Management of metastatic esophagogastric junction adenocarcinoma
Tasuku Toihata, Yu Imamura, Masayuki Watanabe, Hideo Baba
1Department of Gastroenterological Surgery, Graduate School of Medical Science, Kumamoto University, Kumamoto 860-0811,Japan.
2Gastroenterological Surgery, Cancer Institute Hospital, Tokyo 135-8550, Japan.
Abstract The prognosis of metastatic disease of esophagogastric junction adenocarcinoma remains poor, despite using a variety of regimens using cytotoxic agents. Recent understanding of molecular characteristic and tumor microenvironment of this cancer is currently instigating new therapeutic options. In this review, we summarized previous evidences of cytotoxic agents widely used worldwide, and updated recent developments of molecular targeted drugs, and immune checkpoint inhibitors.
Keywords: Esophagogastric junction, adenocarcinoma, advanced, molecular targeted drug, immune checkpoint inhibitor,immunotherapy
INTRODUCTION
The esophagogastric junction (EGJ) adenocarcinoma is defined as tumors which have their center within 5 cm proximal or distal to the anatomical esophagogastric junction[1-3]. In Western countries, the incidence of EGJ adenocarcinoma has been increasing rapidly over the last few decades, in the background of decreasing rate of Helicobacter pylori infection, and increasing trends of obesity and gastroesophageal reflux disease(GERD). EGJ adenocarcinoma is usually diagnosed with unresectable disease because of difficulty in early detection. Even after curative resection, many cases experience recurrent disease, resulting in lower survival rates of this tumor[4,5]. In spite of multidisciplinary treatments, median overall survival (OS) is around 12 months in advanced EGJ or gastric adenocarcinoma[6,7]. Therefore, the treatment goal for metastatic disease of this tumor should include survival benefit with symptom relief, and systemic chemotherapy is a major treatment option for those cases[8]. Treatments for advanced EGJ adenocarcinoma has been developed as a type of advanced gastric cancer, and many clinical trials were conducted targeting both EGJ and gastric adenocarcinoma. Recent comprehensive molecular analysis for upper GI cancers reveals molecular differences between EGJ and gastric adenocarcinoma[9,10]. Here, we update recent evidences of treatments for advanced EGJ adenocarcinoma, and discuss future perspective.
CYTOTOXIC AGENTS (FOR HER2-NEGATIVE TUMORS)
Fluoropyrimidine (ftorafur, S-1, or capecitabine), platinum (cisplatin, or oxaliplatin), irinotecan, and taxanes (paclitaxel, or docetaxel) are globally used for metastatic disease of EGJ adenocarcinoma. In addition, trastuzumab is a humanized monoclonal antibody that selectively binds with high affinity to the extracellular domain of the human epidermal growth factor receptor, and approved for tumors with HER2+[protein overexpression by immunohistochemistry (IHC) or gene amplification by in situ hybridization(FISH)] EGJ adenocarcinoma. Considering chemotherapeutic managements, tumor HER2 status is a valuable information for adding trastuzumab to cytotoxic agents. As a first-line therapy, there is no widely accepted first-line standard regimen for advanced EGJ adenocarcinoma.
In the USA and Europe, fluorouracil and platinum-based agents (CF) or docetaxel, fluorouracil, and cisplatin (DCF) is widely used regimen based on the clinical trial. In 2006, the V-325 study group showed no superiority between DCF and DC (docetaxel and cisplatin) in OS. Median OS was 9.6 months for DCF, and 10.5 months for DC. The incidence of hematologic toxicities was high, but it was comparable between DCF and DC. Grade 3 or 4 neutropenia was the most common in hematologic toxicity; it occurred in 86% in the patients with DCF, and 87% in DC cases, although non-hematologic toxicities of DCF had a higher incidence than that of DC[11].
