Evaluation of possible mechanisms of Cordia dichotoma fruits for hyperlipidemia controlling in Wistar albino rats
Samah A. El-Newary, Abeer Y. Ibrahim, Samir M. Osman, Michael Wink
1Medicinal and Aromatic Plants Researches Department, National Research Centre, El-Buhouth St., Dokki (12622), Giza, Egypt
2Department of Pharmacognosy, Faculty of Pharmacy, October 6 University, Giza, Egypt
3Institute of Pharmacy and Molecular Biotechnology, Heidelberg University, Im Neuenheimer Feld 364, 69120; Heidelberg, Germany
1. Introduction
In cells, the production of reactive oxygen species (ROS) during metabolism is a normal and necessary process. ROS have important physiological functions, some ROS acting as cellular messengers in redox signaling. Oxidative stress is an imponderables between the systemic production of ROS and a biological system’s ability to remove and detoxify the reactive intermediates or to remedy the produced damage, which produces peroxides and free radicals. The peroxides or free radicals have a toxic effect and damage the proteins,lipids, and DNA of cells. Many human diseases have been attributed with oxidative stress, including atherosclerosis, heart failure and myocardial infarction[1]. ROS can damage proteins or change their functions by damaging metal-binding sites and disulfide bonds,fragmentation and cross-linking. An increase in the intracellular generation of ROS plays an essential role in the atherosclerosis incidence[2]. Hypercholesterolemia causes an increment in low density lipoprotein (LDL) in the artery wall, which is followed by oxidative modifications. Therefore, antioxidant substances can stop the initiation and propagation of atherosclerosis[3]. The atherosclerosis process starts by the oxidation of accumulated LDL in arteries. The presence of Ox-LDL is associated with a production of superoxide anion, hydrogen peroxide (H2O2), and peroxynitrite, which make up the evidence for the oxidation of lipids in human atheroma lesions[4].
Many plants have a powerful antioxidant capacity, such as Cordia dichotoma (C. dichotoma) Forst. (Boraginaceae), also known as sebestan plum or soap berry; and it has been known in Egypt as a mokhate[5]. The fruit is divided into a pulpy part and one kernel seed. The kernel seed is surrounded by a viscid sweetish transparent pulp. Traditionally, C. dichotoma has been used for treating anemia, impotence, gastric pain, asthma, mouth ulcers, bronchitis,diarrhea, rheumatism, and dental caries; it has also been used as an anti-inflammatory agent, an anti-oxidant, an anticancer agent, an expectorant, as a demulcent, an anti-arthritic agent, an antidepressant,an anti-ulcer, an anti-fertility, a cosmetic agent and also used in healing wounds. Fruits have ulcer protective, hepatoprotective and woundhealing characteristics, showing antidiabetic and anthelmintic activities as well as hypolipidemic ability[6]. C. dichotoma fruit contains many vital components, such as polyphenols, flavonoids, alkaloids and mucilage[5,6]. Rosmarinic acid, chlorogenic acid, caffeic acid, rutin,quercetin, and kaempferol are the main polyphenols that occur in viscid layer aqueous extract[6]. According to our results which have been published in 2016[6], it was found that the hypolipidemic effect of the aqueous extract of C. dichotoma fruits including decreasing total cholesterol (TC), triglycerides (TG) and low density lipoprotein cholesterol (LDL-C) levels as well as increasing high density lipoprotein cholesterol (HDL-C) level in rats fed on high fat diet for 10 weeks.
So, the current work was carried out to complete the previous study to investigate the possible mechanisms of C. dichotoma fruits extract for hyperlipidemia control in high-fat diet induced hyperlipidemic Wistar albino rats.
2. Materials and methods
2.1. Chemicals
Ferrous chloride, potassium hexacyanoferrate, trichloroacetic acid, 1,1-diphenyl-2-picryl-hydrazyl (DPPH), 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acid)-1,2,4-triazine (ferrozine), nicotinamide adenine dinucleotide, phenazine methosulphate, nitroblue tetrazolium salt,butylatedhydroxytoluene (BHT), ascorbic acid (Vit C), 2,2-azinobis(3-ethylbenzthiazolin-sulphonic acid) diammonium salt, peroxidase,cholesterol and cholic acid were obtained from the Sigma-Aldrich Company (St. Louis, MO, USA). A chow-based commercial diet was purchased from the animal house of National Research Centre, Dokki,Giza, Egypt. Camel fat was obtained from local market. All chemicals used were of analytical grade.
2.2. Plant material and preparation of the extract
Fruits were obtained from Cordia trees in the Sharkia area, Egypt, on August 2015. Fruits were washed and were separated into two parts; the external part (pulp) and internal part (the kernel seed surrounded with a viscid layer enriched with mucilage). Using cold soaking by water the viscid layer was extracted at room temperature for a period of 24 h. The aqueous extract was concentrated in rotary evaporators (Buchi Rotavapor)[6] and then was lyophilized. The remaining powder was kept on -20 ℃ until use.
2.3. In-vitro study
Determination of the antioxidant activity of C. dichotoma extract was carried out with five methods as free radicals scavenging, reducing power, ferrous ion chelating activity, superoxide anion scavenging activity, and total antioxidant capacity. The antioxidant activities of the extract were compared with those of ascorbic acid and BHT which were taken as reference materials. Four concentrations of each material and the extract were used as 1 000, 500, 250 and 100 μg/mL. IC50, the concentration of the extract that inhibited the 50% of free radicals, was calculated using obvious concentrations.
2.3.1. Free radical scavenging activity
The effect of C. dichotoma aqueous extract and standards (ascorbic acid and BHT) on DPPH radicals was determined using the method of Yamaguchi et al.[7].
2.3.2. Reduction capability
The reduction capability of the aqueous extract and standard materials were determined by using the method of Oyaizu[8].
2.3.3. Ferrous ion chelating activity
The ferrous ion chelating effect of the extract and standard compounds were assayed according to Dinis et al.[9].
2.3.4. Superoxide anion scavenging activity
The measurement of the superoxide anion scavenging activity of the extract was based on the method described by Liu et al.[10].
2.3.5. Total antioxidant capacity
The total antioxidant capacity of the extract and standard compounds was estimated based on the methods of Miller and Rice-Evans[11] and Arnao et al.[12].
2.4. In-vivo study
2.4.1. Ethical and animals
The experimental protocol was carried out based on the institutional guidelines for the care and use of laboratory animals, National Research Centre, Dokki, Giza, Egypt. This study was approved by the Medical Research Ethics Committee, National Research Centre, Egypt, under registration No. 16/ 032. The experiment was performed in the animal house of the National Research Centre, Dokki, Giza, Egypt.
