Effects of black chokeberry extracts on metastasis and cell-cycle arrest in SK-Hep1 human liver cancer cell line
Nhuan Do Thi, Eun-Sun Hwang
Department of Nutrition and Culinary Science, Hankyong National University, 327 Chungang-ro, Anseong-si, Gyeonggi-do 17579, Korea
1. Introduction
Black chokeberry (Aronia melanocarpa) originated in the former Soviet Union and in eastern Canada[1-3]. Black chokeberry possesses large quantities of anthocyanins in the fruits, as well as tannins, organic acids,pectins, flavonoids, procyanidins, and other bioactive compounds[4-9].It has been proven that black chokeberry juice, even its leaves or pomace have protective effects in oxidative stress, viral and bacterial infection,mutagenesis, inflammation, cancer, hypertension, and cardiovascular problems in many studies[4-6,8-10]. Cancer refers to a wide range of diseases associated with unregulated cell growth, especially cell division cannot be controlled, and cells grow to form malignant tumors and invade surrounding body parts. Cancer is recognized as a very deadly disease because cancer cells migrate around other cells and transfer the surrounding cells to cancer cells. According to statistics, about half of people receiving invasive cancer treatment are known to die[11]. Finding a way to inhibit cancer metastasis is one of the most important challenges today. In the case of liver cancer, most of the cancer cells found inmetastatic liver tumors are also present in cells other than the area where the cancer started. Therefore, to investigate the spread of cancer, liver metastasis seems to be the best option for researchers.
Interestingly, the onset of cancer is known to be caused by environmental factors of 90%-95% and genetics factors of 5%-10%, thus the environment is very important[12]. Many studies have shown that intake of foods rich in whole grains, fruits and vegetables is associated with a lower incidence of cancer, and increased intake of red meat or processed foods increases the risk of developing cancer[13]. Go et al.[14] reported that the risk of various cancers, such as liver, prostate,and gastrointestinal cancers, is reduced in the group that consumes large amounts of vegetables and fruits. Therefore, many researches are carried out on prevention of cancer and inhibition of cancer cell growth by using physiologically active substances extracted from fruits and vegetables. Several experiments have reported the anticancer and chemo-preventive effects of black chokeberry fruit and its juice[15-17].As a result of treatment of cancer stem cells with 0.3% black chokeberry juice, the cell cycle of S-phase was arrested, and cell death was occurred by inhibiting the proliferation of cancer cell growth[17]. Bermudez-Soto et al.[18] reported that Caco-2 human colon cancer cells were changed in cell transcription after repeated exposure at doses below the toxic concentration of black chokeberry juice. However, the effects of black chokeberry fruit on liver cancer cells have not been fully investigated.
In this study, we investigated the effect of black chokeberry fruit extract on the growth, migration and invasion of SK-Hep1 cancer cells. In addition, we examined the effects of black chokeberry fruit extract on the expression of MMP-2/-9 and MT-1 MMP when treated with SKHep1 cells at protein and gene level.
2. Materials and methods
2.1. Chemicals
Standard reagents for anthocyanin and polyphenol for HPLC analysis were purchased from Extrasynthese (Genay, France) and Sigma Chemical (St. Louis, MO, USA), and the solvent for HPLC analysis was from Fisher Inc. (Fair Lawn, NJ, USA). Cell culture supplies and chemicals were obtained from certified companies.
2.2. Sample preparation
Black chokeberry fruits were harvested at the optimal aging period on a local farm in Korea. The sample was lyophilized (PVTFD20R,Ilshin Lab Co., Kyunggi-do, Korea) and ground to a fine powder.The powdered sample (5 g) was mixed with 125 mL of 80% ethanol and extracted in a water bath at 85 ℃ for 2 h. The extraction process was repeated three times and the extracts were combined and vacuum filtered using Whatman #2 paper (Whatman International Limited,Miadstone, UK). The solvent was evaporated under reduced pressure at 40 ℃ using a rotary evaporator (EYELA Co., Tokyo, Japan). The extract was completely dissolved by adding 50 mL of distilled water to the evaporated extract, and the solution was lyophilized and stored in powder form at -20 ℃ for subsequent experiments.
2.3. Anthocyanin and polyphenol analysis
Anthocyanin and polyphenol were extracted with reference to Thi and Hwang[19], analyzed by HPLC and their contents were calculated.
2.4. Cell culture
SK-Hep1 cells were purchased from the Korean Cell Line Bank(Seoul, Korea), cultured in a 5% CO2, incubated at 37 ℃, and grown in DMEM supplemented with 10% FBS and penicillin/streptomycin.
2.5. MTT cell proliferation assay
The cells were seeded in a 96-well plate and 200 µL of DMEM medium was added to each well to give a concentration of 1 × 105cells/well.After the cells were attached to the wells, the black chokeberry extract was dissolved in fresh DMEM to 0-400 µg/mL and added to each well.Control group did not contain black chokeberry extract. After 24 or 48 hours of culture, the viability of cultured SK-Hep1 cells was determined by the method of Hwang and Lee[20]. The values were analyzed and were presented as percentages of the control values.
