaculator from five unilaterally cryptorchid (UC) and five normal (non-cryptorchid)WAD bucks and analyzed for gross, microscopic and biochemical characteristics. Results:Gross semen evaluation showed no differences between the groups in semen color, viscosity and pH, whereas the normal bucks yielded semen with significantly higher specific gravity(P=0.043 6) and volume (P=0.038 8) than the UC group. Following semen microscopic evaluation, the percentage of sperm vitality (live sperm) was not significantly different between both groups. However, UC bucks yielded semen with significantly lower sperm motility(P=0.038 7), sperm concentration per mL (P=0.002 0) and total sperm count per ejaculate (P=0.007 4). The percentage total sperm abnormality was also higher (P<0.000 1)in the semen of UC goats. Abnormalities observed included sperm with cytoplasmic droplets,looped tails, coiled tails and tailless heads. Sperm morphometry showed no differences in the sperm head length and head width between the groups. Biochemical semen evaluation did not reveal any differences between the groups in the concentration of seminal plasma total protein, catalase activity and lipid peroxidation level. Conclusions: Unilateral cryptorchidism significantly affected the quantity and quality of semen and spermatozoa in affected WAD bucks. Due to the hereditary attribute of the condition, it is recommended that animals with this condition should not be used in breeding to forestall increasing prevalence of cryptorchidism in goats.
1. Introduction
Abnormalities of the male reproductive system can negatively impact on reproductive efficiency and animal production.Cryptorchidism is a male abnormality in which there is failure of one or both testes to descend into the scrotum at the time normal for the species of interest, before or shortly after birth[1]. The retained testis is located in the abdominal, inguinal or subcutaneous regions of the body[2]. Cryptorchidism may be unilateral or bilateral, although unilateral cryptorchidism is more frequent in small ruminants and mainly affects the right testis[3,4]. The etiology of cryptorchidism is a complex interaction of genetic, anatomic and other acquired factors such as fetal exposure to some endocrine disruptors[1,5].
A prevalence of 0.6%-3.3% of cryptorchidism has been reported in goats in several parts of the world[3,6-9]. The West African Dwarf(WAD) goats are small, short-legged goats found in the region south of latitude 14oN across West Africa in the coastal area[10].Unilateral cryptorchidism is prevalent in WAD goats in southeastern Nigeria[11-13] and ranged from 33%-63% in some areas where farmers preferred breeding with unilaterally-cryptorchid (UC)bucks[14]. Goat farmers considered the UC bucks to have better libido and reproductive efficiency than bucks with both scrotal testes. This perception is also buttressed in the livestock markets, for these bucks have higher financial values than non-cryptorchid goats.
Importantly, cryptorchidism has been associated with short and long term deleterious effects including altered testicular function and spermatogenesis and the risk of testicular tumors[2]. There are reports of either reduced production of spermatozoa or increased proportion of abnormal spermatozoa in cases of unilateral cryptorchidism in men[15], dogs[16] and boars[17,18]. In goats, unilateral cryptorchidism is marked by structural changes in both the cryptorchid and scrotal testes[3,14,19]. In one study, unilateral cryptorchidism did not affect the quantity and quality of semen and spermatozoa in bucks[13].However, another study reported normal production of semen and spermatozoa but reduced quality due to increased production of abnormal spermatozoa in UC bucks[20]. It is not clear if the functions of accessory sex glands are impaired in unilateral cryptorchidism.Thus, there is limited information on the possible effects of unilateral cryptorchidism on semen and sperm characteristics in WAD goats particularly on sperm morphometry and the biochemical properties of seminal plasma. Therefore, the main objective of the study was to compare the gross, microscopic and biochemical semen characteristics between UC and normal (non-cryptorchid) WAD goats. The significance is to provide more information on the possible effects of unilateral cryptorchidism on semen and sperm quality in WAD goats.