Adult male Wistar albino rats (thirty-six), weighing between 140 and 160 g, were used and were maintained at (25±2) ℃, moisture 60%-65% with a 12-h light: 12-h dark cycle. The animals were individually deposited in wire-bottomed metabolic cages and maintained at room conditions. The animals were fed standard laboratory rat food, with free access to tap water. Rats were weighed and randomly assigned to the different experimental groups (six animals per group).
2.4.2. Diet
The standard diet was a chow-based commercial diet containing;4.60% fat, 25.00% crude protein, 4.78% crude fiber and 6.71% crude ash (the diet composition analyzed according to AOAC[13]. High-fat diet was an obvious standard diet with fat modification to 20% using camel fat, and supplemented with 1% cholesterol and 0.25% cholic acid[6].
2.4.3. Experimental design
The animals were acclimatized for 7 d under laboratory conditions before the experiment period. The rats were divided into six groups,each group with six rats.
Group Ⅰ: Rats fed standard diet for four weeks and were kept as a negative control.
Group Ⅱ: Rats fed standard diet and were force fed extract at dosage 0.5 g/kg/day for four weeks and were kept as positive control for this dosage.
Group Ⅲ: Rats fed standard diet and were force fed extract at dosage 1.0 g/kg/day for four weeks and were kept as positive control for this dosage.
Group Ⅳ: Rats fed high-fat diet for four weeks and were kept as a hyperlipidemic control.
Group Ⅴ: Rats fed high-fat diet and were co-administrated extract at dosage 0.5 g/kg/day orally for four weeks and were kept as protective group.
Group Ⅵ: Rats fed high-fat diet and were co-administrated extract at dosage 1.0 g/kg/day orally for four-weeks and were kept as protective group.
After four weeks, the animals were fasted and maintained with tap water over night. To achieve anesthesia rats were injected 87 mg ketamine/ kg of body weight and 13 mg/ xylazine, beginning 10-15 min after simultaneous injection intraperitoneal and lasting 15-30 min.The two drugs were dissolved in normal saline and each rats received 0.2 mL/100 g body weight[14]. Animals were scarified after anesthesia and the blood samples were collected from the retro-orbital plexus as well as the liver for biochemical analysis. Sera were collected after blood centrifugation at 4 000 g for 10 min by using Sigma Laborzentrifugen(Osterode am Harz, Germany). Livers were weighed and were used to prepare liver homogenate using tissue homogenizer ultrasonic, and then centrifuged at 4 000 g, at 4 ℃ for 15 min[15]. Supernatants were removed to use for the determination of antioxidant parameters and oxidative stress parameters.
2.4.4. Nutritional parameters
Over the experimental period, faeces were collected daily and weighed before and after drying; body weight and food intake were also monitored daily. Feed efficiency is evidence of the extent of benefit from ingested food, or how much of the food eaten has benefitted the animal was calculated. Diets and faeces protein, fat and TC were analyzed. Crude protein and fat were determined according to the methods adapted in AOAC[13]. The diet and faecal TC was estimated by the procedure developed by Folch et al.[16]. TC concentration was analyzed with the same enzymatic kits as used in the serum analysis.
2.4.5. Biochemical analysis
The lipid profile was determined in serum samples. The determination of TC, HDL-C and TG was carried out according to the methods of Allain et al.[17], Naito and Kaplan[18] and Fossati and Prencipe[19],respectively using kits obtained from Biodigonestic Co (Egypt). The calculation of LDL-C [LDL-C= TC-(VLDL + HDL)], very low density lipoprotein cholesterol (VLDL-C) (VLDL-C= TG/5 and the risk ratio(LDL-C/ HDL-C) was carried out according to Friedewald et al.[20],Naito and Kaplan[18] and Kikuchi et al.[21] respectively.
Glutathione (GSH) concentration and antioxidant enzymes activities of liver, such as glutathione reductase (GR), glutathione-S-transferase(GST), glutathione peroxidase (GPx), catalase (CAT) and superoxide dismutase (SOD), were estimated in liver homogenate according to Griffith[22], Goldberg and Spooner[23]; Habig et al.[24]; Paglia and Valentine[25]; Beers and Sizer[26] and Fridovich[27], respectively using kits obtained from Biodigonestic Co (Egypt). Oxidative stress parameters: malondialdehyde (MDA) and H2O2in the liver were analyzed according to Ohkawa et al.[28] and Chance and Maehly[29],respectively using kits obtained from Biodigonestic Co (Egypt).
Liver TC and TG were extracted, using the procedure developed by Folch et al.[16]. The concentrations of TC and TG in the liver were analyzed with the same enzymatic kits as used in the serum analysis.
2.5. Statistical analysis
All studied data were statistically analyzed using the Co-Stat 6.303 Software Computer Program 2004 hypothesis; testing methods included one-way analysis of variance (ANOVA) test.
3. Results
3.1. In-vitro results
3.1.1. DPPH radical scavenging activity
C. dichotoma extract showed weak dose-dependent radical scavenging.A high concentration of extract, 1 000 μg/mL, showed low DPPH radical scavenging, compared with ascorbic acid and BHT, respectively.IC50value of the extract was 2 235 μg/mL, compared with 41.25 and 48.66 μg/mL by ascorbic acid and BHT, respectively (Table 1).
3.1.2. Reducing power
In the reducing power assay, the reducing power of C. dichotoma extract was higher than that of ascorbic acid and BHT (Table 1).
3.1.3. Ferrous ion chelating activity
The ferrozine method was used to determine the ability of C.dichotoma extract to bind to Fe+2ion catalyzing oxidation; as data shown in Table 1. The extract showed Fe+2chelation ranged between 23.86% at the lowest concentration (100 μg/mL) to 71.30% at the highest one (1 000 μg/mL). IC50value was 522.90 μg/mL, compared with IC50of ascorbic acid (35.80 μg/mL) and BHT (42.10 μg/mL).
3.1.4. Superoxide anion radical scavenging activity
C. dichotoma extract showed potent scavenging properties against superoxide radicalsin a concentration-dependent manner(Table 1). Extract (IC50: 178.41 μg/mL) was required to inhibit 50%generated in a phenazine methosulphate-nicotinamide adenine dinucleotide system, compared with 55.50 μg/mL for ascorbic acid and 52.00 μg/mL for BHT.
