Effectsofzincand silicon on cadmium toxicityand mineralelement translocation in two rice(Oryza sativa)genotypes1
MAPODZEKE James Mutemachani,SAGONDATichaona,SEHAR Shafaque,ZHANG Xin,HUANG Yuqing,ZVOBGO Gerald,MAODZEKA Antony,LWALABA WA LWALABA Jonas,SHAMSI Imran Haider*(.Key Laboratory of Crop Germplasm Resource of Zhejiang Province/College of Agriculture and Biotechnology,Zhejiang University,Hangzhou 30058,China;.School of Marxism,Zhejiang University,Hangzhou 3008,China)
Thetoxiceffectsofcadmium(Cd)onplantgrowth and developmenthave been well-documented[1].Accumulation of Cd in cultivated rice(Oryza sativa)affects rice growth and development and endangers human health via food chain[2-4].Cd is a non-essential,toxic microelement and thus has no biological benefits for plants and animals.Furthermore,it is an extremely stable and non-biodegradable heavy metal[4-7],which enters the food chain through plant uptake in soils[1,7].Increased Cd contamination can occur by natural means or anthropogenic sources,including industrial waste disposal,chemical fertilizers,pesticide usage,atmospheric deposition,sewage sludge disposal or irrigation of fields using Cd-polluted wastewater[1-2,8-9].In generality of heavy metal elements,Cd is more movable and toxic to organisms to be taken up easily by plant roots and translocated to shoots,thereby negatively affecting a wide range of cellular structures and plant metabolic processes essential for plant growth[10-11].Cd pollution in China is a huge concern with a large portion of arable land being contaminated[3].Previous reports have indicated more than 1.3×105hm2of Chinese agricultural soils are underCd pollution[10].Furthermore,the farmed products contaminated by Cd were 1.46×108kg which included 5×104t of rice[10].Recent reports of soil contamination in China from the Ministry of Environmental Protection(MEP)and the Ministry of Land and Resources(MLR)of China shows that soils around mining and industrialareasare highly contaminated,which raised deep concern for the agricultural soils[12].Surveys spanning from 2005 to 2013 covered 70%of Chinese land area showed that 16%of soil samples and 19%of agricultural soils were contaminated mainly with heavy metals,but 7%of the soil was contaminated by Cd and thus exceeded all other heavy metals whose contamination limit was set by MEP[12].
Zinc (Zn) is a vital micronutrient with indispensable metabolic roles in maintaining protein functions such as structural co-factor and in planta biochemistry[13-17].Zn is a close chemical equivalent to Cd[5],that can possibly counteract or modulate Cd uptake[18].Previous studies have showed that Zn in Cdstressed rice plants can create Zn-Cd antagonistic effects to reduce Cd toxicity in plants,and thereby improve plant growth even though Zn increases shoot Cd concentrations at higher Cd supply[19].Some of the Zn mitigation effects on Cd toxicity include enhanced chlorophyllformation,photosynthetic rate,antioxidative enzyme activities,and reduced malondialdehyde(MDA)content in rice[19].Furthermore,the application of Zn in Cd-stressed plants can slow down Cd absorption and translocation in barley[20].Likewise,the effects of Zn on Cd toxicity have been reported in wheat using a continuous supply of supplemental Zn,which effectively reduces the Cd concentrations in tissue and grain by inhibition of Cd uptake in roots[21-22].Similarly,decreased Cd accumulation and grain Cd concentration of wheat grown in Cd-contaminated soil by foliar Zn application have been observed[23].The Zn-Cd interactions revealed that Cd and Zn can influence the uptake of each other by synergistic and antagonistic interactions,which may depend on concentrations and tissue types involved[16,21,24].This also relates to both Zn and Cd sharing the same transporters such as OsIRT1 in rhizosphere and xylem loading transporter OsHMA2[25-26].