2.6. Cell adhesion and wound migration assay
The effect of black chokeberry extract (0-200 µg/mL) on cell attachment and wound migration in SK-Hep1 cells was measured by cell adhesion and migration assay as described by Hwang and Lee[20].
2.7. Zymography assay
The effect of black chokeberry extract (0-200 µg/mL) on the expression of MMP-2/-9, the major enzymes involved in cancer metastasis, was examined in SK-Hep1 cells by the method of Hwang and Lee[20]. After completion of the reaction, the white band that appeared after decolorization in an aqueous solution containing ethanol and acetic acid was quantified to confirm proteolysis by MMP-2/-9.
2.8. Semiquantitative RT-PCR
The effect of black chokeberry extract (0-200 µg/mL) on the expression of MMP-2/-9 and MT-1 MMP in SK-Hep1 human liver cancer cells was determined by the method of Hwang and Lee[20].
2.9. Cell cycle analysis
The effect of black chokeberry extract on cell cycle was measured in SK-Hep1 cells and the method was followed by Hwang and Lee[20].
The cell cycle was measured using the FACS Vantage flow cytometer(BD FACSCalibur, Franklin Lakes, NJ, USA) and analyzed with Win-MDI 2.8 software (Franklin Lakes, NJ, USA). The cell cycle was expressed as percentage of cells in the G1, S, and G2/M phases by analyzing 10 000 cells per group.
2.10. Western blot assay
The effect of black chokeberry extract (0-200 µg/mL) on the expression of Bcl-2 and Bax protein in SK-Hep1cells was determined by the method of Hwang and Lee[20].
2.11. Statistical analysis
All results were expressed as mean ± SD and compared with one-way analysis of variance in SPSS software package Version 17.0.
3. Results
3.1. Anthocyanin and polyphenol analysis
HPLC analysis revealed four anthocyanins in black chokeberry extract and cyanidin-3-O-galactoside occupied the largest amount of 7 980.8 mg/kg dry weight. On the other hand, cyanidin-3-O-glucoside showed the least amount of 491.6 mg/kg of dry weight.
Three polyphenols were identified from the black chokeberry extract,and the content of chlorogenic acid was the highest, occurring in a quantity of 4 600.9 mg/kg dry weight, while vanillic acid (89.1 mg/kg dry weight) and rutin hydrate (97.1 mg/kg dry weight) were present in lower but similar quantities.
3.2. Cell viability
The effect of black chokeberry extract on survival of SK-Hep1 cancer cells was measured by MTT proliferation assay. The growth of cancer cells was reduced by 5.5%-29.4% compared to the control group after culturing 24 h with SK-Hep1 cells supplemented with 0-400 µg/mL of black chokeberry extract (Figure 1). That is, the growth of cancer cells was 93.6% when treated with 25 µg/mL of black chokeberry extract,83.6% when treated with 100 µg/mL and 70.6% with 400 µg/mL of black chokeberry extract, respectively, while the control group without the black chokeberry extract was 100.0%. Similar to the results of the 24-hour culture, the cancer cells death rate decreased from 9.9% to 48.4%when black chokeberry extract was at 0-400 µg/mL concentration after 48 hours of culture. In SK-Hep1 cells, after 24 hours of treatment, the number of cells was decreased by 19.8% and 29.4% at 200 µg/mL and 400 µg/mL of black chokeberry fruit extract respectively, compared to the control. When the black chokeberry extract was treated for 48 h, the cancer cell inhibition rate was decreased to a significant level as compared with the 24 hours of treatment. That is, the cancer cell growth was suppressed to 34.7% and 48.4% at 200 µg/mL and 400 µg/mL of black chokeberry extract in comparison with the control group.