3.1.5. Total antioxidant capacity
The total antioxidant capacity of the extract was evaluated according to the ABTS/ H2O2discoloration method, corresponding to standard material (ascorbic acid and BHT) data in Table 1. C. dichotoma at different concentrations displayed a noteworthy antioxidant capacity.The total antioxidant capacity, represented as IC50of the extract, was 37.43 μg/mL compared with the natural antioxidants, ascorbic acid(41.88 μg/mL), and synthetic ones BHT (33.10 μg/mL).
3.2. In-vivo results
3.2.1. Nutritional parameters of rats co-administrated C.dichotoma extract
3.2.1.1. Total body weight gain (g/4 weeks)
Administration of high-fat diet elevated the total body weight gain of hyperlipidemic control rats [(95.60±3.40) g/4 weeks], compared to that of the negative control[(59.60±1.30) g/4 weeks] (Table 2). The co-administration of the extract was in two dosages: 0.5 and 1.0 g/kg; this reduced the total body weight gain of rats fed high-fat diet by(79.80±3.40) and (60.14±6.37) g/4 weeks, respectively, compared with the hyperlipidemic control. In contrast, the C. dichotoma extract insignificantly increased the total body weight gain of positive control rats compared to the negative control.
3.2.1.2. Total food intake (g/4 weeks)
Rats fed high-fat diet consumed more to reach 10.61% over that consumed by the negative control rats [(367.7±9.9) g/4 weeks]. Co-administration of C. dichotoma reduced the amount of high-fat diet which rats consumed [(383.9±11.5) and (381.7±11.5) g/4 weeks,respectively] at different dosages, compared with the hyperlipidemic control. The total feed intake of positive controls, which was administered C. dichotoma extract, nearly remained the same as that of the negative control.

Table 1 Antioxidants characters of C. dichotoma extract and standard compounds.

Table 2 Effect of C. dichotoma extract on nutritional parameters in rats fed high-fat diet for 4 weeks.
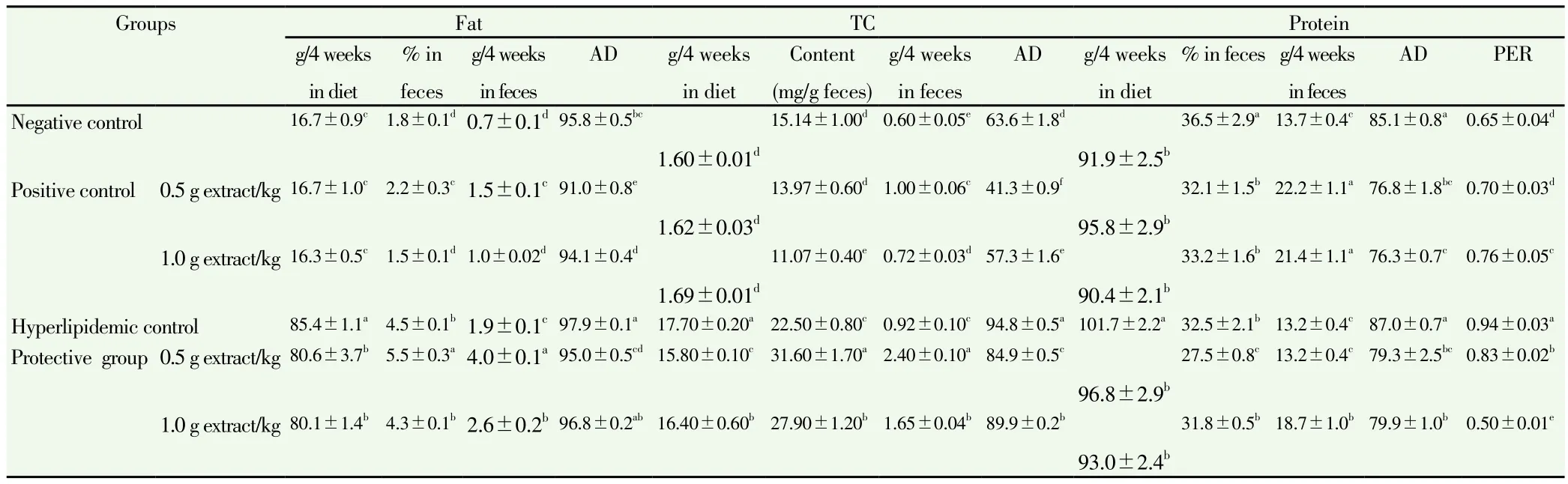
Table 3 Effect of C. dichotoma extract on fat, TC, and protein intake and faecal excretion of rats fed high-fat diet for four weeks.
3.2.1.3. Feed efficiency
Feed efficiency was significantly raised as a response to the administration of high-fat diet (0.24±0.02)%, compared with the negative control at (0.16±0.01)%. High dosages of C. dichotoma extract reduced feed efficiency significantly (0.16±0.03)%, while low dosages did not change it significantly compared to the hyperlipidemic control. The feed efficiency of positive control rats did not show any significant change compared with the negative control.
3.2.1.4. Total faecal excretion (g/4 weeks)
Neither the fresh nor dry weight of faecal excretion significantly changed with the administration of high-fat diet compared with that of the negative control: (68.8±1.3) and (37.6±2.0) g/4 weeks(Table 2). Fresh faecal excretion was significantly elevated by the coadministration of C. dichotoma extract with two dosages, 0.5 and 1.0 g/kg, by about (113.0±3.7) and (101.9±2.5) g/4 weeks, respectively,compared to the hyperlipidemic control. Dry faecal excretion was also significantly higher in the rats which were fed high-fat diet and coadministrated C. dichotoma extract [(72.7±3.5) and (58.8±2.5) g/4 weeks, respectively]. In the positive control rats, C. dichotoma extract with two dosages (0.5 and 1.0 g/kg) raised fresh and dry faecal excretion compared to the negative control.
3.2.2. Intake and excretion of fat, TC and protein
3.2.2.1. Fat
A significant increase was recorded in the fat intake during 4 weeks(+411.38%), fat faecal excretion (+171.43%), fat faecal percentage(+150.0%), and apparent fat digestibility (AD) (+2.20%) in the hyperlipidemic control as a response to the high-fat diet administration,in comparison with the negative group (Table 3).
Co-administration of C. dichotoma extract at 0.5 and 1.0 g/kg in concurrence with feeding high-fat diet significantly reduced fat intake[(80.6±3.7) and (80.1±1.4) g/4 weeks, respectively], compared with that of the hyperlipidemic control rats [(85.4±1.1) g/4 weeks](P<0.05). The fat intake of positive control rats force fed C. dichotoma at two dosages was not significantly influenced by C. dichotoma administration compared with the negative control.
