Molecular cloning and characterization of a serine palmitoyltransferase gene from rice(Oryza sativa)and its gene expression in defense response to brown planthopper
BEGUM Mahfuj Ara,SHI Xiaoxiao,BAI Yueliang,JIANG Yandong,ZHOU Wenwu,MAO Cungui,ZHU Zengrong*(.InstituteofInsectSciences,CollegeofAgricultureandBiotechnology,ZhejiangUniversity,Hangzhou30058,China;.Department of Medicine and Stony Brook Cancer Center,The State University of New York at Stony Brook,New York 794,USA)
Sphingolipids are major structural components of endomembranes and dynamic regulators of basic cellular processes in all eukaryotes and a few prokaryotes[1-6].The biosynthesis of sphingolipids is initiated in endoplasmic reticulum(ER)with the condensation of serine and fatty acyl-CoA.In particular,sphingolipidsplay a crucialrole in divergent signaling events,including differentiation,senescence,proliferation,apoptosis,programmed cell death(PCD)and stress responses[7-12].Recent studies have revealed that sphingolipids also showed antimicrobial activity[13].Long-chain bases(LCBs)are metabolic precursors of complex sphingolipids.Serine palmitoyltransferase(SPT)is the key enzyme of sphingolipids de novo biosynthesis.This enzyme catalyzes the first reaction in LCB synthesis from the condensation of serine and palmitoyl-CoA to form 3-ketosphinganine.SPT is the rate-limiting step for biosynthesis of sphingolipids,which comprises three subunits, LCB1, LCB2a and LCB2b[14].Disruption of SPT in Nicotiana benthamiana activates salicylic acid-dependentresponses and compromises resistance to Alternaria alternata f.sp.lycopersici[15].Plants have evolved a complex innate immune system that can effectively protect plants against various biotic stresses.It is believed that individual plant cells have the capacity to detect a pathogen attack and to activate onsite defense responses. In fact, sphingolipids as bioactive moleculeshavebeen shown to beextensively involved in plant defense-associated programmed cell death(PCD),and more recently,sphingolipids have been implicated in regulation of membrane trafficking and/or formation of membrane subdomains during defense responses[16].Recent evidence suggests that the OsLCB2a1 subunit of SPT is involved in plant defense against herbivores[17].
In this study,we cloned SPT gene from rice.The transcription profiles of one SPT gene,OsLCB2a1,under different levels of brown planthopper(BPH)infestation were analyzed by quantitative real-time polymerase chain reaction(qRT-PCR).
1 Materials and methods
1.1 Plant growth
The rice genotype Xiushui 11(Oryza sativa japonica variety)was used for gene cloning.Eight varieties(Xiushui 11,IR64,Taiping,HybridSy63,Mudgo,Wuyugen2,Guanglu’ai and TN1)were used for varietal expression study.Seeds were reared in a plastic box containing rice nutrient solution[18],and maintained in a controlled climate room at(26±2)℃,12 h light phase and 80%relative humidity.All the collected samples were immediately frozen in liquid nitrogen and maintained at-80℃for RNAextractions.There were five replications for each treatment.
1.2 Insects
Colonies of the brown planthopper(Nilaparvata lugens)were maintained on Taichung Native 1(TN1,an indica variety without any resistant gene to herbivores and pathogens)rice seedlings in a controlled climate room at(26±2)℃,12 h light phase and 80%relative humidity.
1.3 Sequence analysis
We performed PCR reaction(Toyobo,Japan)to amplify the specific genes using primers in Table 1.PCR products were sequenced by a commercial company(Sunny,China).SPT homolog sequences were obtained by BLASTp(https://blast.ncbi.nlm.nih.gov)and Phytozome 10.3(https://phytozome.jgi.doe.gov),and the representative sequences were aligned by CLUSTALW using GENETYX program(Software Development Co.Ltd.,Japan).The aligned sequences were used for phylogenetic analysis with neighborjoining(NJ)method by MEGA 6.0 and bootstrapping for 1 000 replicates[19].Accession numbers of different organisms used in this tree contraction from the NCBI and Phytozome were given in Table S1(http://www.zjujournals.com/agr/EN/abstract/abstract30409.shtml).

Table 1 Primers used in PCR assays
1.4 Statistical analysis
Data were analyzed using analysis of variance(ANOVA)and presented as mean values for each treatment.Statistical analysis was performed using Data Processing System(DPS)statistical software package[20-21].TheTukey’stest(P<0.05)wasperformed to evaluate the treatment effects.