In Europe, epirubicin, cisplatin, and fluorouracil (ECF), epirubicin, cisplatin, and capecitabine (ECX),epirubicin, oxaliplatin, and fluorouracil (EOF), or epirubicin, oxaliplatin, and capecitabine (EOX) is a major regimen for advanced EGJ or stomach adenocarcinoma. The REAL-2 trial assessed above-mentioned four regimens with different three-drug combination, and showed median OS of 9.9 months with ECF,9.9 months with ECX, 9.3 months with EOF, and 11.2 months with EOX, respectively. One-year-survival rates were 37.7%, 40.8%, 40.4%, and 46.8%. The trial showed capecitabine and oxaliplatin were as effective as fluorouracil and cisplatin[12].
In Asia, the recommended first-line treatment is S-1 plus cisplatin (SP) or capecitabine plus cisplatin (XP). In the SPIRITS trial [phase III, including advanced gastric adenocarcinoma (n = 298)], OS was better in patients treated with SP than with S-1 alone. Median OS was 13.0 months [interquartile range (IQR) 7.6-21.9] in those assigned to SP compared with 11.0 months (IQR 5.6-19.8) in those assigned to S-1 alone [hazard ratio (HR)0.77; 95% CI 0.61-0.98; P = 0.04]. Progression-free survival (PFS) was significantly longer in those assigned to SP than S-1 alone [median PFS 6.0 months (3.3-12.9) for SP vs. 4.0 months (2.1-6.8) for S-1 alone; P < 0.0001].The trial showed more grade 3 or 4 adverse events including leucopenia, neutropenia, anemia, nausea, and anorexia, in patients assigned to SP than in patients assigned to S-1 alone[13]. However, the incidence of EGJ cancer remains low in Japan, and this clinical trial included only gastric cancer patients. Therefore, the standard treatment for EGJ cancer has not yet been established in Japan and patients with EGJ cancer are usually treated based on the evidence for gastric cancer.
MOLECULARLY TARGETED DRUG
In the first decade of this century, molecularly targeted drugs have been developed for advanced EGJ adenocarcinoma [Table 1]. To date, trastuzumab and ramucirumab are the only molecularly targeted drugswith confirmed survival benefit in phase III trials. In this section, we focus on the results of phase III clinical trials.