Administration of low dosage of the C. dichotoma extract (0.5 g/kg)magnified fat faecal excretion percentages in rats fed high-fat diet or fed a standard diet, with (5.5±0.3)% and (2.2±0.3)%, respectively,compared with fat excreted in the hyperlipidemic and the negative control rats, respectively. High dosage did not affect the fat faecal excretion percentages of rats fed high-fat diet and positive control with respect to each comparison group.
Rats fed high-fat diet and administrated with C. dichotoma at two dosages [(4.0±0.1) and (2.6±0.2) g/4 weeks, respectively] excreted fat much higher than that excreted by rats of the hyperlipidemic control[(1.9±0.1) g/4 weeks]. The same was recorded in the positive control rats, where C. dichotoma extract elevated fat faecal excretion, compared with the negative control, P<0.05.
C. dichotoma administration decreased the fat AD either in rats fed high-fat diet or in rats of positive control, compared to each control(P<0.05).
3.2.2.2. TC
The TC intake was much higher in animals fed high-fat diet and coadministrated low and high dosage of C. dichotoma extract (Table 3), with (15.80±0.10) and (16.40±0.60) g/4 weeks, respectively in comparison with TC intake of the hyperlipidemic control[(17.70±0.20) g/4 weeks]. The TC intake by the positive control rats was close to that of the negative control.
Both TC faecal content and TC faecal excretion were significantly increased when rats were fed high-fat diet and administrated with C.dichotoma extract, compared to the hyperlipidemic control. TC faecal content and excretion of rats force-fed the low dosage of the extract was much higher, with (31.6±1.7) mg/g faeces and (2.4±0.1) g/4 weeks, respectively, than that of rats co-administrated the high dosage[(27.9±1.2) mg/g faeces and (1.65±0.04) g/4 weeks, respectively]compared with hyperlipidemic control [(22.5±0.8) mg/g faeces and (0.92±0.10) g/4 weeks, respectively]. Compared to the negative control, C. dichotoma extract with two dosages elevated the TC faecal excretion of the positive control rats.
Therefore, C. dichotoma extract significantly decreased the AD of TC on rats fed high-fat diet or positive control rats in comparison with the hyperlipidemic and negative controls, respectively.
3.2.2.3. Protein
Rats fed high-fat diet registered a significant elevation of protein intake and protein efficiency ratio (PER) in comparison with the negative control value. Only the percentage of the faecal excretion of protein was reduced significantly, corresponding to the negative control (Table 3).
The faecal excretion of protein and the AD of protein did not change significantly on the administration high-fat diet. C. dichotoma extract at two dosages (0.5 and 1.0) g/kg reduced protein intake of rats fed highfat diet as a result of decreased food intake basically. No significant difference was noticed between the protein intake of all positive control groups and the negative control.
Low dosages of extract significantly decreased the faecal percentages of protein in hyperlipidemic rats, while high dosages did not show this effect compared with the hyperlipidemic control. In positive control of rats administrated C. dichotoma extract with two dosages, the faecal percentage of protein was decreased compared with negative control(P<0.05).
In addition, the faecal excretion of protein was significantly elevated in hyperlipidemic rats force fed high dosages of extract, while two dosages of extract significantly elevated faecal excretion of protein in positive control rats as a result of increasing faecal excretion in general.
According to the data mentioned above, C. dichotoma extract at low and high dosages remarkably decreased AD in hyperlipidemic rats and positive control rats as a response to elevated faecal excretion, in comparison with hyperlipidemic and negative control, respectively.
C. dichotoma extract decreased the PER of hyperlipidemic rats as a result of decreasing total body weight. In an opposite trend of positive control rats, force feeding of high dosages significantly increased PER of rats as a response to total body weight gain in positive control rats,compared with the negative control.
3.2.3. Hypolipidemic parameters of serum and liver of rats co-administrated C. dichotoma extract
3.2.3.1. Serum lipid pattern
The group fed high-fat diet caused a significant hyperlipidemia (data presented in Table 4). Serum lipid profile of hyperlipidemic control rats showed elevated levels of serum TC, TG and VLDL-C and LDL-C and decreased level of HDL-C compared with those of the negative control. Therefore, the risk ratio of hyperlipidemic control[(7.23±0.42)%] were elevated significantly compared to the negative control [(0.73±0.09)%].
TC levels were much lower in rats fed high-fat diet and coadministrated C. dichotoma extract at low and high dosages with(125.30±3.69) and (138.29±2.87) mg/dL, respectively, in comparisonwith (234.39±4.92) mg/dL TC in the hyperlipidemic control rats. Also,TC of the positive controls was lower than that of the negative control at(73.56±3.50), (77.75±1.60) and (87.89±2.69) mg/dL, respectively,P<0.05.
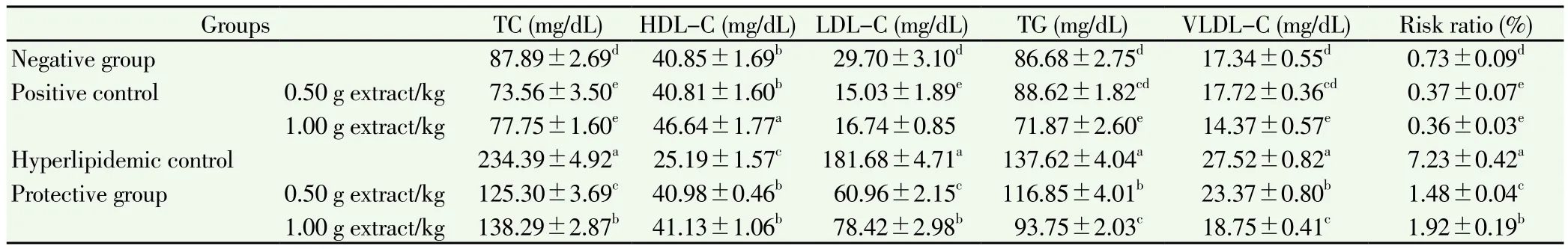
Table 4 Effect of force-feeding of C. dichotoma extract on lipid profile in rats fed high-fat diet after 4 weeks.
The TG and VLDL-C of animals fed high-fat diet and coadministrated with C. dichotoma extract at low and high dosages were significantly decreased with (116.85±4.01) and (93.75±2.03) mg/dL for TG and (23.37±0.80) and (18.75±0.41) mg/dL for VLDL-C,respectively, compared with those of the hyperlipidemic control[(137.62±4.04) and (27.52±0.82) mg/dL]. Low extract dosages did not change the TG and VLDL-C of positive control rats, while high dosages reduced these significantly, with (71.87±2.60) and(14.37±0.57) mg/dL, respectively, compared with the negative control[(86.68±2.75) and (17.34±0.55) mg/dL] (P<0.05).