Silicon(Si)is beneficial for improving growth and development in various plants preferably rice(a high silicon accumulator)due to mitigation of biotic and multiple abiotic stresses[27-28].It has been documented that Si enhanced activities for improving plant tolerance to heavy metal toxicity[29].Fertilization with Si can improve rice yield with lowered Cd[27].Previous study on rice seedlings showed Si can prevent Cd accumulation by reducing H2O2and MDA contents,thereby enhancing antioxidantenzyme capacity[30]and maintaining the integrity of plant tissue[31].In wheat,Si application as amorphous silica is able to boost plant biomass and Si concentrations in plant tissues thereby reducing Cd availability and shoot translocation[32].Similar attributes of Si were also detected in both rice and wheatagainst chromium(Cr)toxicity by inhibiting the uptake and translocation of Cr in shoots and enhancing defense mechanisms against oxidative stress[33-34].
Rice(Oryza sativa L.)is a main cereal in the world after wheat(Triticum aestivum L.)with the production over 150 million hm2committed to growing rice alone[10].Therefore,studies aimed at reducing Cd toxicity effects in rice are vital to crop productivity and sustainability.The main objective of the present study is thus to investigate the longterm effects of Zn-Si on Cd toxicity in rice,and to determine how Zn-Si affect mineral element uptake in Cd-stressed rice plants.Heavy metal toxicity alleviation greatly relies on how plants resist the stress in the long term.The present paper describes results obtained from 90 d experiment in which two rice genotypes grown in hydroponic nutrient solution and given Cd stress combined with different exogenous Zn and Si levels.
1 Materials and methods
1.1 Seedling growth and experimental design
Two rice(Oryza sativa)genotypes,Xiushui 110(cadmium tolerant)and HIPJ 1(cadmium sensitive),were grown in a greenhouse at Zijingang Campus,Zhejiang University,Hangzhou,China.Rice grains were first surface sterilized with 2%H2O2solution for 15 min,washed with distilled water for 10 times and soaked for at least 2 d at 25℃in darkness.Healthy soaked grains were placed on a germinating tray and covered with damp filter paper,then incubated at 30℃in darkness for 24 h.Afterwards,the germinating grains were planted in sand and grown in a controlled chamber with 85%humidity,30℃/22℃(day/night),photoperiod of 16 h/8 h(light/dark)and light intensity of(225±25)µmol/(m2·s)for 14 d.The seedlings were selected for uniform size and transplanted(4 seedlings per pot)into 5 L plastic pots filled with half strength nutrient solution and acclimatized for 5 d in a greenhouse with controlled temperature and humidity.After 5 d,the seedlings were transferred into full strength nutrient solution.The nutrient solution comprised 2.9 mmol/L NH4NO3,1.7 mmol/L MgSO4·7H2O,1.0 mmol/L K2SO4,1.0 mmol/L CaCl2,0.32 mmol/L NaH2PO4·2H2O,36µmol/L EDTAFeNa,18 µmol/L H3BO3,9.1 µmol/L MnCl2·4H2O,0.52 µmol/L(NH4)6Mo7O24·4H2O,0.16µmol/L CuSO4·5H2O,and 0.15 µmol/L ZnSO4·7H2O.The pH of nutrient solution was adjusted to 6.0 using HCl or NaOH solution as necessary.Because EDTA could readily form complexes with Zn thereby affecting Zn uptake,36 µmol/L FeCl3·6H2O replaced EDTAFeNa before applying treatments. The treatments comprised two Cd levels[Cd0(0µmol/L)and Cd15(15µmol/L)]as CdCl2,three Zn levels[Zn0(0µmol/L),Zn1(1µmol/L)and Zn10(10µmol/L)]as ZnSO4·7H2O,and three Si levels[Si0(0 µmol/L),Si5(5 µmol/L)and Si15(15 µmol/L)]as Na2SiO3·9H2O.Cd,Zn and Si were applied into the nutrient solution to produce eight treatment combinations:(Cd0-Zn0-Si0,control),(Cd15-Zn0-Si0),(Cd15-Zn10-Si0),(Cd15-Zn0-Si15),(Cd15-Zn1-Si5),(Cd15-Zn10-Si5),(Cd15-Zn1-Si15)and(Cd15-Zn10-Si15).The plants were treated for 90 d and the whole experiment was set up on a randomized block design with three replications for each treatment.To sustain the treatment levels,every 5 d the nutrient treatment solution was renewed.