2. Materials and methods
2.1. Animals
Ten apparently healthy bucks comprising 5 normal bucks (N; both testes present in the scrotum) and 5 UC bucks (one scrotal testis and one undescended testis) were obtained from the local market.Unilateral cryptorchidism was confirmed retrospectively at the end of the study during post-mortem examination. All animals were between 12-14 months old by the use of dental aging. The study was performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals[21].The experimental protocol was approved by the Experimental Animal Ethics Committee of the Faculty of Veterinary Medicine,University of Nigeria, Nsukka 410001, Nigeria (approval number:UNFVM/2017/11-178314; dated 20/07/2017). Animals were housed in conducive, well-ventilated pens and were provided water and feedad libitum. The animals were acclimatized for a month, during which they were screened for blood and gastrointestinal parasites,and were treated accordingly. The mean body weight of the N group was 7.1 kg (range 6.5-8.5 kg) while the UC group was 7.3 kg (range 6.0-9.0 kg). Physiologic parameters (rectal temperature, pulse and respiratory rates) were within the normal reference. General physical examination, chest auscultation and abdominal palpations did not reveal any signs of illness. Examination and palpation of the reproductive organs (prepuce, penis, scrotum and testes and spermatic cord) did not reveal any obvious abnormalities.
2.2. Semen collection
Animals were initially trained by using the electro-ejaculator to collect semen once a week for three consecutive weeks. Following this, semen samples were collected for analysis. All animals were sampled twice with an interval of 5 days. Briefly, the animal was restrained in lateral recumbency and the hairs around the preputial opening were trimmed and cleaned properly. The rectal probe of the electro-ejaculator was lubricated with inert petrolatum and gently inserted into the rectum. The voltage regulator was applied to stimulate the floor of the rectum and the underlying accessory sex glands to cause penile erection followed by semen ejaculation into a clean wide mouthed plastic container that was non-toxic to spermatozoa. Total ejaculates were collected by avoiding the loss of any semen portion. Semen samples were maintained between 28 ℃-37 ℃ before laboratory evaluation.
2.3. Gross semen evaluation
Semen color was assessed visually by subjectively using a color chart. Semen viscosity was also assessed by swirling the semen in the sample bottle and values were assigned subjectively on a scale of 1 to 4. Semen pH was measured using a digital pH meter (Hanna;Woonsocket, RI, USA). Semen specific gravity was measured using a clinical refractometer (Atago; Bellview, WA, USA). Semen volume was determined using a graduated pipette (Volac, England).
2.4. Microscopic semen evaluation
Semen evaluation was performed in accordance with the World Health Organization laboratory manual for the examination and processing of human semen[22]. Progressively forward sperm motility (%) was evaluated by examining a diluted wet mount of the semen sample at ××400 using a phase-contrast microscope (Motic B3; Motic, Carlsbad, CA, USA) equipped with a stage slide warmer set at 37 ℃ (TCS-100; Amscope, Ivrine, CA, USA). Sperm vitality(%) was determined after staining with eosin-nigrosin vital stain.Live sperm (unstained head) and dead sperm (red-stained head) were identified using light microscopy at ×1 000 magnification under oil immersion. Sperm morphology was first evaluated at ×1 000 magnification using phase-contrast microscopy. Semen samples were also smeared on a slide, dried, fixed, stained with Papanicolaou staining method (Harris’s hematoxylin, G-6 orange and EA-50 green stains) before examination for sperm abnormalities using light microscopy at×× 1 000 magnification under oil immersion. All percentage values were determined by examining 200 sperm cells in replicates. Sperm concentration (/mL) was determined by diluting the semen in a semen-dilution fluid containing a fixative followed by counting of sperm cells using a hemocytometer (Weber, England).Total sperm count in each ejaculate was determined by multiplying the volume of the ejaculate by its sperm concentration (/mL). Sperm morphometry was performed following calibration and measurement of the sperm head length and sperm head width using the Moticam 2.0 image system and software (Motic, Carlsbad, CA, USA). For each semen sample, seven to ten measurements were made in three different random fields and in replicates. All micrographs were captured using Moticam 2.0 image system.
2.5. Biochemical evaluation of seminal plasma
Semen sample was centrifuged at 1 000 ××gfor 10 min. The sperm-free supernatant (seminal plasma) was then removed and evaluated for total protein concentration and oxidative stress using catalase and lipid peroxidation activities. Due to the small volume of ejaculates, semen samples were diluted accordingly and the dilution factors were used in the calculation of the test results. Total protein concentration in seminal plasma was measured using a clinical refractometer (Atago; Bellview, WA, USA).
Catalase activity in seminal plasma was determined according to the method described previously by Sinha[23] and modified by Hadwan[24]. In this method, dichromate in acetic acid was reduced to chromic acetate when heated in the presence of hydrogen peroxide,with the formation of perchromic acid as an unstable intermediate.Hydrogen peroxide concentration was directly proportional to the concentration of chromic acetate that was produced from the reaction.The chromic acetate produced was measured colorimetrically at 570 nm using a spectrophotometer (Jenway 6305; Jenway, Essex,UK) and catalase activity was expressed in karmen unit.