1.5 Quantitative RT-PCR(qRT-PCR)
The total RNA from leaf or seedling samples was extracted using a extraction kit(Tiangen,Shanghai)and reversely transcribed to cDNA using a Prime Script RT reagent kit(Takara)according to the manufacture’s instruction.Gene-specific primers(Table 2)were designed with Primer-3-Plus software.qRT-PCR was performed using SYBR Green Supermix Reagent(BioRad,USA),and run on an ABI 7500 Real-Time PCR System for one cycle of 95℃ for 3 min,followed by 40 cycles of 95℃for 10 s,and 60℃for 30 s.Normalized gene expression was calculated by the 2-ΔΔCTmethod[22].
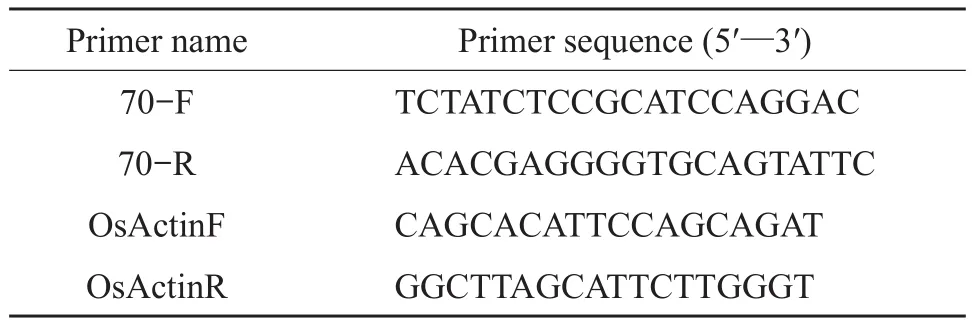
Table 2 Primers used in qRT-PCR assays
1.6 Evaluation for plant resistance against BPH
A seedling bulk test was conducted to evaluate the BPH resistance ofrice varietiesfollowing previously described methods[23]. Seeds were randomly sown in a plastic box in three 26 cm-long rows,with 2.5 cm between rows.The varieties IR64 and TN1 were used as resistant and susceptible check,respectively.At the third-leaf stage,the seedlings were infested with the second to third-instar nymphs of BPH with 10 insects per seedling.When all of the seedlings of susceptible control(scored as 9)died,the plants of other rice were examined and each seedling was given a score of 1 to 9 according to the method[23];the lowerscoresindicate higher resistance to the insect.
2 Results
2.1 Cloning of the SPT genes
To investigate the possible function of SPT in insect resistance response,we cloned genes from O.sativa encoding the LCB1,LCB2 and the subunit of SPT.The full-length cDNA of three LCB1 and three LCB2 genes were amplified by RT-PCR.
The lengths of the deduced LCB proteins ranged from 481 to 497 amino acid residues.To identify LCB homology,weusedBLASTptoconduct sequence similarity searches using the sequence of Arabidopsis thaliana(AtLCB1,AT4G36480)and A.thaliana(AtLCB2a,AT5G23670).The results of the BLASTX search are shown in Table 3.Sequence alignment showed that LCB1.1 had the highest identity(89%)to the LCB protein found in Sorghum bicolor.LCB1.2 showed the highest identity(88%)to the serine palmitoyltransferase 1 found in Zea mays.LCB1.3 showed the highest identity(94%)to the LCB found in Dichanthelium oligosanthes.On the other hand,LCB2a.1 and LCB2a.2 showed high identity to the LCB protein in Z.mays,with 96%and 88%,respectively;whereas LCB2a.3 showed the highest identity(97%)to LCB protein in S.bicolor.Most conserved regions of LCB1 and LCB2 in different species were showed in multiple alignment(Fig.1A-B).
To investigate the evolutionary relationships,phylogenetic analysis was performed with the LCBprotein from O.sativa and other species(Fig.2).The NJ tree revealed that three LCB1 genes clustered with one sub-branch had very closely related homologues with LCB of Brachypodium distachyon and Z.mays.Moreover,we found that two LCB2a genes were clustered with B.distachyon in one single branch,and had very closely related homologues with Z.mays and S.bicolor.On the other hand,another LCB2a gene clustered in separate clad had very closely related homologues with Z.mays and B.distachyon.The accession number of proteins were listed in Table S1(http://www.zjujournals.com/agr/EN/abstract/abstract30409.shtml).