1.2 Measurements of physiological parameters
Measurements of net photosynthetic rate(Pn),stomatal conductance (Gs), intercellular CO2concentration(Ci),transpiration(Tr),and soil and plant analyzer development(SPAD)values were carried out on the second fully expanded leaves after 90 d of treatment using an infrared analyzer(LI-6400 System,Li-COR Company,USA)and a chlorophyll meter(Minolta SPAD-502,Japan).All measurements were done on a sunny day between 10:00 a.m.and 1:00 p.m.,with air temperature of 25℃to 28℃,relative humidity of 50%to 70%,CO2concentration of400 µmol/L and photosyntheticphoton flux density(PPFD)of 1 000 µmol/(m2·s).
The root length(RL),shoot height(SH)and fresh mass were immediately measured at plant harvest.The RL was measured from the primary root tip to the root-shoot junction,while the SH was measured from the root-shoot junction to the shoot tip.Plants were separated into roots,shoots and leaves,and dried in a hot air oven at 105℃for 3 h and at 80℃for 24 h[35].Thereafter,dry mass was measured.Other harvested plants were stored at 4℃for enzyme activity and other parameter tests.
1.3 Measurements of antioxidant enzyme activities and lipid peroxi-dation
The activities of superoxide dismutase(SOD)and catalase(CAT)in both roots and leaves were assayed according to previous literature with some modifications[33,35-36].For SOD determination,0.5 g plant tissue samples were grinded in pre-cooled mortar on ice and 3 mL of 1 mol/L Tris-HCl buffer solution(pH 7.4)was added.The samples were homogenized on ice and filled up to 5 mL with buffer solution.After centrifugation of the samples at 1.2×104r/min for 20 min at 4℃,the enzyme extract(supernatant)was mixed in glass tubes with reaction solution prepared using 75 µmol/L nitroblue tetrazolium(NBT),20µmol/L riboflavin,100µmol/L EDTA-Na2and 130 mmol/L methionine.Light and dark controls having reaction solution and distilled water were used for CK and zero reading,respectively.The control light and all other samples positioned under light conditions at 4 000 lx for 20 min,while the control dark sample was in 100%dark condition. The SOD activity was measured spectrophotometrically at 560 nm against a reagent blank.The CAT reaction solution consisted of 300 mmol/L H2O2and 1 mol/L Tris-HCl buffer.Solution mix of 2.8 mL Tris-HCl buffer,0.1 mL sample enzyme extract and 0.1 mL H2O2was shaken gently and measured spectrophotometrically at 240 nm within the time range of 0-30 s.
Lipid peroxidation was measured and expressed as malondialdehyde(MDA)concentration(nmol/g)using a modified thiobarbituric acid(TBA)method according to previous studies[33,35-36].MDA reaction solution contained 5%trichloro-acetic acid(TCA)and TBA.The reaction solution consisted of 2.5 g TBA in 500 mL solution of 5%TCA.Solution mix of 1.5 mL sample enzyme extract and 2.5 mL reaction solution was added in small tubes,heated in water bath at 95℃for 15 min,and then immediately given in ice bath.After centrifugation at 4 800 r/min for 10 min at 4℃,the MDA content was measured specrophotometrically at 532 and 600 nm using distilled water for zero reading.
1.4 Element analysis
Approximately 0.1 g dried samples were placed in tube to determine the concentrations of cadmium(Cd),zinc(Zn),silicon (Si)and other mineral elements.The 5 mL 65%HNO3and 1 mL 30%(V/V)H2O2were added into the dried samples,which were subsequently digested in a microwave(Mars 6,CEM Technologies,USA)[3].Thereafter,the tubes of samples were placed on a block heater for 2 h at 160℃in a fume hood,afterwards diluted to 20 mL with Milli-Q water.The inductively coupled plasmaoptical emission spectrometer(ICP-OES,Optima 8000DV,PerkinElmer,USA)with the reference standard of 1 000 mg/L of quality control standard of 21elementssuppliedbyPerkinElmerused for elemental content detection.Translocation factor(TF)is the ratio of metal concentrations in aerial plant parts to plant roots[37].TFs of elements from root to shoot and from shoot to leaf were calculated using the following equations:
TF of element from root to shoot=[Element]shoot/[Element]root.