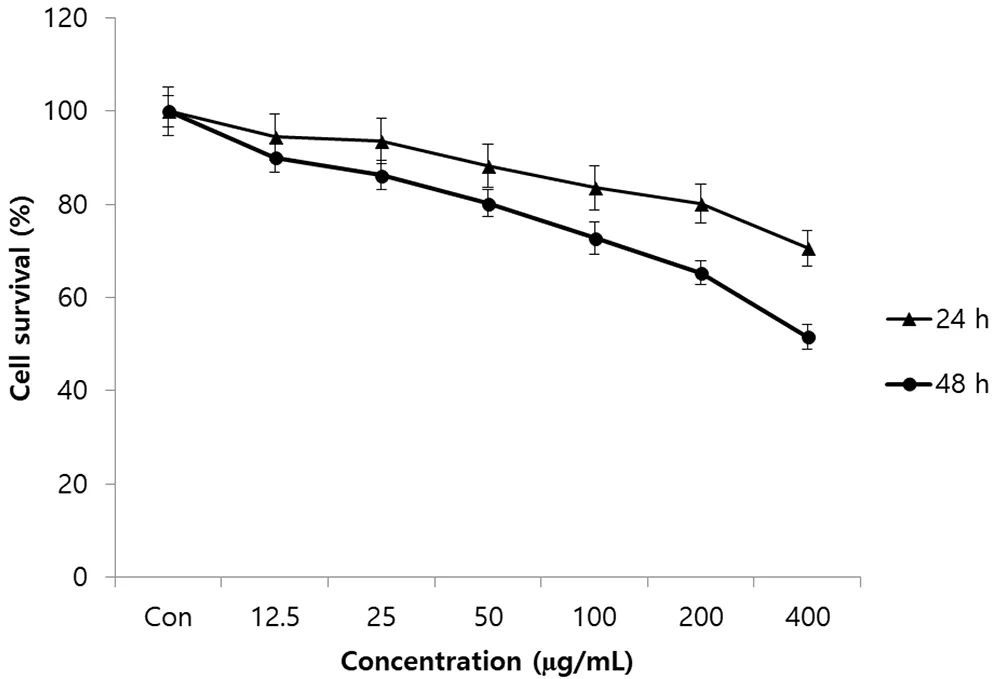
Figure 1. Effect of black chokeberry extract on SK-Hep1 human hepatoma cancer cell proliferation as measured by MTT assay.
3.3. Cell adhesion and migration
SK-Hep1 cells incubated for 60 min with 100 µg/mL of black chokeberry extract resulted in significant inhibition of cell adhesion and adhesion rate was proportional to the concentration of black chokeberry extract (Figure 2). The reduction was 87.6% and 75.3%with 100 µg/mL and 200 µg/mL black chokeberry extract, respectively.SK-Hep1 cells cultured on a 6-well plate were treated with the black chokeberry extract at different concentrations and the cell migration to the wound was measured. As shown in Figure 3, the black chokeberry extract significantly inhibited SK-Hep1 cell migration. When SKHep1 cells were treated with black chokeberry extract for 24 h, the cell migration was inhibited to 78.4% at 100 µg/mL and to 93.2% at 200 µg/mL compared to the control. It was confirmed that black chokeberry strongly inhibited the migration of the cancer cells to other sites.
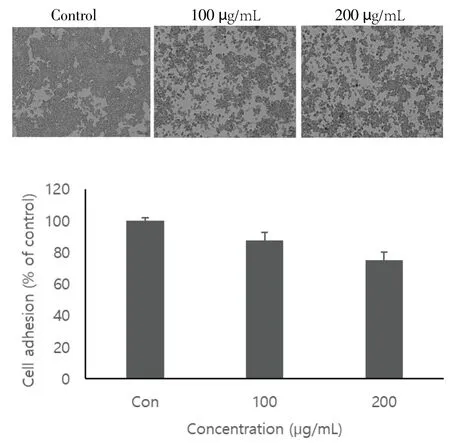
Figure 2. Effect of black chokeberry extract on cell adhesion on Matrigel of SK-Hep1 cells.

Figure 3. Effect of black chokeberry extract on wound healing migration of SK-Hep1 cells.
3.4. MMP expression
The effect of black chokeberry extract on MMP-2/-9 expression was measured by zymography and shown in Figure 4a. When SK-Hep1 cells were cultured for 48 h, the control group without black chokeberry extract secreted more MMP-2 and MMP-9 than the other group.However, in black chokeberry treated group, MMP-2/-9 expression was remarkably decreased in proportion to the concentration (50-200µg/mL) of black chokeberry extract. Treatment with black chokeberry extract at 50, 100, and 200 µg/mL concentrations decreased MMP-9 expression by 2.6%, 5.8%, and 11.3%, respectively. MMP-2 was more highly down-regulated than MMP-9 by black chokeberry extracts.MMP-2 expression was reduced by 41.7% in 50 µg/mL of black chokeberry extract treatment, 83.2% in 100 µg/mL and 96.8% at 200µg/mL treatment compared to the control group.
To investigate the effect of black chokeberry on MMP-2/-9 and membrane type-1 (MT1)-MMP messenger RNA (mRNA), the cells were treated with 50, 100, and 200 µg/mL of extract and measured. The mRNA levels in the SK-Hep1 cells were also measured using RT-PCR(Figure 4b).
When cells were treated with extract, MMP-9 mRNA expression decreased by 26.3% and by 85.0% at 50 µg/mL and 100 µg/mL of extract, respectively. MMP-2 mRNA expression was more suppressed than that of MMP-9 mRNA, and it was completely inhibited when treated with 100 µg/mL of black chokeberry extract. Treatment with black chokeberry extracts at concentrations of 50, 100, and 200 µg/mL decreased MT-1 MMP expression by 3.1%, 9.4%, and 84.4%,respectively. It was confirmed that MMP-2/-9 and MT-1 MMP expressions were apparently inhibited depending on the concentration of black chokeberry extract.