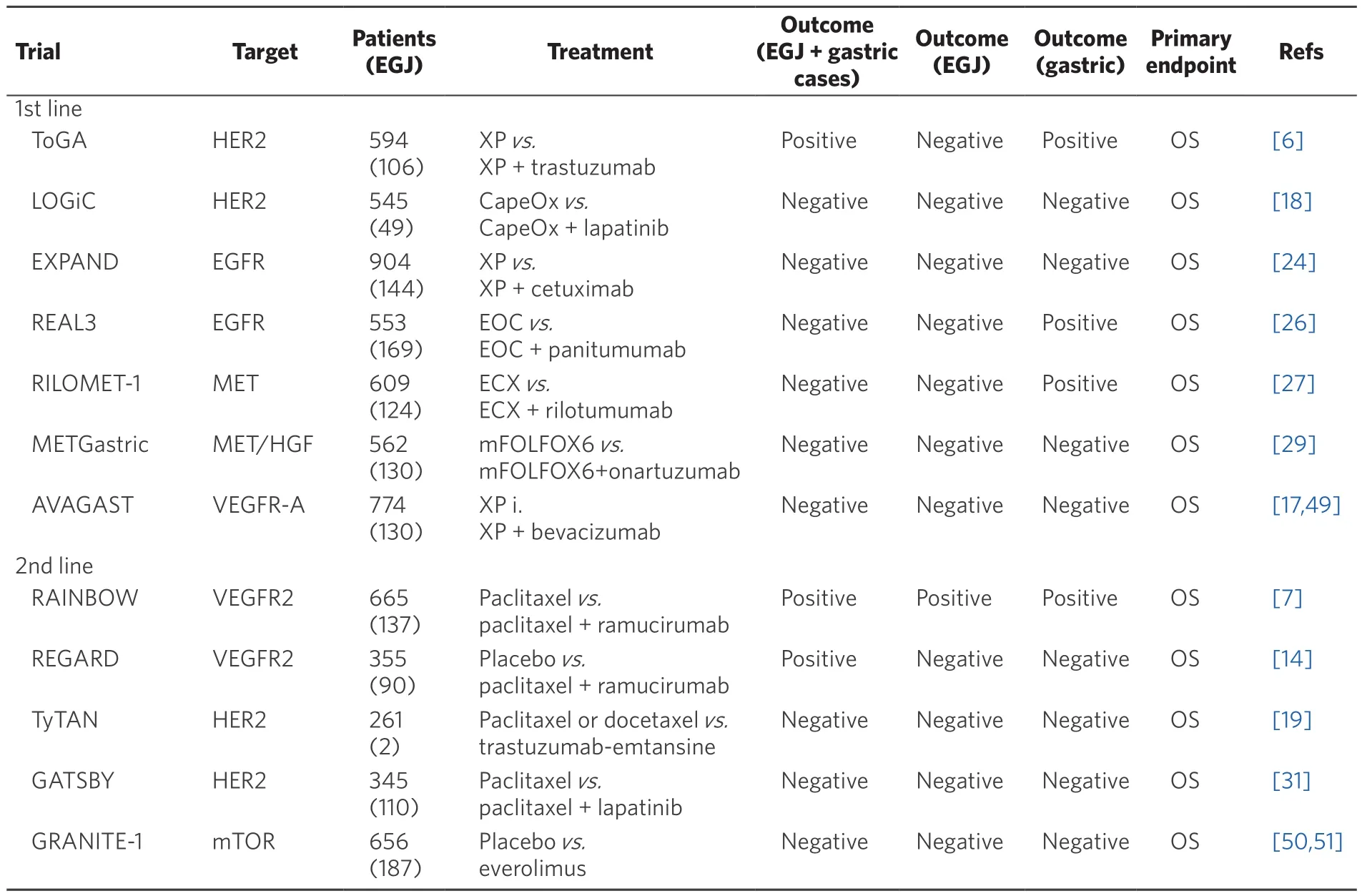
Table 1. Clinical trials testing targeted therapies for esophagogastric junction and gastric adenocarcinoma
Trastuzumab (anti-HER2 antibody)
Trastuzumab is a monoclonal antibody targeting HER2. In 2010, ToGA trial [phase III, including EGJ(n = 106) and advanced gastric adenocarcinoma (n = 478)] was to assess the efficacy and safety of trastuzumab plus first-line chemotherapy (XP or FP) of advanced HER2 positive 106 EGJ and 478 gastric adenocarcinoma.HER2 status was tested by IHC and FISH. HER2 positivity was defined as samples with 3+ by IHC, or those with both 2+ IHC and FISH positive. HER2 positivity was frequently observed in tumors located at EGJ,compared to those in stomach (33.2% for EGJ vs. 20.9% for stomach; P < 0.001). Median OS was significantly longer in trastuzumab plus chemotherapy groups than in chemotherapy alone [median 13.8 months(95% CI 12-16) vs. median 11.1 months (95% CI 10-13), HR 0.74; 95% CI 0.60-0.91; P = 0.0046]. However, in a subgroup analysis of EGJ adenocarcinoma, there were no survival benefit of trastuzumab (trastuzumab plus chemotherapy groups vs. chemotherapy alone, HR 0.67; 95% CI 0.42-1.08). The most common adverse events in both groups were nausea, vomiting and neutropenia. Rate of overall grade 3-4 adverse events (68%in trastuzumab plus chemotherapy groups vs. 68% in chemotherapy alone) and cardiac adverse events (6% in trastuzumab plus chemotherapy groups vs. 6% in chemotherapy alone) did not differ between the groups[6].NCCN guideline recommends the addition of trastuzumab to any chemotherapy combination for patients with HER2-positive tumors.
Ramucirumab (VEGFR-2 inhibitor)
Ramucirumab is a human IgG1 monoclonal antibody VEGFR-2 antagonist. The REGARD trial and the RAINBOW trial showed a significant benefit of ramucirumab for advanced EGJ and gastric adenocarcinoma,as the second-line chemotherapy.