TF of element from shoot to leaf=[Element]leaf/[Element]shoot.
The percentages for TF of Cd from root to shoot and from shoot to leaf were calculated using the following equation:
Cd TF from root to shoot/%=[Cd]shoot/[Cd]root×100.
Cd TF from shoot to leaf/%=[Cd]leaf/[Cd]shoot×100.
1.5 Statistical analysis
Experimental data were statistically analyzed using SAS university edition,with the PROC ANOVA and PROC GLM procedures.Significant effects of genotypes,Cd,Zn and Si were evaluated using analysis of variance(ANOVA)at P<0.05.Mean values were compared using least significant difference(LSD)test at P<0.05 per genotype[34].
2 Results
2.1 Effects of Zn and Si combination treatments with Cd on plant growth
Table 1 shows the plant growth parameters measured after 90-d treatments,and Fig.1 shows the plants after treatments.Cd toxicity significantly(P<0.05)suppressed plant growth as was expected.Xiushui 110 had significantly(P<0.05)greater root length(RL)and shoot height(SH)compared with HIPJ 1 when exposed to Cd(15µmol/L)treatment.The combination treatment of Cd(15µmol/L)and Si(15 µmol/L)showed significantly(P<0.05)higher SH and RL in both the genotypes compared with Cd(15µmol/L)alone treatment.The same was observed in Zn(10 µmol/L)and Cd(15 µmol/L)combined treatment.No significant difference was observed in shoot height and root length between the two genotypes under the combination treatment of Cd(15 µmol/L),Zn(10 µmol/L)and Si(15 µmol/L).Sole Zn alleviation was highly effective on biomass increase than sole Si for both the genotypes as supported by dry mass,tillerand panicle formation.Zn-Sicombined treatmentsdid not improve growth parameters comparing with Cd15-Zn10-Si0 treatmentin both the genotypes.Si addition caused reduction of both root and shoot heights compared with Cd15-Zn10-Si0 treatment.The dry mass data signified the treatment Cd15-Zn10-Si15 having reduced shoot dry mass compared with Cd15-Zn10-Si0 in HIPJ 1.Similarly,Si5 addition reduced biomass production in Zn10 treatmentsbutmainly affected Xiushui110 by reducing dry mass of both root and shoot.