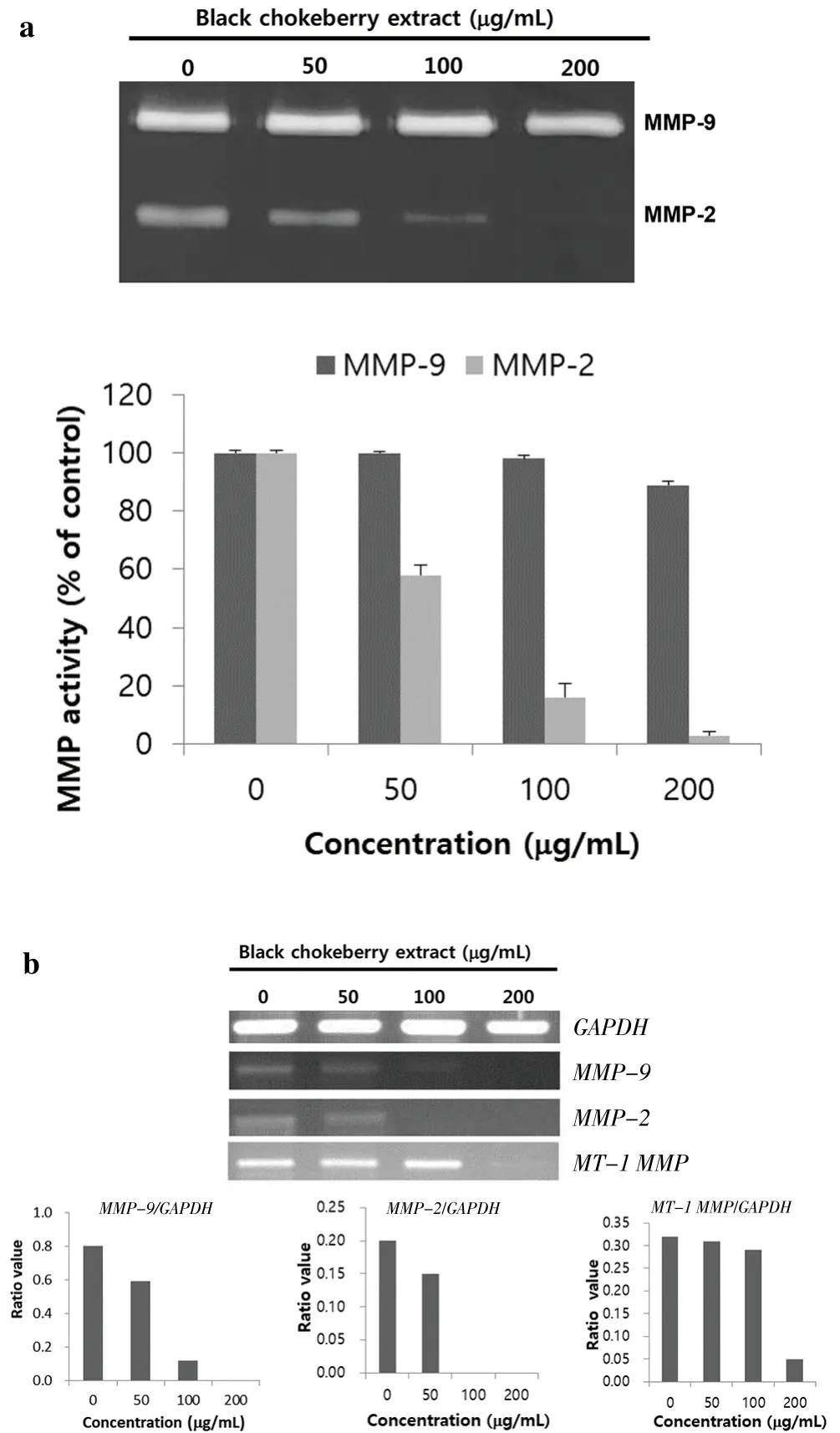
Figure 4. Effects of black chokeberry extract on MMP-2 and MMP-9 expressions analyzed by gelatin zymography (a) and mRNA expression as measured by RT-PCR (b) in SK-Hep1 cells.
3.5. Cell cycle
By flow cytometry analysis, we tried to determine whether black chokeberry extract was involved in cell cycle regulation of SK-Hep1 cells (Figure 5). DNA histogram showed that black chokeberry extracts increased cell number in the G2/M phase from 3.32% to 3.46% in the control and 200 µg/mL black chokeberry-treated cells, respectively.However, at the same time, it was confirmed that Sub G0/G1phase(from 68.28% to 59.68% in the control group and 200 µg/mL black chokeberry-treated cells, respectively) and S phase (from 18.12% to 17.54% in the control group and 200 µg/mL black chokeberry-treated cells, respectively) decreased in concentration dependent manner in the SK-Hep1 cells. This was probably due to the cell cycle progression to the G2/M stage when black chokeberry extract was treated. As shown in Figure 4, the black chokeberry extract inhibited the growth of SK-Hep1 cells by arresting the cell cycle in the G2/M phase and decreasing the number of cells in the Sub G1phase. The cell growth inhibitory effect was proportional to the concentration of the black chokeberry extract.