The REGARD trial [phase III, including advanced EGJ (n = 90) and gastric adenocarcinoma (n = 265)]exhibited a significant benefit of ramucirumab (OS 5.2 months for ramucirumab vs. OS 3.8 months for placebo; HR 0.776, 95% CI 0.603-0.998; P = 0.047). However, in a subgroup analysis of EGJ adenocarcinoma,the trial did not exhibit any significant benefit of ramucirumab (ramucirumab groups vs. placebo groups,HR 0.76; 95% CI 0.47-1.21). The incidence of hypertension was higher in the ramucirumab group than in the placebo group (16% vs. 8%)[14]. In the RAINBOW trial [phase III, including advanced EGJ (n = 137) and gastric adenocarcinoma (n = 528)], the ramucirumab plus paclitaxel conferred a significantly prolonged OS,compared to the placebo plus paclitaxel group (9.6 vs. 7.4 months, HR 0.807; 95% CI 0.678-0.962; P = 0.017).In a subgroup analysis of EGJ adenocarcinoma, the trial revealed survival benefit of adding ramucirumab,either (ramucirumab plus paclitaxel groups vs. placebo plus paclitaxel groups, HR 0.39; 95% CI 0.26-0.59).Grade 3 or 4 adverse events that occurred in more than 5% of patients in the ramucirumab and paclitaxel group vs. placebo and paclitaxel group were as follow; neutropenia (41% vs. 19%), leucopenia (17% vs. 7%),hypertension (14% vs. 2%), fatigue (12% vs. 5%), anemia (9% vs. 10%), and abdominal pain (6% vs. 3%)[7].
Bevacizumab (anti-VEGF antibody)
Bevacizumab, which is a monoclonal antibody that targets vascular endothelial growth factor A (VEGF-A),inhibiting tumor growth in preclinical studies[15,16]. In AVAGAST trial [phase III, including advanced EGJ(n = 103) and gastric adenocarcinoma (n = 671)], bevacizumab did not confer any survival benefit (median OS 12.1 months in bevacizumab plus XP; vs. median OS 10.1 months in XP alone). In a subgroup analysis of EGJ adenocarcinoma, there was no survival benefit (data not available)[17].
Lapatinib
Lapatinib is the dual inhibitor of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) tyrosine kinases. In the TRIO-013/LOGiC trial [phase III, including advanced EGJ(n = 46), esophageal (n = 20) and gastric adenocarcinoma (n = 424)], lapatinib plus capecitabine and oxaliplatin(CapeOX) showed no additional efficacy as the first-line treatment for HER2 positive patients [median OS 12.2 months in CapeOX + lapatinib groups (95% CI 10.6-14.2) vs. median OS 10.5 months in CapeOX groups(95% CI 9.0-11.3), HR 0.91; 95% CI 0.73-1.12; P = 0.35]. In a subgroup analysis of EGJ adenocarcinoma, there was no survival benefit (CapeOX + lapatinib groups vs. CapeOX groups, HR 0.90; 95% CI 0.44-1.85; P = 0.77)[18].
TyTan study [phase III, including advanced EGJ (n = 2) and gastric adenocarcinoma (n = 259)] demonstrated that lapatinib plus paclitaxel did not improve OS in HER2-positive patients compared to paclitaxel alone[median OS 11.0 months in lapatinib plus paclitaxel group (95% CI 9.5-14.5) vs. median OS 8.9 months in paclitaxel alone group (95% CI 7.4-11.1), HR 0.84; 95% CI 0.64-1.11; P = 0.1044][19].