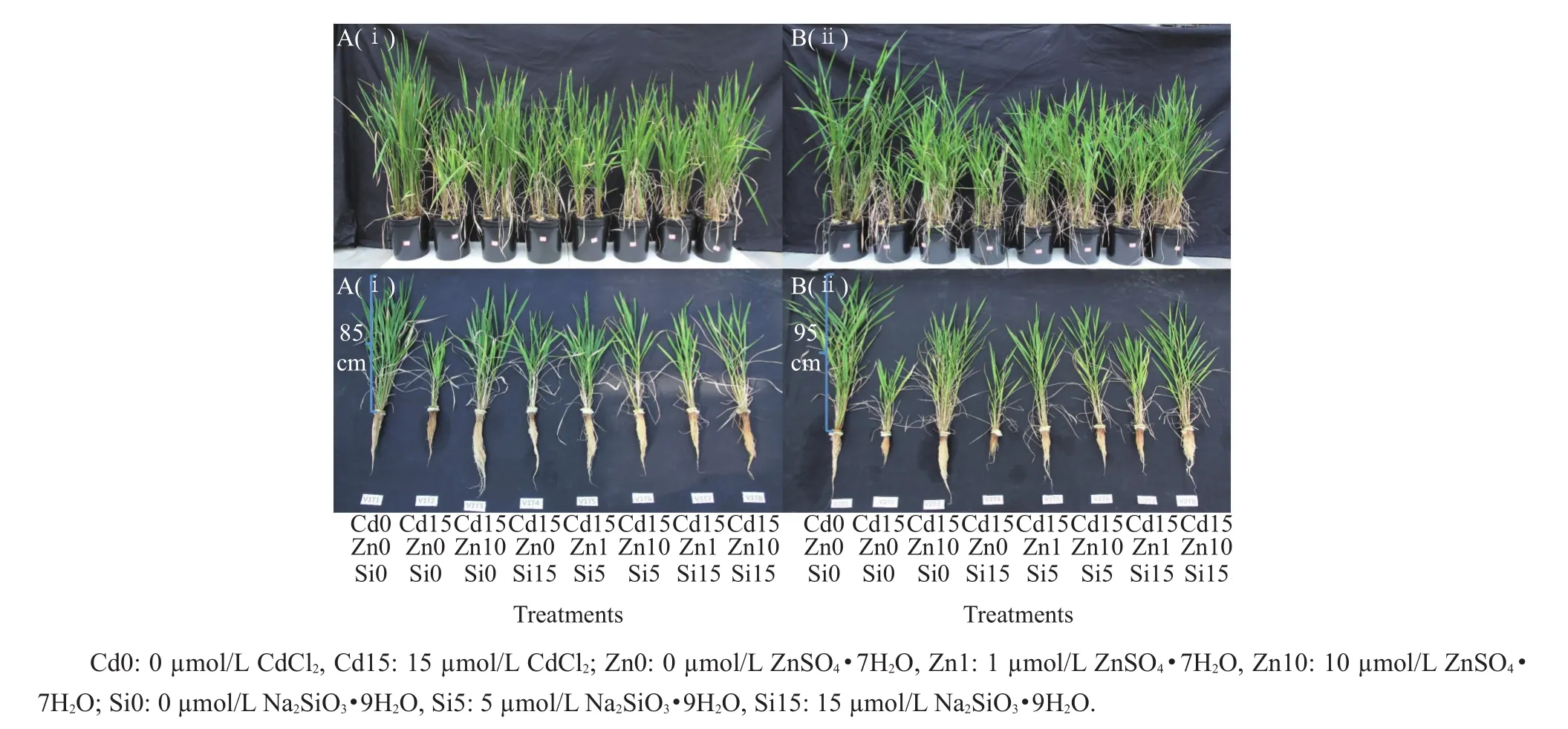
Fig.1 Differences in growth of two rice genotypes,Xiushui 110(A)and HIPJ 1(B),after 90-d treatment
2.2 Effects of Zn and Si combination treatments with Cd on photosynthetic parametersand transpiration rate
Table 2 shows measurements of photosynthesis related attributes,chlorophyll contents(expressed as SPAD value)and transpiration rate at 90-d treatment.Cd-induced stress was evident by a significant reduction(P<0.05)in SPAD,net photosynthetic rate(Pn),and stomatal conductance(Gs).However,Zn and Si effects sustained increased Pn and SPAD values in all Zn-Si combined treatments for both the genotypes as compared with Cd alone treatment(Cd15-Zn0-Si0).The sole Cd treatment(Cd15-Zn0-Si0)produced the lowest Gs in HIPJ 1,but the treatment Cd15-Zn1-Si15 had significantly higher Gs(P<0.05)in Zn-Si combined treatments.Alternatively,Xiushui 110 produced significantly higher Gs(P<0.05)with sole Zn alleviation(Cd15-Zn10-Si0)as compared with all other Cd-stressed plants.This obviously depictscleardistinction ofgenotypic difference between the two genotypes with response to stomatic stability.IntercellularCO2concentration decreased in Xiushui110 plantsunderZn-Si treatments.In HIPJ 1,intercellular CO2concentration also decreased under Zn-Si and sole Zn and Si treatments relative to mock control and 15µmol/L Cd treatment.Different responses between Xiushui 110 and HIPJ 1 on transpiration(Tr)was observed,in which the treatment Cd15-Zn10-Si0 highly increased Tr towards the mock control in Xiushui 110,but the Cd15-Zn1-Si15 highly increased the Tr toward mock control in HIPJ 1.This suggests high Zn for Xiushui 110 and high Si for HIPJ 1 can increase transpiration rates.However,there was a general increase in Tr towards the mock control for both the genotypes using theZn-Sicombinedtreatments.