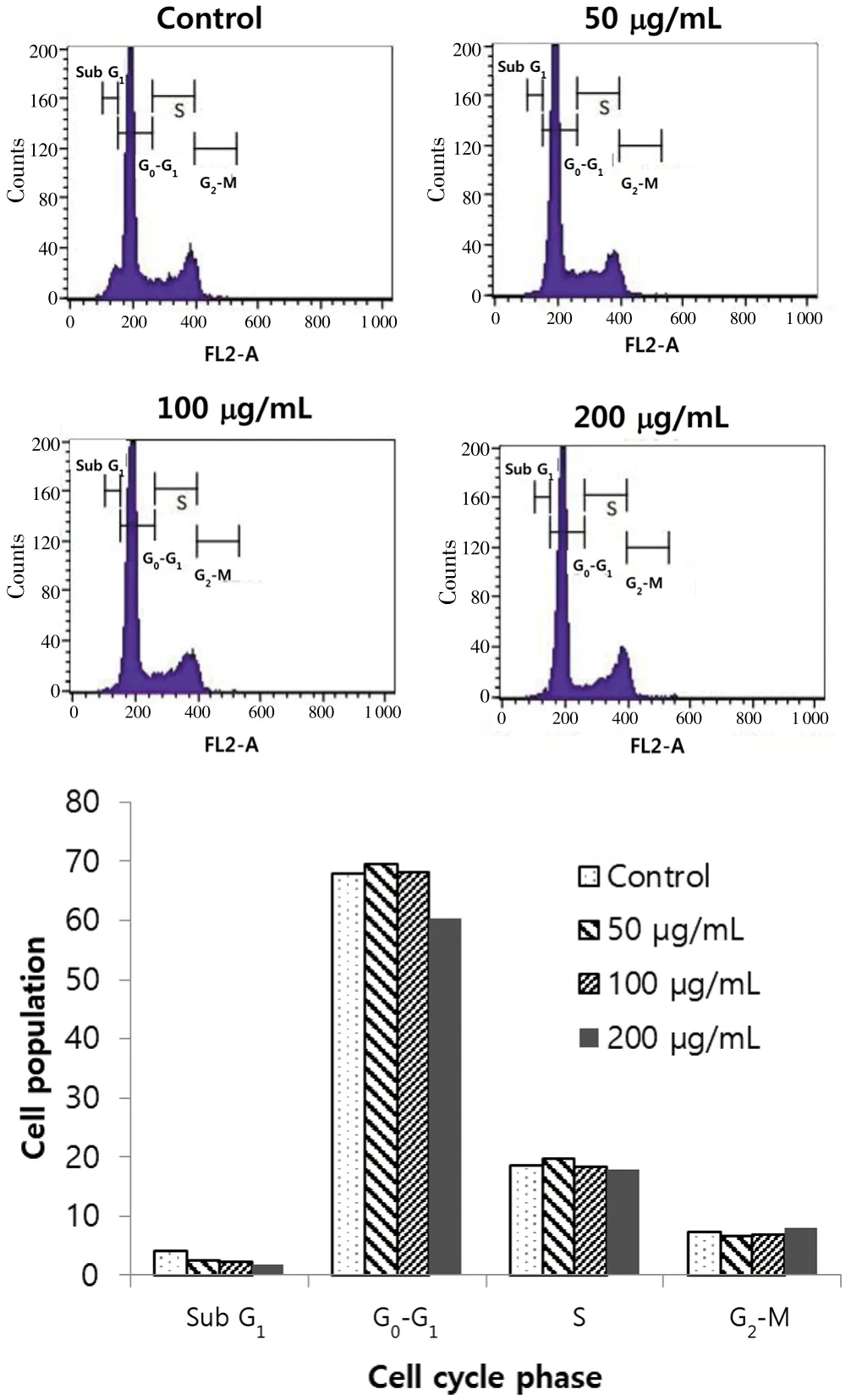
Figure 5. Effects of black chokeberry extract on SK-Hep1 cell-cycle arrest.
3.6. Bcl-2 and Bax expression
After treatment of black chokeberry extract (50-200 µg/mL) in SK-Hep1 cells for 24 h, the Bcl-2 and Bax proteins were measured by Western blot. The Bcl-2 expression decreased with increasing concentrations of black chokeberry extract (Figure 6). In other words,compared to control, Bcl-2 expression was decreased 0.6-fold and 0.4-fold, respectively, when SK-Hep1 cells were treated with 100 and 200 µg/mL of extract. The secretion of Bax protein showed a constant pattern regardless of the concentration of black chokeberry extract.