Cetuximab, or panitumumab (anti-EGFR antibody)
Cetuximab is an EGFR antibody, widely used for patients with KRAS wild-type metastatic colorectal cancer[20,21], recurrence or metastatic squamous-cell carcinoma of the head and neck[22], and advanced nonsmall-cell lung cancer[23]. In the EXPAND trial [phase III, including advanced EGJ (n = 144) and gastric adenocarcinoma (n = 747)], the efficacy of adding cetuximab to capecitabine plus cisplatin was examined.However, there was no benefit to adding of cetuximab to chemotherapy compared to chemotherapy alone in the first-line treatment [median PFS 4.4 months in cetuximab plus capecitabine and cisplatin groups(95% CI 4.2-5.5); vs. median PFS 5.6 months in capecitabine and cisplatin alone groups (95% CI 5.1-5.7); HR 1.09; 95% CI 0.92-1.29; P = 0.32]. In a subgroup analysis of EGJ adenocarcinoma, there was no benefit to add cetuximab, either [median PFS 5.6 months in cetuximab plus capecitabine and cisplatin groups vs. median PFS 5.6 months in capecitabine and cisplatin alone groups; HR 1.12; 95% CI 0.73-1.71][24].Panitumumab is a fully human immunoglobulin G2 monoclonal antibody targeting EGFR. In advanced colorectal adenocarcinoma, panitumumab significantly improved PFS[25]. The REAL3 trial [phase III,including advanced EGJ (n = 169), esophageal (n = 220) and gastric adenocarcinoma (n = 167)] revealed no survival benefit of adding panitumumab to epirubicin, oxaliplatin and capecitabine (EOC) chemotherapy[median OS 11.3 months in EOC alone groups (95% CI 9.6-13.0) vs. median OS 8.8 months in panitumumab plus EOC groups (95% CI 7.7-9.8), HR 1.37; 95% CI 1.07-1.76; P = 0.013]. In a subgroup analysis of EGJ adenocarcinoma, the trial revealed no survival benefit of adding panitumumab, either (EOC alone groups vs.panitumumab plus EOC groups, HR 1.27; 95% CI 0.78-2.07)[26].
Rilotumumab, and onartuzumab (MET/HGF inhibitor)
Rilotumumab is a fully human monoclonal antibody that selectively targets the ligand of the MET receptor,hepatocyte growth factor (HGF). In the RILOMET-1 trial [phase III, including advanced EGJ (n = 124), distal esophageal (n = 67) and gastric adenocarcinoma (n = 63)], median OS was 8.8 months (95% CI 7.7-10.2) in the rilotumumab group, compared with 10.7 months (95% CI 9.6-12.4) in the placebo group (HR 1.34, 95% CI 1.10-1.63;P = 0.003), demonstrating that rilotumumab conferred no survival benefit. In a subgroup analysis of EGJ adenocarcinoma, rilotumumab conferred no survival benefit (the rilotumumab group vs. the placebo group,HR 1.28; 95% CI 0.83-1.98)[27].
Onartuzumab is a recombinant, fully humanized, monovalent monoclonal antibody that binds the extracellular domain of MET, blocking interaction with HGF[28]. In METGastric trial [phase III, HER2-negative and MET-positive tumors, including advanced EGJ (n = 130) and gastric adenocarcinoma(n = 432)], no survival benefit was observed in onartuzumab plus mFOLFOX group, compared to placebo plus mFOLFOX (median OS 11.3 months in placebo plus mFOLFOX group vs. median OS 11.0 months in onartuzumab plus mFOLFOX group, HR 0.82; 95% CI 0.59-1.15; P = 0.24). In a subgroup analysis of EGJ adenocarcinoma, no survival benefit was observed in onartuzumab plus mFOLFOX group (median OS not estimable in placebo plus mFOLFOX group vs. median OS 11.0 months in onartuzumab plus mFOLFOX group, HR 1.12; 95% CI 0.58-2.19)[29].
Everolimus (mTOR inhibitor)
Everolimus is an oral mTOR inhibitor. In GRANITE-1 [phase III, including advanced EGJ (n = 187) and gastric adenocarcinoma (n = 656)], everolimus did not significantly improve OS, compared to placebo alone(median OS, 5.4 months in everolimus vs. median OS, 4.3 months in placebo, HR 0.90; 95% CI 0.75-1.08; P =0.124). In a subgroup analysis of EGJ adenocarcinoma, everolimus did not significantly improve OS, either(everolimus vs. placebo, HR 0.84; 95% CI 0.61-1.16)[30].