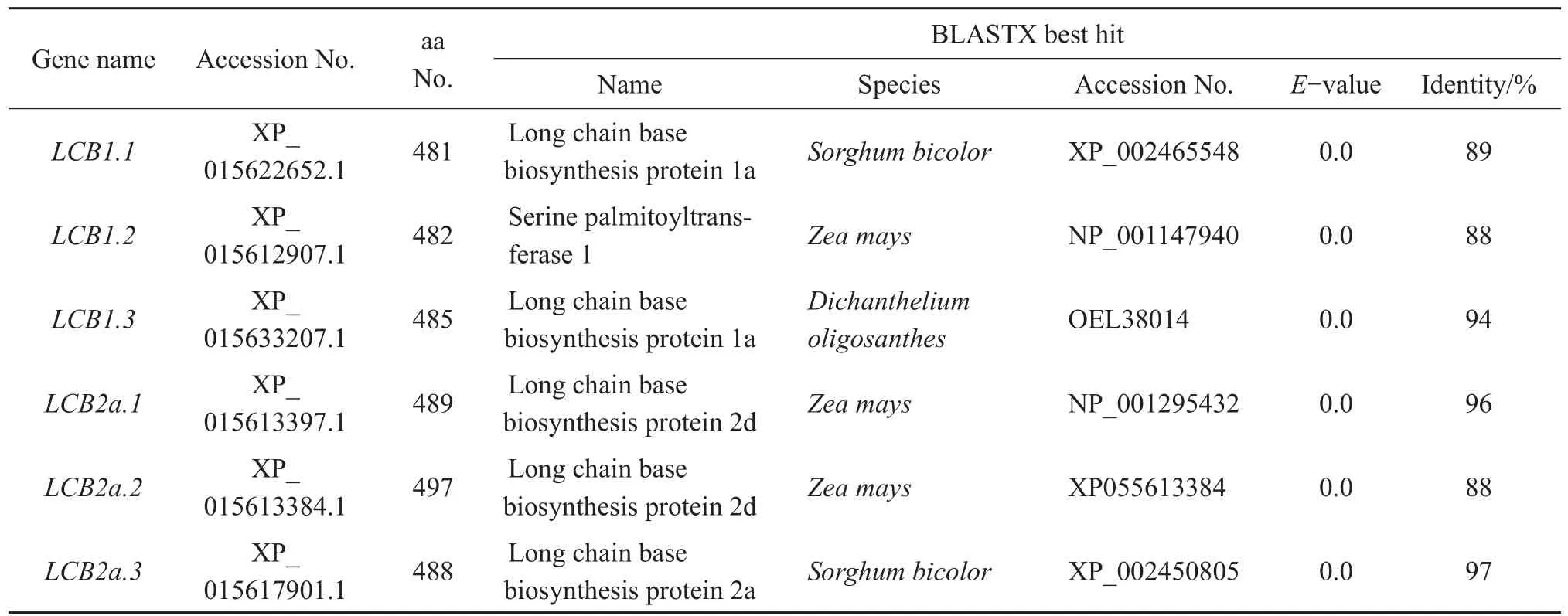
Table 3 BLASTX results of SPT genes in Oryza sativa
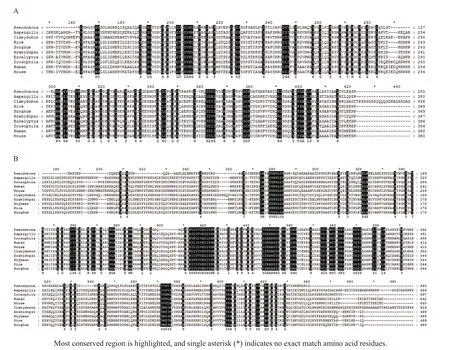
Fig.1 Multiple alignment of deduced amino acid sequences of LCB1(A)and LCB2(B)genes in different species
2.2 Expression analysis of SPT in different levels of BPH infestation
When we subjected rice plants to BPH feeding,the results showed that OsLCB2a1 transcripts increased at 4 h after infestation and then gradually decreased from 8 to 24 h(Fig.3).Plants subjected the physical wound had much lower expressions.