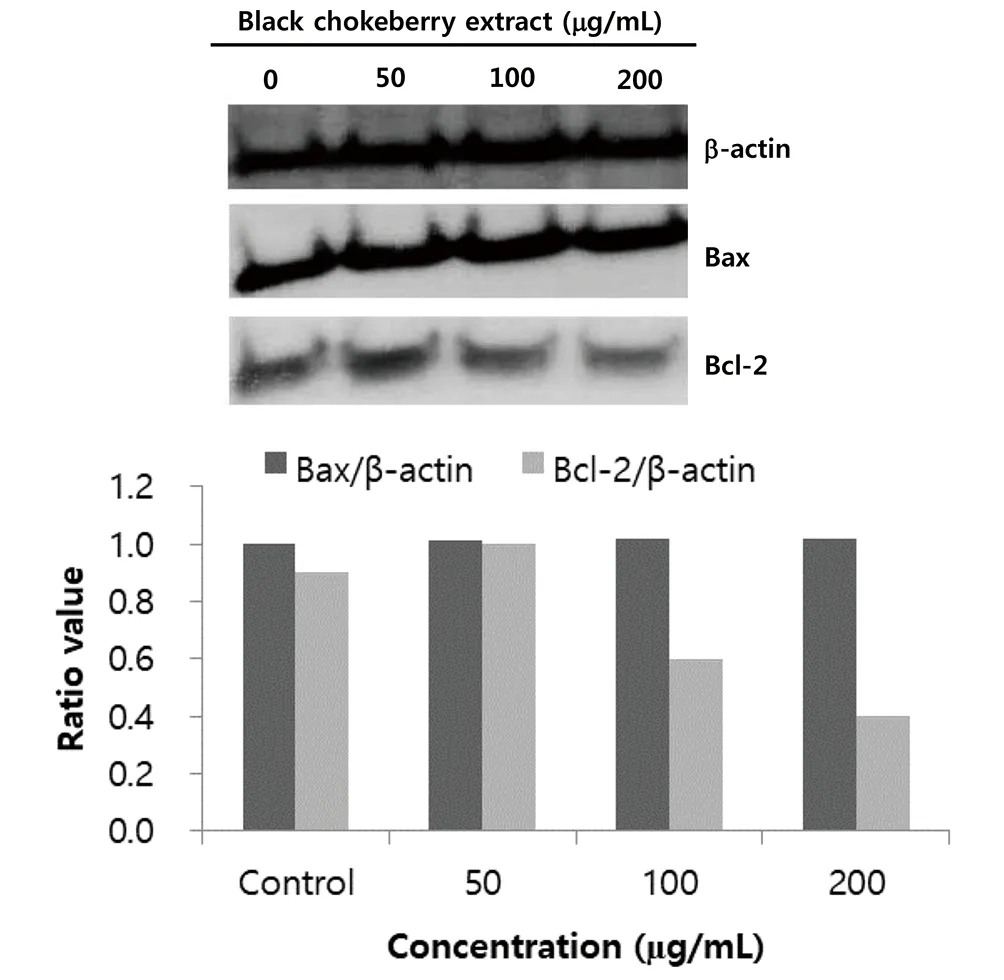
Figure 6. Expression of Bax and Bcl-2 protein expression in SK-Hep1 cells after treatment with black chokeberry extract for 24 and 48 h, as measured by Western blot analysis.
4. Discussion
The anthocyanins naturally contained in purple fruits and vegetables have been widely studied and it has been proven through various experiments that anthocyanins in berries can prevent various chronic diseases, including cancer, cardiovascular diseases, and diabetes[3,21].Anthocyanins have been shown to have nutraceutical functions[3,21].
Our results show that treatment of black chokeberry extract with SKHep1 cells, which has a high metastatic potential, inhibits the growth of cancer cells in proportion to the concentration of black chokeberry extract and treatment time. Similar to the results of our experiment,when the black chokeberry extract was treated to U373 brain tumor cells at a concentration of 600 µg/mL, the cell growth was inhibited by 70% and the degree of cell growth inhibition was proportional to the concentration of black chokeberry extract[22]. In another study, black chokeberry extracts inhibited HT29 colon cancer cell growth by up to 50%, which showed the highest cancer cell growth inhibition than grape, blueberry, elderberry, and radish fruit extracts[23]. We examined the wound healing assay in SK-Hep1 cells to investigate the effectof black chokeberry extract on the migration of cancer cells. Black chokeberry extract affects cancer cell migration, and previous studies have shown that cell migration was inhibited up to 46.5% at 25 µg/mL of black chokeberry extract compared to the control[24].