Lipid peroxidation in seminal plasma was quantified by measuring the formation of thiobarbituric acid reactive substances based on the method of Stocks and Dormandy[25] modified by Sicinskaet al[26]. Briefly, 0.1 mL of seminal plasma was mixed with 1.0 mL of 20% trichloroacetic acid and incubated at room temperature.Samples were centrifuged at 2 500××gfor 10 min. Thiobarbituric acid (1%) was added to the supernatant and samples were heated at 100 ℃ for 15 min followed by cooling and centrifugation for 10 min at 2 500××g. The absorbance of the supernatant was read at 532 nm against a reagent blank using a spectrophotometer and lipid peroxidation (thiobarbituric acid reactive substances) was expressed in absorbance unit.
2.6. Data analysis
Statistical analysis was performed using GraphPad Prism version 6.01 (GraphPad Software Inc.). Animals were sampled twice and the mean values for each animal were compared between the N and UC groups using thet-test. Values represent Mean ± Standard Error(Mean ± SE) and were considered significant whenP< 0.05.
3. Results
3.1. Gross semen characteristics
Semen samples from N and UC groups of goats were milky or creamy in color with no differences in appearance. And the results of the evaluation of viscosity, pH, specific gravity and volume in the N and UC goats were presented in Table 1. Semen viscosity and pH were not significantly different between the two groups. Specific gravity of semen was significantly higher in the N goats compared to the UC group (P= 0.043 6). The N goats also yielded a significantly higher semen volume than the UC group (P= 0.038 8).

Table 1 Comparison of gross semen characteristics of normal and unilaterallycryptorchid WAD goats.
3.2. Microscopic semen characteristics
The results of semen analysis for sperm motility, sperm vitality,total sperm abnormality, sperm concentration, total sperm count and sperm measurements in N and UC goats were presented in Table 2.The percentage of motile sperm (sperm motility) was significantly reduced (P= 0.038 7) in the UC goats compared to the N group.
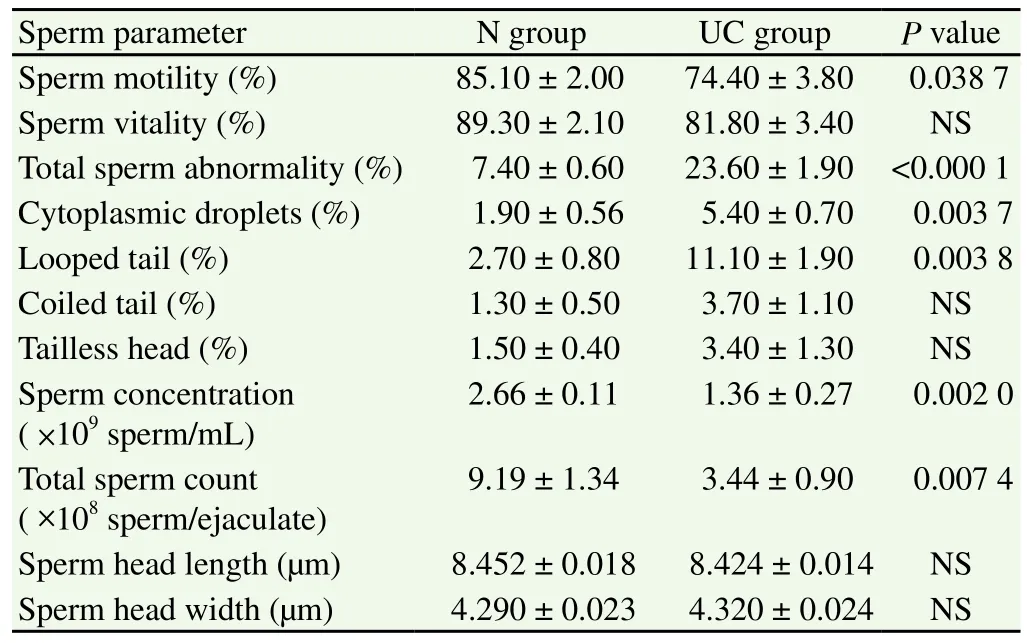
Table 2 Comparison of microscopic semen characteristics of normal and unilaterallycryptorchid WAD goats.