We also studied OsLCB2a1 gene expression in different varieties,different plant parts and growth stages of rice.Among eight different rice varieties(Xiushui 11,Wuyugen2,IR64,Taiping,HybridSy63,Mudgo,Guanglu’ai and TN1,all are indica varieties except the first two),the gene expression levels were significantly higher in Taiping(P<0.05)and lower in TN1,a susceptible rice variety for BPH;it was also high in BPH-resistant varieties like Mudgo and IR64,but was low in the other four varieties(Fig.4A).Gene expression was negatively correlated with BPH resistance scores(Fig.4B).
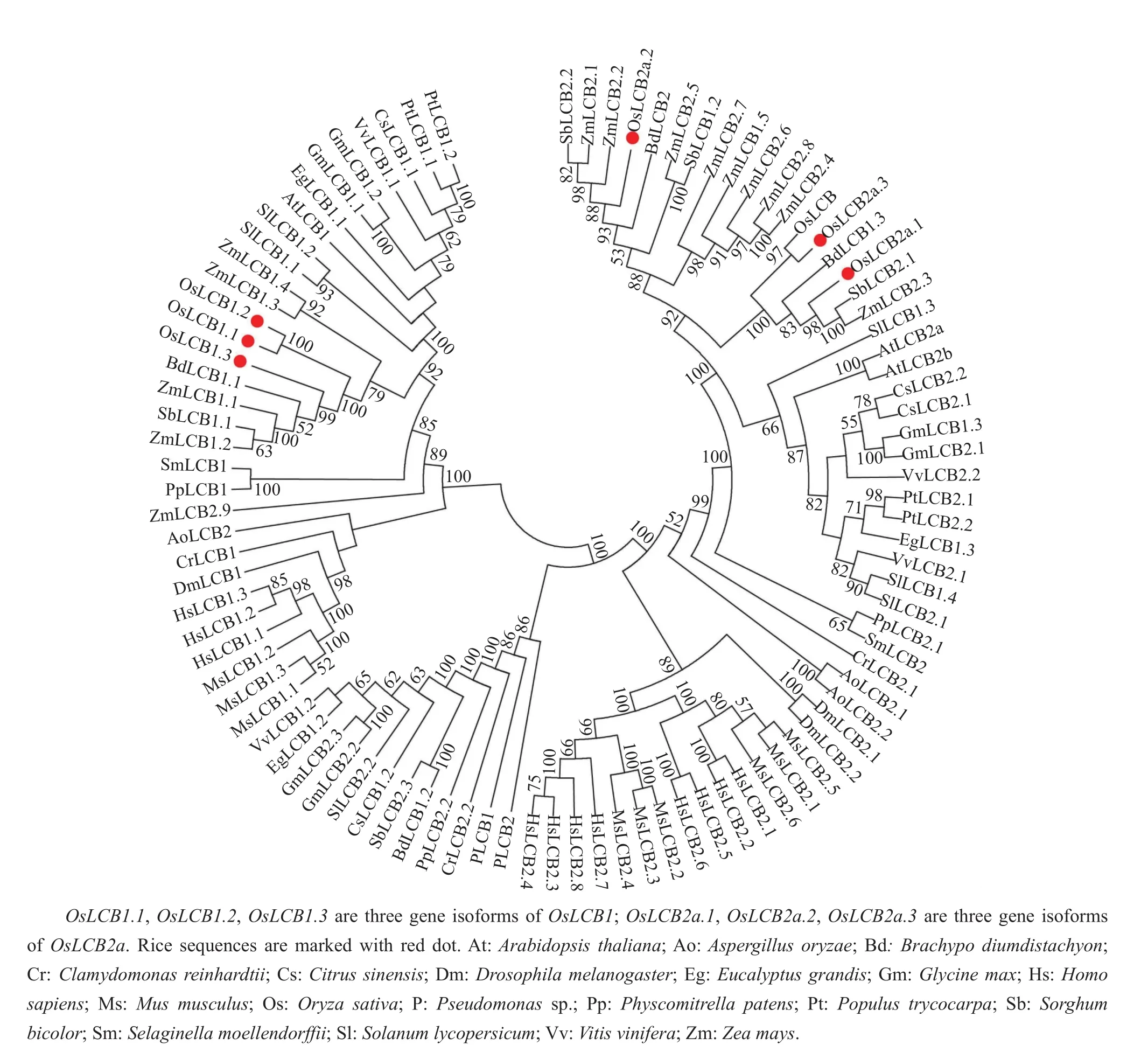
Fig.2 Phylogenetic relationships of serine palmitoyltransferase protein(LCB1 and LCB2)from various species
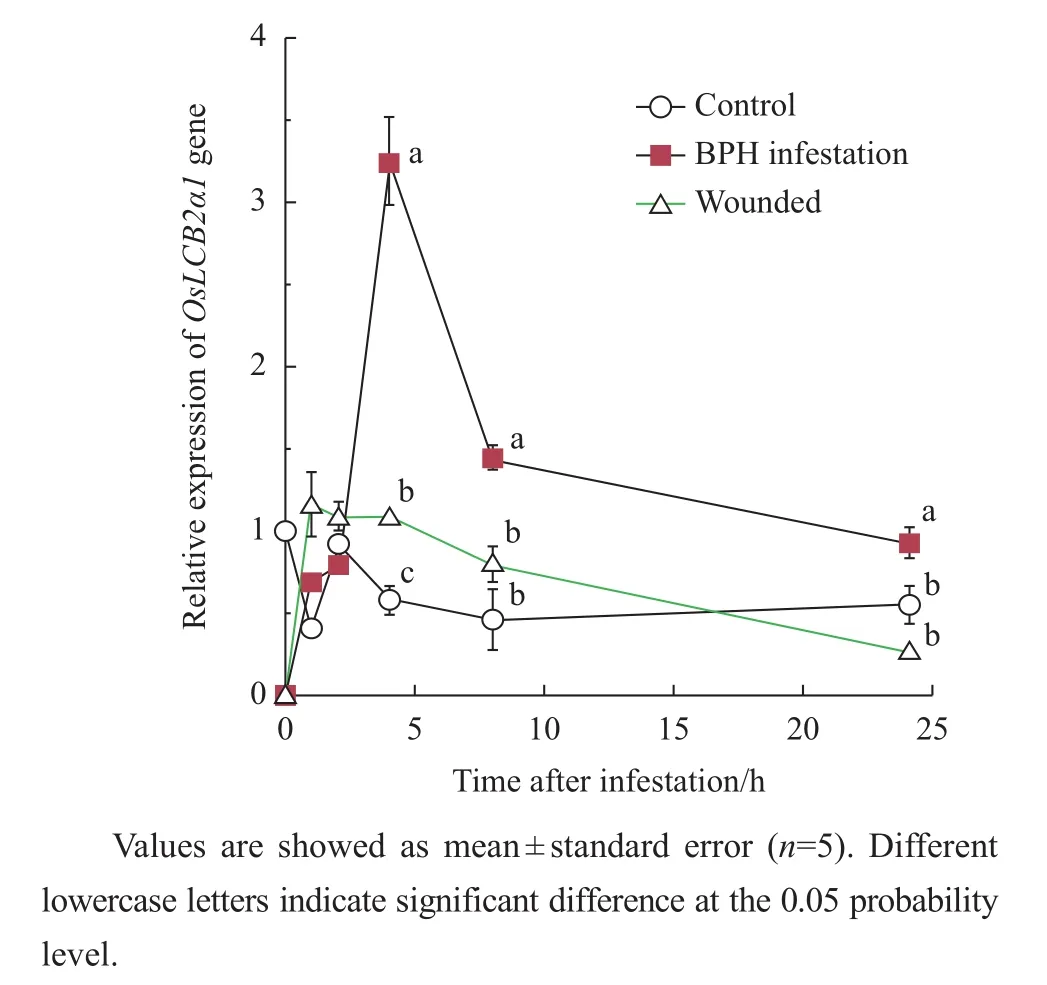
Fig.3 Analysis of OsLCB2a1 expression under BPH infestation and wounding inducement
Gene expression of OsLCB2a1 was determined in leaf blade,leaf sheath and roots of three rice varieties,i.e.IR64,Taiping and TN1.Among different plant parts of rice varieties,gene expression was found to be significantly higher in the leaf blade of IR64 and the leaf sheath of Taiping,and comparatively lower in the roots compared with the leaf blade and leaf sheath of IR64 and Taiping.In TN1,gene expression was significantly low in all plant parts(Fig.5A).Transcript levels of OsLCB2a1 gene were also compared at seedling,vegetative and reproductive stages of three rice varieties.The results showed that significantly higher gene expression was observed at the maximum tillering stage of IR64 and Taiping(Fig.5B).