On the other hand, HDL-C levels were much higher in the animals ingested high-fat diet and co-administrated with two extract dosages of 0.5 and 1.0 g/kg, with (40.98±0.46) and (41.13±1.06) mg/dL, respectively,in comparison with (25.19±1.57) mg/dL in the hyperlipidemic control.In the positive control rats, high dosages of C. dichotoma extract raised HDL-C significantly, while the low dosage had not significant effect on HDL-C compared with the negative group.
Based on the previous results, LDL-C levels were significantly lowered,when rats were force fed with 0.5 and 1.0 g/kg extract and fed high-fat diet, to (60.96±2.15) and (78.42±2.98) mg/dL, respectively, compared to (181.68±4.71) mg/dL showed in the hyperlipidemic control. LDL-C levels of the positive control rats showed the nearly same results in comparison with the negative group.
Accordingly, the risk ratio was significantly reduced, when hyperlipidemic rats were force-fed by two dosages of extract; (1.48±0.04)% and (1.92±0.19)%,respectively, compared with the hyperlipidemic control (7.23±0.42)%. The risk ratios of positive control rats receiving low and high dosages of extract were reduced significantly compared with that of the positive control(P<0.05).
3.2.3.2. Hepatic cholesterol and TG
Administration of high-fat diet significantly increased relative weight of rat’s liver compared to that of the negative control. C.dichotoma extract did not exert an effect on relative weight of liver of rats fed standard diet or high-fat diet compared with the negative or hyperlipidemic controls.
In comparison with animals administrated a standard diet, rats fed high-fat diet showed significant elevation of hepatic TC and TG,respectively (Table 5). The TC and TG stored in the hepatic tissue were much lower in the rats fed high-fat diet and co-administrated C. dichotoma extract in low and high dosages [(44.84±2.63) and(49.25±1.49) mg/g liver tissue for TC and (50.88±2.51) and(55.47±4.22) mg/g liver tissue for TG, respectively] compared to(83.04±4.43) and (84.13±4.19) mg/g liver tissue that were recorded in the hyperlipidemic control, P<0.05.
In positive control rats, neither TC nor TG changed significantly when rats were administrated extract with two dosages 0.5 and 1.0 g/kg compared with the negative group.
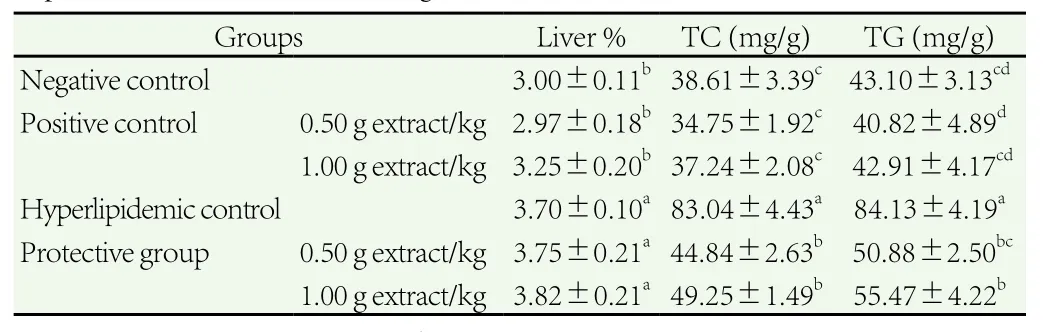
Table 5 Effect of force-feeding of C. dichotoma extract on relative weight of liver and hepatic TC and TG in rats fed high-fat diet.
3.2.4. Antioxidant parameters of liver of rats coadministrated C. dichotoma extract
Liver GSH concentration and antioxidant enzymes activities,GR, GST, GPx, CAT and SOD in all groups were documented in Table 6. Feeding high-fat diet caused a significant reduction of GSH concentration and GR, GST, GPx, CAT and SOD activities in comparison wiht the negative group (P<0.05).
Co-dminstration of the extract restored GSH concentration of hyperlipidemic rats higher than GSH of hyperlipidemic control by about 73.41% and 82.96% with low and high dosage respectively.Significant increase was observed in GSH of rats fed standard diet and co-adminstrated the extract compared with the negative control.
The GR activity was much higher in rats fed high-fat diet and coadministrated with low and high dosages of extract, with (7.83±0.50)and (8.26± 0.32) μmol/mg protein/ min, respectively, compared with(4.52±0.52) μmol/mg protein/min of the hyperlipidemic control. A moderately higher GR activity was observed in the positive control rats[(9.65±0.52) and (8.96±0.33) μmol/mg protein/min at two dosages,respectively] compared with the negative group: (6.85±0.42) μmol/mg protein/min.
The GST activity was significantly increased in rats fed high-fat diet and co-adminstarted C. dichotoma extract at low and high dosages[(3.95±0.25) and (6.34±0.23) μmol/mg protein/min, respectively]compared to the hyperlipidemic control: (3.19±0.12) μmol/mg protein/min. GST activity in the positive control rats was significantly increased, compared with negative control.
GPx exhibited an elevated activity in rats of protective groups[(2.46±0.24) and (3.36±0.15) μmol/mg protein/min at two dosages,respectively] compared with the hyperlipidemic control. The same increased activity of GPx was also observed in positive controls when GPx in negative control was (3.99±0.32) μmol/mg protein/min.
CAT activity was significantly increased when rats were coadminstrated C. dichotoma extract. The CAT activity of rats fed high-fat diet was increased by low and high dosages of extract, with(12.80±0.86) and (15.09±0.47) U/mg protein, respectively, in comparison with (4.66±0.11) U/mg protein of CAT activity of the hyperlipidemic control. In positive control rats, a high dosage of 1.0 g/kg caused a remarkable increment in CAT activity, compared with the negative control.
The SOD activity was also significantly enhanced in rats of protective groups by about +236.27% and +249.67%, respectively, in comparison with the hyperlipidemic control: (3.06±0.18) U/mg protein. The same observation was recorded in positive control rats; SOD activity significantly increased by about +38.36% and +26.14%, respectively,compared with the SOD activity in the negative control: (7.69±0.15)U/mg protein.