The percentage of live sperm (sperm vitality; Figure 1) was not significantly different between both groups although the N goats yielded a higher mean % of live sperm compared to the UC group.The percentage total sperm abnormality was significantly higher(P< 0.000 1) in the semen of UC goats compared to the N group.Sperm abnormalities observed included sperm with cytoplasmic droplets, looped tails, coiled tails and tailless heads (Table 2;Figure 2). The percentage of sperm with cytoplasmic droplets and looped tails were significantly higher in UC goats but there were no differences in the percentage of sperm with coiled tails and tailless head between both groups. Sperm concentration per mL was significantly reduced (P= 0.002 0) in UC goats compared to the N group. Similarly, total sperm count was also lower in the UC goats compared to the N group (P= 0.007 4). There was no significant difference in the sperm head length and sperm head width of the N goats compared to the UC group, respectively (Table 2; Figure 3).
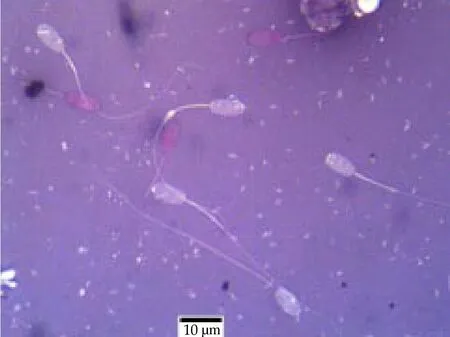
Figure 1. Evaluation of sperm vitality using eosin-nigrosin staining.Live sperm were unstained whereas dead sperm were stained red.
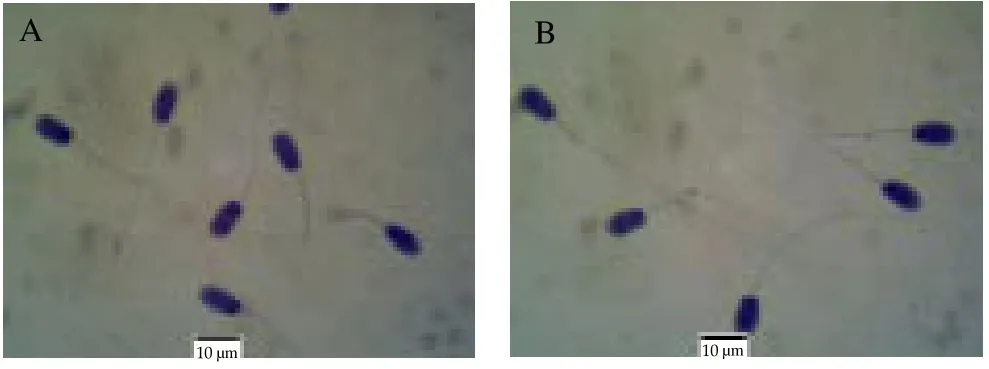
Figure 2. Evaluation of sperm morphology using Papanicolaou staining.

Figure 3. Evaluation of sperm morphometry using Papanicolaou staining.
3.3. Biochemical semen characteristics
The results of the evaluation of total protein concentration, catalase activity and lipid peroxidation level in the seminal plasma of N and UC bucks were presented in Table 3. There was no significant difference between both groups in the concentration of seminal plasma total protein, and also in the catalase activity and lipid peroxidation levels in the seminal plasma.
4. Discussion
The findings in this study provided evidence that unilateral cryptorchidism significantly affected the quantity and quality of semen and spermatozoa in WAD goats. The observed gross and microscopic semen parameters in the normal goats were comparable to the values reported previously for the WAD breed[13,27,28].Unilateral cryptorchidism did not alter the semen color and pH as also reported by others[13,20], and semen viscosity did not also differ from normal. The specific gravity of semen in UC bucks was lower than that of normal bucks, most probably due to lower density of spermatozoa in the semen of these bucks. Semen volume comprises spermatozoa and other cell components but is mainly determined by secretions of the caudal epididymis and accessory sexual glands[29].Semen volume was also lower in UC bucks whereas other studies observed no differences in semen volume in Sahel goats[20] and WAD goats[13]. This discrepancy may be due to differences in the breed of goat in the former study (Sahel goats also weighed anaverage of 18 kg compared to 7 kg of WAD goats) or due to the number of goats sampled in the latter study.

Table 3 Comparison of total protein concentration, catalase activity and lipid peroxidation level in the seminal plasma of normal and unilaterally-cryptorchid goats.