Table 2 Effect of Cd,Zn and Si treatments on chlorophyll content(SPAD),net photosynthetic rate(Pn),stomatal conductance(Gs),intercellular CO2concentration(Ci),and transpiration(Tr)of the top-most secondary fully expanded leaves in two rice genotypes,Xiushui 110 and HIPJ 1,at 90-d treatment
2.3 Effects of Zn and Si combination treatments with Cd on antioxidant enzyme activities and MDAcontents
Table 3 shows the enzyme activities and MDA contents recorded in both roots and leaves.The Cd alone treatment(Cd15-Zn0-Si0)increased leaf SOD for both the genotypes as compared with the mock control.The treatmentCd15-Zn1-Si15 caused significantly higher root SOD(P<0.05)in HIPJ 1,but Cd15-Zn10-Si5 recorded the lowest.Xiushui 110 did not attain much change in treatments for root SOD.Both Xiushui 110 and HIPJ 1 attained the lowest leaf SOD in treatment Cd15-Zn10-Si0,suggesting that Zn10 decreased the leaf SOD as compared with Si which stimulated leaf SOD in Zn-Si combined treatments.The results of root CAT activity on genotype response to treatments which showed the highest root CAT activity was in treatment Cd15-Zn1-Si5,and the lowest was in Cd15-Zn10-Si5 for Xiushui 110,while there was a reverse trend in the same treatments for HIPJ 1.Hence,increased Zn in treatments reduced the root CAT activity in Xiushui 110,but in HIPJ 1 was on the contrary.It might relate to genotypic differences by which the same treatment produces opposite effects on distinct genotypes.Xiushui 110 had overall higher leaf CAT in all treatments than HIPJ 1,which correlated to increased Cd tolerance than HIPJ 1,which augments the increased leaf CAT activity in Cd alone treatment(Cd15-Zn0-Si0)for Xiushui 110 as compared with HIPJ 1.Hence,the upsurge of leaf CAT activity is maybe one of the mechanisms utilized by Xiushui 110 for Cd tolerance.However,the CAT activity in leaves both the genotypes was greatly reduced in treatment Cd15-Zn10-Si5,but only HIPJ 1 attained statistically higher leaf CAT levels with Cd15-Zn1-Si5 treatment,implying that increased Zn levels could reduce CAT activity.Root MDA distinguished the genotypes by mainly affecting Xiushui 110 than HIPJ 1.The treatment Cd15-Zn1-Si15 lowered root MDA contentsinXiushui110.MDA contents significantly increased by Cd in treatments(P<0.05)as observed by the higher leaf MDA of both the genotypes found in Cd alone treatment(Cd15-Zn0-Si0)as compared with the control.Both Zn and Si independent and combined treatments lowered the leaf MDA contents as compared with Cd15-Zn0-Si0.Combined Zn-Si treatment of Cd15-Zn10-Si15 reduced the leaf MDA of both the genotypes as compared with Cd alone treatment(Cd15-Zn0-Si0).The above results showed MDA contents were mostly affected in leaves than roots.