Trastuzumab emtansine (anti-HER2 antibody)
Trastuzumab emtansine (T-DM1) is anti-HER2 monoclonal antibody consisting of trastuzumab linked to emtansine (DM1), which is a microtubule inhibitor. In GATSBY [phase II/III, including HER2-positive advanced EGJ (n = 110) and gastric adenocarcinoma (n = 235)], there was no superiority of T-DM1 to taxane[median OS 7.9 months with T-DM1 (95% CI 6.7-9.5) vs. median OS 8.6 months with taxane (95% CI 7.1-11.2),HR 1.15; 95% CI 0.87-1.51; P = 0.86]. In a subgroup analysis of EGJ adenocarcinoma, similarly to the above,there was no superiority of T-DM1 to taxane (median OS 7.1 months with T-DM1 vs. median OS 8.5 months with taxane, HR 1.18; 95% CI 0.70-2.01)[31].
Future prospect of molecularly targeted drugs
Although precision medicine still remains developing for the upper gastrointestinal malignancies, there are some new approaches such as VIKTORY, and PANGEA trials. PANGEA is a phase II trial that gastroesophageal tumors are classified into the following six categories (HER2+, MET+, FGFR2+, VEGFR2+,MSI-H, and EGFR+), and then paired specific targeted therapies (trastuzumab, TBD, anti-EGFR antibody ABT-806, TBD2, ramucirumab, and nivolmab) are assigned according to the biomarkers, along with standard chemotherapy[32]. VIKTORY is a screening trial without drug intervention for metastatic GC patients who failed or progressed on first-line chemotherapy, using cancer panel/nanostring CNV and immunohistochemistry[33]. These efforts may create new algorithms in upper gastrointestinal cancers.
IMMUNOTHERAPY
The most advanced of the emerging development in EGJ and gastric adenocarcinoma is immunotherapy.Programmed death protein 1 (PD1), programmed cell death 1 ligand 1 (PD-L1) and cytotoxic T lymphocyte protein 4 (CTLA4) are the key drugs to regulate cellular immune functions. Pembrolizumab and nivolumab,which are being developed as anti-PD1 antibodies, have been examined in clinical trials.
Pembrolizumab
Pembrolizumab is a selective, humanized, high-affinity IgG4-κ monoclonal antibody. By binding to PD1,pembrolizumab block the interaction between PD-1 and its ligands. In the USA, pembrolizumab was approved by the FDA for the treatment of melanoma, non-small-cell lung cancer and head and neck cancer.In a phase Ib trial (KEYNOTE-012), the safety and activity of pembrolizumab was assessed in patients with PD-L1 positive advanced EGJ and gastric adenocarcinoma. The median PFS and the median OS were 1.9 months (95% CI 1.8-3.5) and 11.4 months (95% CI 5.7) respectively[34]. The KEYNOTE-061 is a phase III trial as a second-line therapy for PD-L1-positive patients, comparing pembrolizumab with paclitaxel. The KEYNOTE-062 is phase III trial of pembrolizumab alone or combination with FP or capecitabine vs. FP or capecitabine alone as a first-line therapy for PD-L1-positive patients. Both of these trials are still in progress.
Nivolumab
Nivolumab is a fully human IgG4 monoclonal antibody inhibitor of PD-1. In the ATTRACTION-2 study,which was a randomized phase III trial, investigating the efficacy and safety of nivolumab as a third-line for unresectable advanced and recurrent EGJ and gastric adenocarcinoma regardless of PD-L1 expression.Median OS was 5.26 months (95% CI 4.60-6.37) in the nivolumab group and 4.14 months (95% CI 3.42-4.86)in the placebo group (HR 0.63, 95% CI 0.51-0.78; P < 0.0001), resulting in a new treatment option for these cancers[35]. The other anti PD-L1 antibody, such as avelumab, durvalumab and atezolizumab have been expected to advanced EGJ and gastric adenocarcinoma. Two randomized phase III trials of avelumab in EGJ and gastric adenocarcinoma are undergoing [Table 2].