3.2.5. Oxidative stress parameters of liver of rats coadministrated C. dichotoma extract
Compared with negative control, feeding rats on high-fat diet caused oxidative stress in liver cells and it was showed by a significant increment in MDA and H2O2concentrations, which were the powerful oxidants in cells (Table 7).
The force-feeding of C. dichotoma extract at low and high dosages showed a remarked decrease in H2O2concentration of rats fed high-fat diet [(8.51±0.76) and (5.71±0.66) mmol/g liver tissue, respectively]in comparison to the hyperlipidemic control: (36.37±2.36) mmol/g liver tissue. In positive control rats, extract significantly decreased H2O2concentration compared to the negative group.
MDA, the lipid peroxidation biomarker of rats fed high-fat diet and co-administrated C. dichotoma extract at low and high dosages was significantly reduced by about 51.37% and 42.14%, respectively,compared with the hyperlipidemic control: (8.78±0.48) mmol/g liver tissue. Also, MDA concentration of positive control rats were significantly decreased by about 49.26% at low dosages of extract compared with negative control.
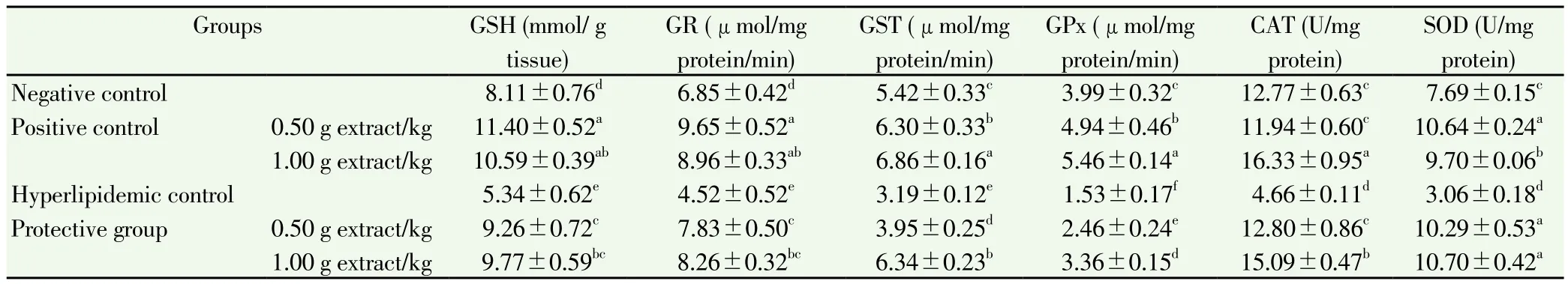
Table 6 Effect of C. dichotoma extract on GSH concentration and antioxidant enzymes activities in liver homogenate of hyperlipidemic rats.
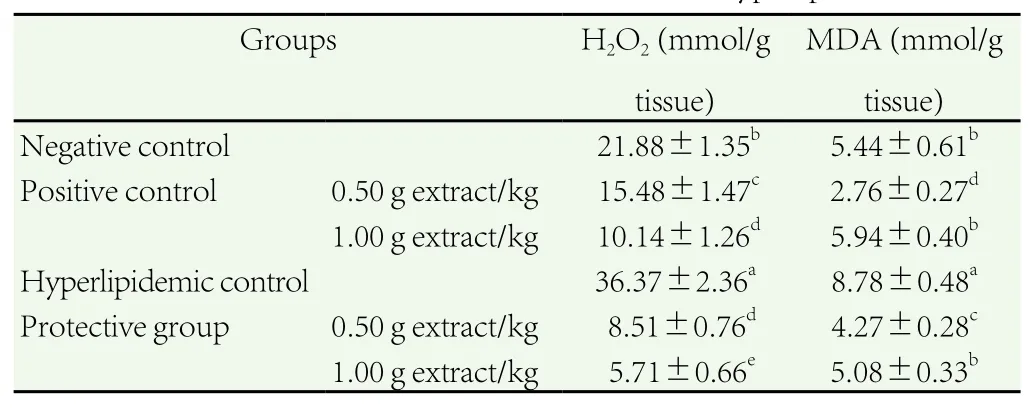
Table 7 Effect of C. dichotoma extract on liver oxidative stress of hyperlipidemic rats.
4. Discussion
This study was conducted to complete our previous study on the hypolipidemic effect of C. dichotoma aqueous extract of the viscid sweetish transparent layer, which surrounds the kernel seeds. We studied the hypolipidemic mechanism of this extract. The rats fed highfat diet only was characterized by elevated TC, TG, VLDL and LDL levels and a reduced HDL level, compared to normal lipid profile of negative control.
Feeding rats on high-fat diet showed a significant rise in total body weight and total food intake, and did not affect the fresh and dry weight of faecal excretion. The force-feeding of the extract decreased the total gain in body weight and the total food intake, while both the fresh and dry weights of faecal excretion of rats fed high-fat diet and coadminstrated the extract were elevated. The positive rats showed the same increase in fresh and dry faecal excretion weight, but did not show any significant effect on body weight gain or feed intake. These data are supported by those of Lecumberri et al.[30] on cocoa fiber, and El-Newary et al.[6] on C. dichotoma.
The decrease in body weight of rats fed high-fat diet and coadminstrated C. dichotoma extract may be elucidated by a decreasing food, fat, and TC intake with an increase in faecal excretion and fat,and TC faecal excretion. This reduction may be associated with the mucilage content in the extract: 20.18% as viscous dietary fibers[6],and a reduction of the availability of diet energy, which is decreased by increasing dietary fiber consumption. Viscous fiber increased the food glueyness and size in the stomach, which induces satiety and sense of fullness and a delay in stomach drainage; this all resulted in decrease in caloric intake[31]. Metabolizable energy is the difference between the total income energy and the outcome energy through faeces, urine and combustible gases which is decreased by increasing dietary fiber consumption. Undoubtedly, phenols such as caffeine suppress appetite and equally stimulate thermogenesis, a physical reaction that increases the number of calories the body burns. Caffeine may cause temporary weight loss due to its diuretic effect. Quercetin causes a reduction in body weight and liver fat accumulation[32]. C. dichotoma extract contains quercetin and caffeic acid at about 4.90 and 13.5 mg/g extract,respectively[6].