3 Discussion
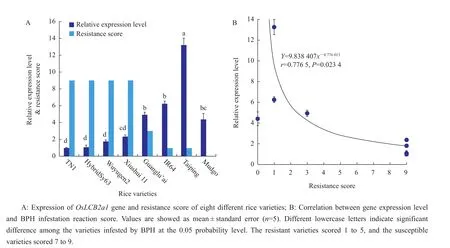
Fig.4 OsLCB2a1 gene expression level and BPH resistance score and their correlation in eight different rice varieties
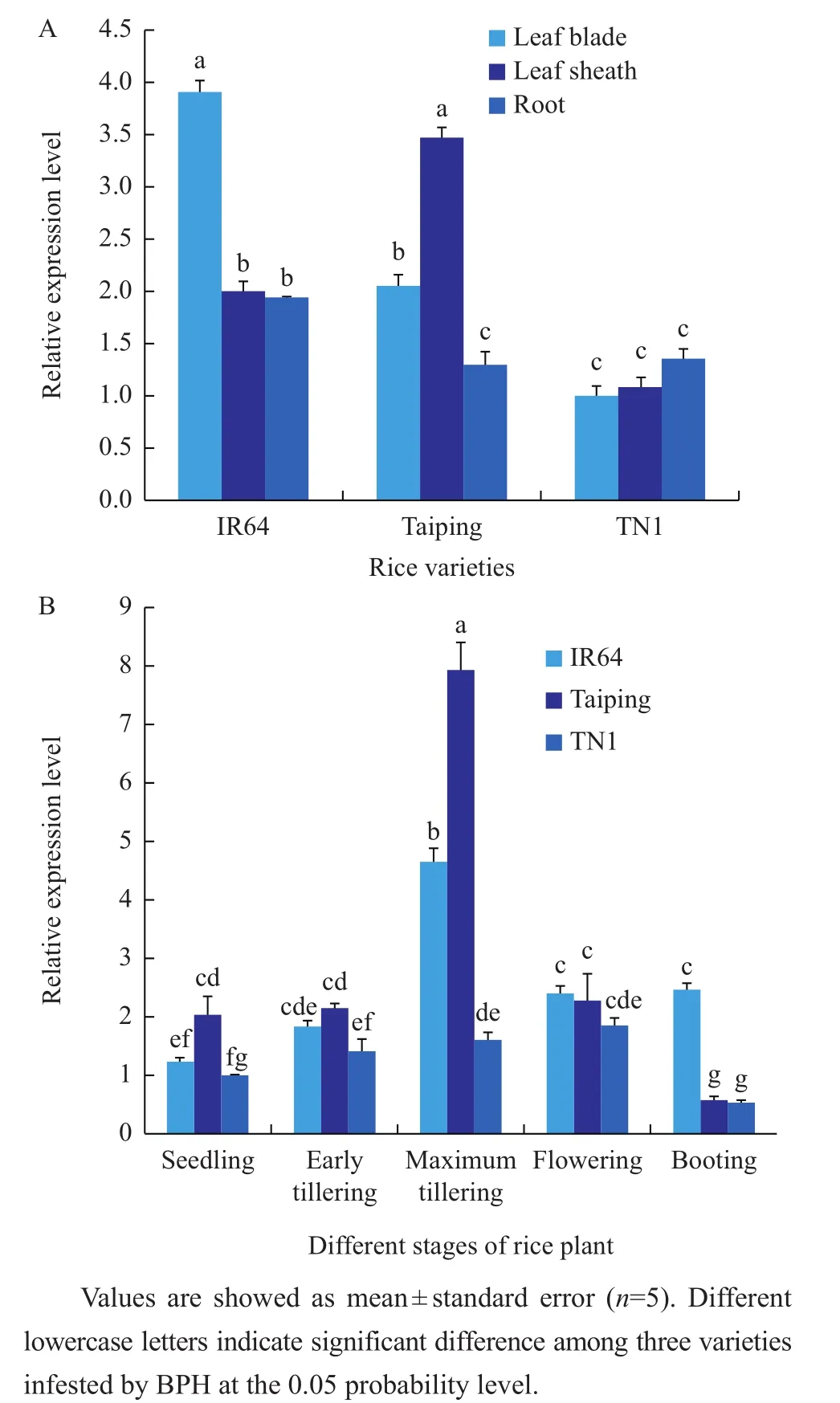
Fig.5 OsLCB2a1 gene expression level at different plant parts(A)and different growth stages(B)of rice
SPT has been suggested to be the key enzyme for the regulation of sphingolipid levels in cells.It regulates not only plant cell death but also the defense responses against non-host pathogen[24].It has been shown that LCB2a gene from serine palmitoyltransferase is required for PCD process that operates as one of the more effective strategies used as defense against pathogens in plants[25].Recent studies have noted the diversity of sphingolipid functions during plant development and stress responses.In plants,LCBs act as bioactive molecules in the immune response[15].In Arabidopsis,fuminosin B1-induced PCD is activated by increased levels of LCB,as demonstrated by a mutation in the LCB1 gene that encodes one of the two subunits of SPT[26].In this study,we characterized the rice SPT genes and studied the gene expression of one SPT gene,OsLCB2a1.Multiple alignment showed that OsLCB2a1 has a conservative GTFTKSFG motif corresponding to the known PLP-binding site like N.banthamiana,A.thaliana,S.cerevisiae,Homo sapiens and other species[17,27,19].The GTFTKSFG motif is completely conservative among the predicted LCB2 proteins from different organisms,suggesting that these LCB2 polypeptides constitute catalytic subunits of SPT.For example,the pyridoxal 5′-phosphate binding site of N.banthamiana LCB2 is required for its function as a cell death inducer[24].TAKAHASHI and his colleagues hypothesized that over-expression of NbLCB2 enhanced the SPT activity,which in turn triggered plant cell death[24].Bioinformatics analysis showed that the SPT gene has high homology with a large number of species from bacteria to humans.