CheckMate-032 is an ongoing trial, evaluating nivolumab alone, and nivolumab in combination with ipilimumab, for various solid tumors including previously treated advanced EGJ and gastric adenocarcinoma,regardless of PD-L1 expression status. Patients were randomly assigned in to the following three groups,NIVO3 group (nivolumab: 3 mg/kg, once every 2 weeks), NIVO1 plus IPI3 group (nivolumab: 1 mg/kg plus ipilimumab: 3 mg/kg, once every 3 weeks) and NIVO3 plus IPI1 group (nivolumab: 3 mg/kg plus ipilimumab: 1 mg/kg, once every 3 weeks). The median OS were 6.2 months (95% CI 3.4-12.4) in NIVO3 group, 6.9 months (95% CI 3.7-11.5) in NIVO1 plus IPI3 group and 4.8 months (95% CI 3.0-8.4) in NIVO3 plus IPI1 group[36,37]. In addition, CheckMate 649 examining nivolumab plus ipilimumab or nivolumab plus chemotherapy compared with patients receiving chemotherapy alone are also in progress[38]. Utilizing nivolumab in combination with the other agents may be a major option for EGJ and gastric adenocarcinoma.
Future prospect of immunotherapy
Many study reported that PD-L1 expression has been related with poor prognosis and associated with response to immunotherapy[39-42]. On the other hands, only a few studies reported that PD-L1 blockade was effective without PD-L1 expression[35]. These results indicated that PD-L1 is not yet established as a biomarker for PD-L1 inhibitors. Recent reports suggested that host microbiome and tumor and stromal genomic profiles may be related with response to immune checkpoint blockade[9,10]. The diversity andabundance of specific bacterial species in the oral and fecal microbiome enhanced systemic and antitumor immunity[43,44]. For example, in the patients with advanced tumor who received immunotherapy, the use of antibiotics caused poor prognosis. In addition, oral administration of bacteria improved anti-tumor effect[45].Some immune checkpoints, such as lymphocyte activation gene 3 protein (LAG3)[46], T-cell immunoglobulin and mucin domain 3 (TIM3)[47], T-cell immune-receptor with Ig and ITIM domains (TIGIT)[48]are being currently investigated in clinical trials, in order to develop new drugs in the near future.

Table 2. The phase III clinical trials of immunotherapy for esophagogastric junction and gastric adenocarcinoma
CONCLUSION
Global standard treatment for metastatic EGJ and gastric adenocarcinoma is the combination of platinumagents and fluoropyrimidine. The availability of targeted agents such as trastuzumab or ramucirumab,have become a new hope to the patients with this aggressive tumor. Immune checkpoint inhibitors have emerged as a novel therapeutic option. Discovering the best combination of these drugs may lead a dramatic improvement of the prognosis of these aggressive tumors.
DECLARATIONS
Authors’ contributions
Concept, design, literature search and manuscript preparation: Toihata T Concept, design, and manuscript editing: Imamura Y
Manuscript review: Watanabe M, Baba H
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Patient consent
Not applicable.
Ethics approval
Not applicable.
Copyright
© The Author(s) 2018.
 Journal of Cancer Metastasis and Treatment2018年5期
Journal of Cancer Metastasis and Treatment2018年5期
- Journal of Cancer Metastasis and Treatment的其它文章
- The combined analysis of solid and liquid biopsies provides additional clinical information to improve patient care
- Conversion surgery for stage IV gastric cancer
- Treatment strategy for metastatic gastric cancer in Japan
- Anti-oxidation properties of leaves, skin, pulp, and seeds extracts from green papaya and their anticancer activities in breast cancer cells
- AUTHOR INSTRUCTIONS
- Hypoxia in prostate cancer