The decrease in amount of ingested food might also be attributed to the C. dichotoma extract mucilage. Soluble fiber as mucilage was not absorbed in the small intestine, and fermented in the large intestine,which produces glucagon-like peptide (GLP-1) and peptide YY (PYY).Both GLP-1 and PYY are gut hormones, which affect satiety. Viscous fibers have been shown to lead to a simple distention of the gastric antrum. Therefore, the feeling of satiety was elevated and stopping of eating through meal times. Viscous fiber delayed a postprandial rise of ghrelin, resulting in the slow absorption of glucose and amino acids[33]. Alexander et al.[34] have reported that alkaloids cause dryness of the mucosa in the upper gastrointestinal tract, also antagonizing the muscarinic acetylcholine receptors, finally leading to inhibition on acetylcholine function. Since acetylcholine is the transmitter answerable for the peristaltic and segmentation movements in the small intestine,blockage of such receptors could cause delay, the slowing down of activities or blocking of the smooth muscle contraction[34]. The extract of C. dichotoma contains 6.87% total alkaloids[6].
The administration of C. dichotoma extract caused significant improvement in the lipid profile of rats fed high-fat diet and coadminstrated extract with two doses towards normalization. Significant drops in TC, LDL-C, TG and VLDL-C, as well as an elevation of HDL-C were recorded. The risk ratio of these rats was significantly diminished. The lipid profiles of positive control rats were improved significantly, too. These results were in accordance with those of El-Newary[35] on Portulaca oleracea stems, and El-Newary et al.[6] on C.dichotoma fruits extract.
This lipid profile amelioration of rats fed high-fat diet and coadminstrated the extract may be due to the reduction of digested fat and TC. Therefore, appendant digestibility of fat and TC was decreased.The same observation was registered in positive rats. We concluded that fat and TC in the diet are not absorbed in the intestines, and hence the delivery from them to the liver is small. The TC and TG of liver tissue significantly lessened when positive rats and rats fed high-fat diet were force-fed C. dichotoma extract. These results may be due to the content of C. dichotoma extract from polyphenols, flavonoids, alkaloids and
mucilage, as well as its improved effect on HDL-C.
Mucilage formed a viscous matrix that trapped bile salts in the gut,forcing the liver to use more cholesterol to synthesis more bile acids.Mucilage reduced the digestion and absorption of dietary fats, causing a decrease in cholesterol delivery to the liver by chylomicron remnants.This caused up-regulation of the LDL receptor and decreased lipoprotein secretion to maintain cholesterol homeostasis in the liver.Mucilage was not digested in the stomach and passed through the small intestines without absorption, but it was fermented as a work of colonic microflora, composing short-chain fatty acids like acetic,propionic, and butyric acids. Propionates hinder cholesterol and fatty acids synthesis in the liver[30]. The extract under study contains 20.18%mucilage.
On the other hand, the extract contains 2.47% flavonoids which demounted as a hypolipidemic[6]. Quercetin, kaempferol and catechin flavonoids are documented as hypolipidemic agents. Quercetin has antidyslipidemia effect, induces lipolysis activity, reduces oxidative stress,and up-regulates the adipocyte gene expression which increases the lipids’ beta oxidation[36]. Quercetin suppressed LDL oxidation and exerted significant veso-relaxation[37]. Kaempferol reduced oxidative stress[38], preventing arteriosclerosis by the inhibition of LDL oxidation.Flavonoids inhibit acyl coenzyme A, cholesterol O-acyltransferase and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase in rats[39]. C. dichotoma extract contains quercetin and kaempferol at about 4.90 and 24.10 mg/g extract, respectively[6].
Polyphenols lowered serum TG levels and contributed to the inhibition of pancreatic lipase, which decreased the intestinal absorption of TG and secretion of apolipoprotein-B, and raised lipoprotein lipase ability[30]. The extract contains 5.52% polyphenols[6]. The lipoprotein lipase has an important role in lipid metabolism, it hydrolyzes the core TG from circulating chylomicrons and VLDLs. Lipoprotein lipase activity is positively correlated with the intake of antioxidants[39].
The protective effect of C. dichotoma may due to its effect on HDL-C.HDL-C represents a key cardioprotective factor which is given its role in reverse cholesterol transport, its effects on endothelial cells, and its antioxidant activity. Adminstration of C. dichotoma extract restored HDL-C of rats fed high-fat diet within normal levels[40].
In the cardiovascular system, cells constantly generate ROS that are utilized as signaling molecules. Oxidant stress promotes atherosclerosis.Oxidant stress causes endothelial dysfunction; activates inflammation,immune responses, and thrombus formation; oxidizes lipids; and initiates a cascade of vascular events that is permissive for the formation of atherosclerotic plaques. The endothelial dysfunction increases the permeation of LDL through the intima layer, resulting in oxidation and formation of atherosclerotic damage. The complexity of the cardiovascular antioxidant system is highlighted by the enzymatic and non-enzymatic antioxidants that reduce cellular ROS. There are a several key cellular and circulating antioxidant systems, including the superoxide
dismutases, glutathione peroxidases, and catalase that collectively reduce superoxide/hydrogen peroxide (or lipid hydroperoxides) to water(or lipid hydroxides). There are also many important small-molecule antioxidants such as reduced GSH[41,42]. C. dichotoma extract showed a high to moderate reducing power, Fe+2chelation ability, free radical scavenging and O-2scavenging activity in vitro. Also, adminsteration of C. dichotoma extract with two doses ameliorated oxidative stress and enhanced antioxidant defence system in rats fed high-fat diet and coadminstrated the extract. These results agreed with that of Singh et al.[43]on Cinnamomum tamala.
We found that the C. dichotoma extract with two doses (0.5 and 1.0 g/kg/day) normalized lipid profile of rats fed on high-fat diet, and the risk ratio of these rats also significantly decreased. The protective effect of extract against hyperlipidemia could be due to the increment of faecal excretion and fat, and TC in the faecal excretion which indicated missing absorption of fat, and TC in intestine. The extract lowered the ability of rats fed high-fat diet to ingest and absorb fat and cholesterol,and enhanced the ability to get rid of them in faecal excretion.
Conflict of interest statement
The authors would like to confirm the absence of any conflict of interest associated with the implementation or the publication of this work.
[1] Dean OM, Van den BM, Berk M, Copolov DL, Mavros C, Bush AI. N-acetyl cysteine restores brain glutathione loss in combined 2-cyclohexene-1-one and D-amphetamine-treated rats: Relevance to schizophrenia and bipolar disorder. Neurosci Lett 2011; 499(3): 149-153.
[2] Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: An overview. Free Radic Biol Med 2000; 28(12): 1815-1826.
[3] Di Carlo G, Mascolo N, Lzzo AA, Capasso F. Flavonoids old and new aspects of class of natural therapeutic drugs. Life Sci 1999; 65(4): 337-353.