Investigations on the gene expression in different varieties,plant parts and growth stages of rice with BPH infestation will help us predict the functions of SPT gene in herbivore defense.We found that OsLCB2a1 expression was up-regulated when plants were infested by BPH,suggesting that OsLCB2a1 had effects on herbivore defense.Gene expression was higher in resistant varieties than in susceptible ones.The transcript level of OsLCB2a1 gene was also found to be higher in the leaf blade and leaf sheath of resistant varieties than the root.This is probably because BPH sucks sap from leaf blade and leaf sheath of rice plants and is not a root feeder.The mRNA level of OsLCB2a1 was found to be higher in maximum tillering stage of resistant varieties,which suggests that this gene is potentially more effective in the vegetative stage.
4 Conclusions
The rice SPT genes were cloned in this study,which has a high degree of sequence and structure similarity with other plant SPT genes.Our data suggest that OsLCB2a1 is involved in herbivore defense.Further studies are warranted to explore the function of SPT as a defense element in the interactions between rice and other pests,and to determine the underlying mechanisms of SPT effecting on pest performance.
AcknowledgementsWe thank Professor K.L.Heong for his critical modification of the earlier version of the manuscript.
:
[1] SPERLING P,HEINZ E.Plant sphingolipids:Structural diversity,biosynthesis,first genes and functions.Biochimica et Biophysica Acta(BBA):Molecular and Cell Biology of Lipids,2003,1632(1/2/3):1-15.
[2] LYNCH D V,DUNN T M.An introduction to plant sphingolipids and a review of recentadvances in understanding their metabolism and function. New Phytologist,2004,161(3):677-702.
[3]MERRILL JR A H,WANG M D,PARK M,et al.(Glyco)sphingolipidology:An amazing challenge and opportunity for systems biology.Trends in Biochemical Sciences,2007,32(10):457-468.
[4]MERRILL A H,STOKES T H,MOMIN A,et al.Sphingolipidomics:A valuable tool for understanding the roles of sphingolipids in biology and disease.Journal of Lipid Research,2009,50(Suppl.):97-102.
[5]PRUETT S T,BUSHNEV A K,HAGEDORN M,et al.Thematic review series:Sphingolipids.Biodiversity of sphingoidbases(“sphingosine”)andrelatedamino alcohols.Journal of Lipid Research,2008,49(8):1621-1639.
[6] HANNUN Y A,OBEID L M.Principles of bioactive lipid signalling:Lessons from sphingolipids.Nature Reviews Molecular Cell Biology,2008,9(2):139-150.
[7] PAYNE S G,MILSTIEN S,SPIEGEL S.Sphingosine-1-phosphate:Dual messenger functions.FEBS Letters,2002,531(1):54-57.