The expression of MMP-2 and MMP-9, the major enzymes involved in the metastasis of cancer cells, was analyzed to explore the antimetastatic effect of the black chokeberry extracts. MMP-2 and MMP-9 are enzymes specific for the type Ⅳ collagen chains and are deeply involved in the degradation of basement membrane and the invasion and metastasis of tumor cells[25]. Many studies have shown that increased MT1-MMP expression indicates tumor progression, such as cell growth, metastasis, migration and invasion of cancer cells[26-28].Thus, reducing the activity of MT-1 MMP may be associated with the delay or inhibition of growth of cancer cells[29]. ProMMP-2 is activated by MT-1 MMP and it is known to activate MMP-2[27]. Recent studies have shown that the MMP-2/-9 is increased in various cancer cells, including lung, liver, and colorectal cancer, and the expression of these MMPs is thought to result in invasion and metastasis of tumor cells[29,30]. MT-1 MMPs specially bind to tissue inhibitor of metalloproteinase by activating pro-gelatinase on the surface of tumor cells[26,27].
The results of this study confirmed that the extract of black chokeberry induced cell cycle arrest of G2/M phase in SK-Hep1 cells and significantly reduced the expression of Bcl-2, anti-apoptotic protein.Bermúdez-Soto et al.[18] determined cell cycle in Caco-2 cells with 5% digested black chokeberry extract treated and showed that the number of cells in the G2/M phase was doubled (from 18.4% to 40.2%)and the G0/G1phase decreased by 2-fold (from 41.5% to 19.5%), but the number of cells in the S phase remained unchanged. In previous study, 50 mg of the anthocyanin monomer extracted from black chokeberry was HT-29 human colon cancer cells and the cell cycle was observed. As a result, the G1/G0phase was blocked and the G2/M phase was arrested[31]. In our experiment, black chokeberry extract did not significantly affect Bax expression. In general, Bax and Bcl-2 protein expressions are known to induce cell death, known as an intracellular signaling pathway, and to induce G2/M phase arrest in the cell cycle[32].From the above results, it was confirmed that the black chokeberry extract that contains high amounts of anthocyanins and phenolic compounds inhibits the proliferation, adhesion, migration and arrests cell cycle to have anti-cancer effect on SK-Hep1 human hepatoma cells.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF-2016R1A2B4014977).
[1] Kulling SE, Rawel HM. Chokeberry (Aronia melanocarpa) - A review on the characteristic components and potential health effects. Planta Med 2008;74(13): 1625-1634.
[2] Sikora J, Broncel M, Mikiciuk-Olasik E. Aronia melanocarpa Elliot reduces the activity of angiotensin i-converting enzyme-In vitro and ex vivo Studies.Oxid Med Cell Longev 2014; 2014(7): 1-7.
[3] Steed LE, Truong VD. Anthocyanin content, antioxidant activity, and selected physical properties of flowable purple-fleshed sweetpotato purees. J Food Sci 2008; 73(5): S215-221.
[4] Braunlich M, Slimestad R, Wangensteen H, Brede C, Malterud KE, Barsett H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013; 5(3): 663-678.
[5] Denev PN, Kratchanov CG, Ciz M, Lojek A, Kratchanova MG.Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A Review. Compr Rev Food Sci Food Safety 2012;11(5): 471-489.
[6] Thi ND, Hwang ES. Bioactive compound contents and antioxidant activity in aronia (Aronia melanocarpa) leaves collected at different growth stages. Prev Nutr Food Sci 2014; 19(3): 204-212.
[7] Sharif T, Alhosin M, Auger C, Minker C, Kim JH, Etienne-Selloum N, et al. Aronia melanocarpa juice induces a redox-sensitive p73-related caspase 3-dependent apoptosis in human leukemia cells. PLoS One 2012; 7(3):e32526.
[8] Sonoda K, Aoi W, Iwata T, Li Y. Anthocyanin-rich Aronia melanocarpa extract improves body temperature maintenance in healthy women with a cold constitution. Springerplus 2013; 2: 626-630.
[9] Zapolska-Downar D, Bryk D, Malecki M, Hajdukiewicz K, Sitkiewicz D.Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur J Nutr 2012; 51(5): 563-572.
[10] Chrubasik C, Li G, Chrubasik S. The clinical effectiveness of chokeberry: A systematic review. Phytother Res 2010; 24(8): 1107-1114.
[11] Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61(2): 69-90.
[12] Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST,Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008; 25(9): 2097-2116.
[13] Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W,Bandera EV, et al. American cancer society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012; 62: 30-67.
[14] Go VL, Wong DA, Wang Y, Butrum RR, Norman HA, Wilkerson L. Diet and cancer prevention: Evidence-based medicine to genomic medicine. J Nutr 2004; 134(12 Suppl): 3513-3516.
[15] Balansky R, Ganchev G, Iltcheva M, Kratchanova M, Denev P, Kratchanov C, et al. Inhibition of lung tumor development by berry extracts in mice exposed to cigarette smoke. Int J Cancer 2012; 131(9): 1991-1997.