[4] Griendling KK, Fitz-Gerald GA. Oxidative stress and cardiovascular injury:Part Ⅰ: Basic mechanisms and in vivo monitoring of ROS. Circulation 2003;108(16): 1912-1916.
[5] Sulieman AM, El-Newary SA. The possible hypolipidemic effect of Cordia dichotoma Forst. pulp in normal and high-fat diet-fed rats. World J Dairy Food Sci 2014; 9: 260-271.
[6] El-Newary SA, Sulieman AM, El-Attar SR, Sitohy MZ. Hypolipidemic and antioxidant activity of the aqueous extract from the uneaten pulp of the fruit from Cordia dichotoma in healthy and hyperlipidemic Wistar albino rats. J Nat Med 2016; 70(3): 539-553.
[7] Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1,-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem 1998; 62(6): 1201-1204.
[8] Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 1986; 44: 307-315.
[9] Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives(acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994; 315(1): 161-169.
[10] Liu F, Ooi VE, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci 1997; 60(10): 763-771.
[11] Miller NJ, Rice-Evans CA. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink. Food Chem 1997; 60(3): 331-337.
[12] Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 2001; 73(2): 239-244.
[13] AOAC. Official methods of analysis. USA: Washington D.C; 2000.
[14] Van Pelt LF. Ketamine and xylazine for surgical anesthesia in rats. J Am Vet Med Assoc 1977; 171(9): 842-844.
[15] Ighodaro OM, Omole JO, Uwaifo AO. Effects of chronic ethanol administration on body weight, reduced glutathione (GSH), malondialdehyde(MDA) levels and glutathione-s-transferase activity (GST) in rats. New York Sci J 2010; 3: 39-47.
[16] Folch J, Lees M, Sloan-Stanley GH. A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497-509.
[17] Allain CC, Poon LS, Chan CS, Richand W, Paul C. Enzymatic determination of total cholesterol. Clin Chem 1974; 20: 470-474.
[18] Naito HK, Kaplan AQ. High-density lipoprotein (HDL) cholesterol clinical chemistry. The C.V. Mosby Co. St Louis: Toronot. Priceton 1984; 437:1207-1213.
[19] Fossati P, Prencipe L. Enzymatic determination of triglycerides. Clin Chem 1982; 28: 2077.
[20] Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18(6): 499-502.
[21] Kikuchi HH, Onodera N, Matsubara S, Yasuda E, Chonan O, Takahashi R, et al. Effect of soy milk and bifidobacterium fermented soy milk on lipid metabolism in aged ovariectomized rats. Biotech Biochem 1998; 62(9):1688-1692.
[22] Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinyl pyridine. Anal Biochem 1980; 106(1):207-212.
[23] Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70(1): 158-169.
[24] Goldberg DM, Spooner RJ. Assay of glutathione reductase. In: Bergmeyer HU. (ed.) Methods of enzymatic analysis. 3rd ed. Florida: Verlog Chemie,Deerfiled Beach; 1983, p. 258-265.
[25] Habig WH, Pabst MI, Jacoby WB. Glutathione-S-transferase. J Biol Chem 1974; 249: 7130-7139.
[26] Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of H2O2by catalase. J Biol Chem 1952; 195: 133-140.
[27] Fridovich I. Superoxide dismutases. Encyclo Biol Chem 2013; 44(10): 352-354.
[28] Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95(2): 351-358.
[29] Chance B, Maehly AC. Assay of catalase and peroxidases. Method Enzymol 1955; 11: 764-775.
[30] Lecumberri E, Goya L, Mateos R, Alía M, Ramos S, Izquierdo-Pulido M, et al. A diet rich in dietary fibre from cocoa improves lipid profile and reduces malondialdehyde in hypercholesterolemic rats. Nutrition 2007;23(4): 332-341.
[31] Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients 2010; 2(12): 1266-1289.
[32] Lee KH, Park E, Lee HJ, Kim MO, Cha YJ, Kim JM, et al. Effects of daily quercetin-rich supplementation on cardiometabolic risks in male smokers.Nutr Res Pract 2011; 5(1): 28-33.
[33] Salim R, F Nazir, Yousf N. Dietary fibre and its effect on health. Inter J Res Anal Rev 2017; 4: 360-362.
[34] Alexander J, Benford D, Cockburn A, Cravedi JP, Dogliotti E. Tropane alkaloids (from Datura sp) as undesirable substances in animals feed.Scientific opinion of the panel on contaminants in the food chain. EFSA J 2008; 69: 1-55.
[35] El-Newary SA. The hypolipidemic effect of Portulaca oleracea L. stem on hyperlipidemic Wister Albino rats. Ann Agr Sci 2016; 61(1): 111-124.
[36] Burns J, Gardner PT, O’Neil J, Crawford S, Morecroft I, McPhail DB,et al. Relationship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J Agr Food Chem 2000; 48(2): 220-230.
[37] Singh R, Singh B, Singh S, Kumar N, Kumar S, Arora S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del.Toxicol In Vitro 2008; 22(8): 1965-1970.
[38] Kurowska N, Borradaile M, Spence JD, Carroll KK. Hypocholesterolemic effects of dietary citrus juices in rabbits. Nut Res 2000; 20(1): 121-129.
[39] Yang RL, Le G, Li A, Zheng J, Shi Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet.Nutrition 2006; 22(11-12): 1185-1191.
[40] Lopes RHO, Macorini LFB, Antunes KÁ, Espindola PPT, Alfredo TM, Rocha PS, et al. Antioxidant and hypolipidemic activity of the hydroethanolic extract of Curatella americana L. Leaves. Oxid Med Cell Longev 2016; 2016(5): 1-6.
[41] Jane A, Leopold MD. Antioxidants and coronary artery disease: From pathophysiology to preventive therapy. Coron Artery Dis 2015; 26(2): 176-183.
[42] Pellegrino D. Antioxidants and cardiovascular risk factors. Diseases 2016;4(1): 11.
[43] Singh V, Singh SP, Singh M, Kumar A. Evaluation of antioxidant,hypoglycemic and hypolipidemic effects of the phytoconstituents of Cinnamomum tamala in rats. Indian J Pharm Sci 2018; 80: 161-172.
 Asian Pacific Journal of Tropical Biomedicine2018年6期
Asian Pacific Journal of Tropical Biomedicine2018年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Probiotic based therapy for atopic dermatitis: Outcomes of clinical studies
- Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators
- Antifungal and cytotoxic activities of extracts obtained from underutilised edible tropical fruits
- Effects of black chokeberry extracts on metastasis and cell-cycle arrest in SK-Hep1 human liver cancer cell line