[8] CHALFANT C E,SPIEGEL S.Sphingosine 1-phosphate and ceramide 1-phosphate:Expanding roles in cell signaling.Journal of Cell Science,2005,118(20):4605-4612.
[9] LYNCH D V,FAIRFIELD S R.Sphingolipid long-chain base synthesis in plants (characterization of serine palmitoyltransferase activity in squash fruit microsomes).Plant Physiology,1993,103(4):1421-1429.
[10]ZAUNER S,TERNES P,WARNECKE D.Biosynthesis of sphingolipids in plants(and some of their functions)//Sphingolipids as Signaling and Regulatory Molecules.New York,USA:Springer,2010:249-263.
[11]DUTILLEUL C,BENHASSAINE-KESRI G,DEMANDRE C,et al.Phytosphingosine:Phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling.New Phytologist,2012,194(1):181-191.
[12]LI S F,SONG L Y,YIN W B,et al.Isolation and functional characterization of the genes encoding Δ8-sphingolipid desaturase from Brassica rapa.Journal of Genetics and Genomics,2012,39(1):47-59.
[13]TANG J,MENG X J,LIU H,et al.Antimicrobial activity of sphingolipids isolated from the stems of cucumber(Cucumis sativus L.).Molecules,2010,15(12):9288-9297.
[14]WU J X,LI J,LIU Z,et al.The Arabidopsis ceramidase AtACER functions in disease resistance and salt tolerance.The Plant Journal,2015,81(5):767-780.
[15]RIVAS-SAN VICENTE M,LARIOS-ZARATE G,PLASENCIA J.Disruption of sphingolipid biosynthesis in Nicotiana benthamiana activates salicylic acid-dependent responses and compromisesresistanceto Alternaria alternata f.sp.lycopersici.Planta,2013,237(1):121-136.
[16]BERKEY R,BENDIGERI D,XIAO S.Sphingolipids and plant defense/disease:The“death”connection and beyond.Frontiers in Plant Science,2012,3:68.
[17]BEGUM M A,SHI X X,TAN Y,et al.Molecular characterization ofrice OsLCB2a1 gene and functional analysis of its role in insect resistance.Frontiers in Plant Science,2016,7:1789.
[18]YOSHIDA S,FORNO D A,COCK J H.Laboratory Manual for Physiological Studies of Rice.Los Banos,Philippines:International Rice Research Institute,1971:61.
[19]TAMURA K,PETERSOND,PETERSONN,etal.MEGA5:Molecular evolutionary genetics analysis using maximum likelihood,evolutionary distance,and maximum parsimony methods.Molecular Biology and Evolution,2011,28(10):2731-2739.
[20]TANG Q Y,ZHANG C X.Data Processing System(DPS)software with experimental design,statistical analysis and data mining developed for use in entomological research.Insect Science,2013,20(2):254-260.
[21]祝增荣,唐启义,吴慧明,等.现代植物保护信息技术实验.杭州:浙江大学出版社,2015:135.ZHU Z R,TANG Q Y,WU H M,et al.Experiments in Modern Information Techniques in PlantProtection.Hangzhou: Zhejiang University Press,2015:135.(in Chinese)
[22]LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod.Methods,2001,25(4):402-408.
[23]HUANG Z,HE G,SHU L,et al.Identification and mapping of two brown planthopper resistance genes in rice.Theoretical and Applied Genetics,2001,102(6/7):929-934.
[24]TAKAHASHI Y,BERBERICH T,KANZAKI H.Serine palmitoyltransferase,the first step enzyme in sphingolipid biosynthesis,is involved in nonhost resistance.Molecular Plant-Microbe Interactions,2009,22(1):31-38.
[25]SAUCEDO-GARCÍA M,GUEVARA-GARCÍA A,GONZÁLEZ-SOLÍS A,et al.MPK6,sphinganine and the LCB2a gene from serine palmitoyltransferase are required in the signaling pathway that mediates cell death induced by long chain bases in Arabidopsis.New Phytologist,2011,191(4):943-957.
[26]SHI L H,BIELAWSKI J,MU J Y,et al.Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis.Cell Research,2007,17(12):1030-1040.
[27]HANADA K.Serine palmitoyltransferase,a key enzyme of sphingolipid metabolism.Biochimica et Biophysica Acta(BBA):Molecular and Cell Biology of Lipids,2003,1632(1/2/3):16-30.