[16] Kedzierska M, Olas B, Wachowicz B, Glowacki R, Bald E, Czernek U, et al. Effects of the commercial extract of aronia on oxidative stress in blood platelets isolated from breast cancer patients after the surgery and various phases of the chemotherapy. Fitoterapia 2012; 83(2): 310-317.
[17] Sharif T, Stambouli M, Burrus B, Emhemmed F, Dandache I, Auger C, et al. The polyphenolic-rich Aronia melanocarpa juice kills teratocarcinomal cancer stem-like cells, but not their differentiated counterparts. J Funct Foods 2013; 5(3): 1244-1252.
[18] Bermúdez-Soto MJ, Larrosa M, Garcia-Cantalejo JM, Espín JC,Tomás-Barberan FA, García-Conesa MT. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of polyphenol-rich chokeberry juice. J Nutri Biochem 2007; 18(4):259-271.
[19] Thi ND, Hwang ES. Effects of drying methods on contents of bioactive compounds and antioxidant activities of black chokeberries (Aronia melanocarpa). Food Sci Biotech 2016; 25(1): 55-61.
[20] Hwang ES, Lee HJ. Phenylethyl isothiocyanate and its N-acetylcysteine conjugate suppress the metastasis of SK-Hep1 human hepatoma cells. J Nutr Biochem 2006; 17(12): 837-846.
[21] Lim S, Xu J, Kim J, Chen TY, Su X, Standard J, et al. Role of anthocyaninenriched purple-fleshed sweet potato p40 in colorectal cancer prevention.Mol Nutr Food Res 2013; 57(11): 1908-1917.
[22] Thani NAA, Sallis B, Nuttall R, Schubert FR, Ahsan M, Davies D, et al.Induction of apoptosis and reduction of MMP gene expression in the U373 cell line by polyphenolics in Aronia melanocarpa and by curcumin. Oncol Rep 2012; 28(4): 1435-1442.
[23] Zhao C, Giusti MM, Malik M, Moyer MP, Magnuson BA. Effect of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J Agric Food Chem 2004; 52(20):6122-6128.
[24] Parzonko A, Oświt A, Bazylko A, Naruszewicz M. Anthocyanins-rich Aronia melanocarpa extract possess ability to protect endothelial progenitor cells against angiotensin Ⅱ induced dysfunction. Phytomed 2015; 22(14): 1238-1246.
[25] Lin YL, Ramanujum R, He S. Infection of Schistosomiasis japanicum is likely to enhance proliferation and migration of human breast cancer cells:Mechanism of action of differential expression of MMP2 and MMP9. Asian Pac J Trop Biomed 2011; 1(1): 23-28.
[26] Torricelli C, Fortino V, Capurro E, Sacchi G, Ponzo P, Pacini A, et al.Role of PTHrp and PTHrp-engaged pathways in MCF-7 cells migration/invasion. Matrix Biol 2006; 25(2): 104-111.
[27] Kim IY, Jeong SJ, Kim ES, Kim SH, Moon A. Type Ⅰ collagen-induced pro-MMP-2 activation is differentially regulated by H-Ras and N-Ras in human breast epithelial cells. J Biochem Mol Biol 2007; 40(5): 825-831.
[28] Barillari G, Iovane A, Bacigalupo I, Labbaye C, Chiozzini C, Sernicola L, et al. The HIV protease inhibitor indinavir down-regulates the expression of the pro-angiogenic MT1-MMP by human endothelial cells. Angiogenesis 2014; 17(4): 831-838.
[29] Liabakk NB, Talbot I, Smith RA, Wilkinson K, Ballewill F. Matrix metalloproteinase 2 (MMP-2) and matrix metalloproteinase 9 (MMP-9)type Ⅳ collagenase in colorectal cancer. Cancer Res 1996; 56(1): 190-196.
[30] Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P,Trangas T, et al. Overexpression of matrix-metalloproteinase-9 in human breast cancer: A potential favorable indicator in node-negative patients. Br J Cancer 2001; 84(11): 1488-1496.
[31] Malik M, Zhao C, Schoene N, Guisti MM, Moyer MP, Magnuson BA.Anthocyanin-rich extract from Aronia melanocarpa E. induces a cell cycle block in colon cancer but not normal colonic cells. Nutr Cancer 2003; 46(2):186-196.
[32] Hwang ES, Lee HJ. Effects of phenylethyl isothiocyanate and its metabolite on cell-cycle arrest and apoptosis in LNCaP human prostate cancer cells. Int J Food Sci Nutr 2010; 61(3): 324-336.
 Asian Pacific Journal of Tropical Biomedicine2018年6期
Asian Pacific Journal of Tropical Biomedicine2018年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Probiotic based therapy for atopic dermatitis: Outcomes of clinical studies
- Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators
- Antifungal and cytotoxic activities of extracts obtained from underutilised edible tropical fruits
- Evaluation of possible mechanisms of Cordia dichotoma fruits for hyperlipidemia controlling in Wistar albino rats