Some microscopic sperm parameters were not altered in UC bucks including sperm vitality and sperm head morphometry. However,other parameters were significantly altered in UC bucks which had decreased sperm motility and concentration and increased proportion of morphologically abnormal sperm. This differed from a previous study that observed no differences in sperm motility, vitality,morphology and concentration in UC WAD bucks[13]. Another study observed no effect on sperm concentration but a high proportion of dead sperm and sperm with primary and secondary morphological abnormalities in UC Sahel goats[20]. Findings in other male species also provided more evidence of the deleterious effects of unilateral cryptorchidism on spermatozoa. In bulls, sperm motility and velocity parameters were significantly reduced in UC animals[30].In boars, unilateral abdominal cryptorchidism was associated with a decreased sperm production and significantly higher frequency of primary abnormalities of spermiogenesis in the testis but not with maturational abnormalities in the epididymis[17]. In men, unilateral cryptorchidism was associated with low sperm concentration and/or abnormal spermiogram in treated cases[15] and in long-standing untreated cases[31,32].
Another consideration is whether a cryptorchid testis affects sperm production and function in the descended testis and epididymis, as suggested by the findings here. One study reported no difference in the sperm concentration of a scrotal testis and epididymis of the normal buck and the scrotal testis of a UC buck[20]. In contrast, Uchenduet al[14] reported no differences in testicular sperm concentration but reduced sperm motility and viability and increased morphological abnormalities in the scrotal epididymis of UC bucks. In other studies,epididymal sperm reserve was also lower in the scrotal testis of UC boar, in addition to the higher percentage of proximal cytoplasmic droplets and acrosomal defects compared to the normal boar[18]. In dogs, semen volume and number of sperms increased gradually, and sperm abnormality decreased after the removal of the cryptorchid testis, implying that the cryptorchid testis disturbed the testicular function of the scrotal testis[16].
Seminal plasma comprises a variety of proteins mainly produced by the accessory sexual glands that have important functions in fertility including sperm transport and capacitation, modulation of the uterine immune response, and gamete interaction and fusion[29,33].Oxidative stress is known to have a major impact on sperm function and fertility, and can occur in the form of damage due to increased levels of reactive oxygen species and lipid peroxidation that result in impairment of sperm morphology and motility, loss of sperm function, and sperm death[34,35]. The seminal plasma is an important source of antioxidants including catalase enzyme that protect the sperm from damage due to reactive oxygen species[36]. Unilateral cryptorchidism did not alter total protein concentration and some oxidative stress and antioxidant indicators in the seminal fluid of WAD bucks. This suggested that the sperm abnormalities observed in the UC bucks may not be related to direct effects of the condition on the secretive and antioxidant function of other accessory sex glands.
The influence of unilateral cryptorchidism on the fertility of affected goats is not clearly defined. However, the occurrence of decreased sperm count and increased proportion of abnormal spermatozoa in UC goats is likely to negatively affect fertility. However, this impact may be less significant in small farms or flocks where affected males are used to serve fewer females, but more significance is found in intensive farms where few males breed a large number of females particularly following estrus synchronization. In summary,unilateral cryptorchidism significantly affected the quantity and quality of semen and spermatozoa in affected WAD goats. Hence,these findings do not support the folk belief that UC bucks have better reproductive indices than non-cryptorchid (normal) bucks.Cryptorchidism is considered to have hereditary attribute, therefore it is recommended that animals with cryptorchidism should not be used in breeding to forestall increasing prevalence of cryptorchidism in goats.
Conflict of interest statement
The authors declare that they have no conflict of interest.
 Asian Pacific Journal of Reproduction2018年4期
Asian Pacific Journal of Reproduction2018年4期
- Asian Pacific Journal of Reproduction的其它文章
- In vitro fertilization: Facts in medical sciences
- Functions of follicular and marginal zone B cells in pregnancy
- Treatment of cows with clinical endometritis Ⅲ as cows affected by pyometra-Non antibiotic treatment of severe clinical endometritis
- Effect of foot and mouth disease vaccination on seminal antioxidant profiles of mithun(Bos frontalis)
- Increase in reproductive ability of high-producing cows, and qualitative parameters of their offspring, under conditions of intensive milk production
- Developmental competence of bovine oocytes with increasing concentrations of nano-copper and nano-zinc particles during in vitro maturation