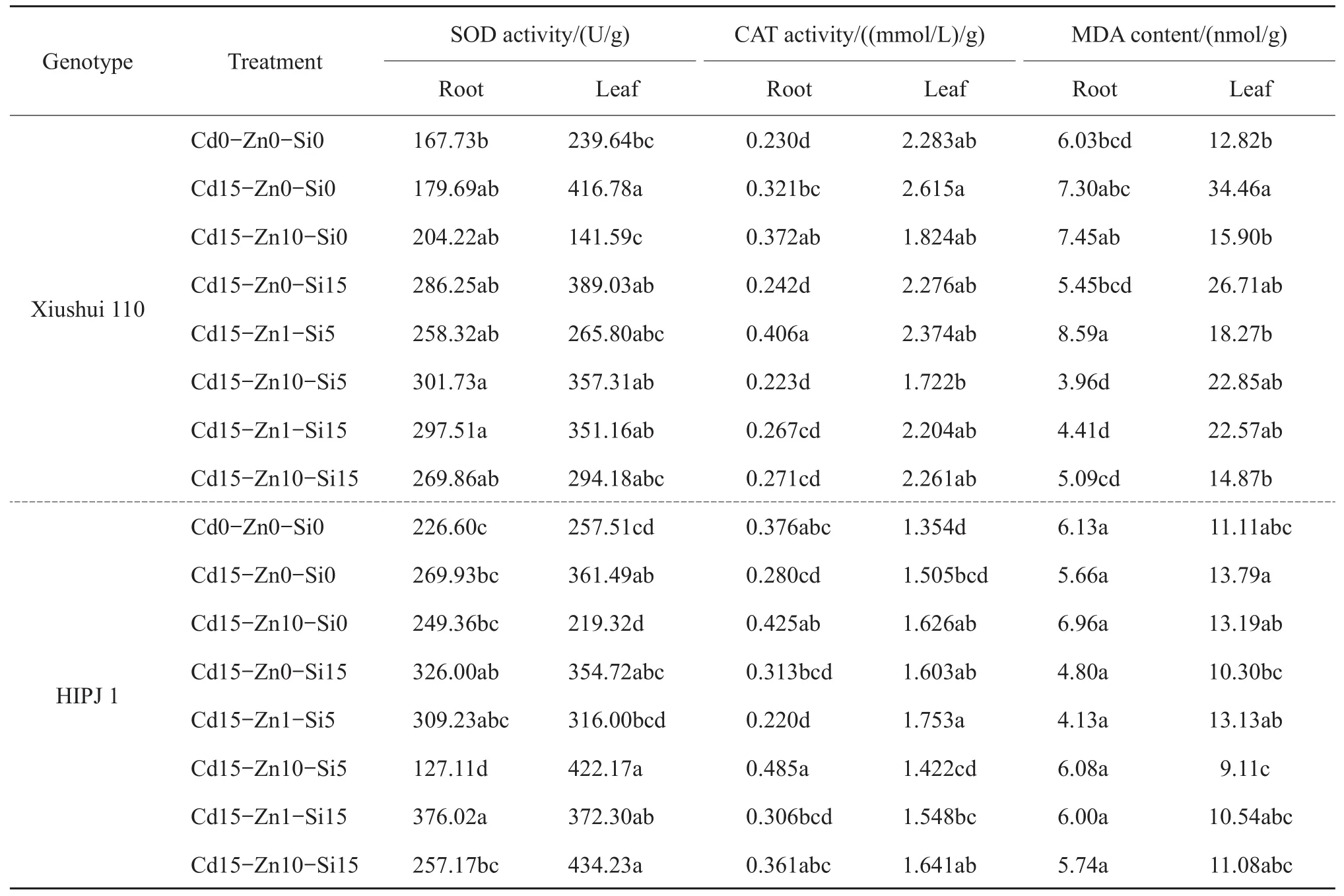
Table 3 Effect of Cd,Zn and Si treatments on superoxide dismutase(SOD)and catalase(CAT)activities,and malondialdehyde(MDA)content in the roots and the top-most secondary leaves of two rice genotypes,Xiushui 110 and HIPJ 1,at 90-d treatment
2.4 Effects of combination treatments on uptake and accumulation of Cd,Zn and Si
Table 4 shows the element concentrations for Cd,Zn and Si in plant tissues after 90-d treatment period for both the genotypes.Cd,Zn and Si uptake and localization in root,shoot and leaves were in a dose and duration manner.The Cd concentration was higher in the root than shoot and leaf respectively(root>shoot>leaf)of both the genotypes.Added Zn stimulated significant(P<0.05)increase of Cd in the roots of both the genotypes in treatment Cd15-Zn10-Si0 compared with Cd15-Zn0-Si0.Si sole alleviation(Cd15-Zn0-Si15)reduced the Cd concentration in the shoot and leaf as compared with Cd15-Zn0-Si0 in HIPJ 1.Combined Zn-Si treatments with Zn10 significantly(P<0.05)increased Cd concentration in the shoot of both the genotypes and in the leaves of HIPJ 1.This also correlates to the same treatments with increased Zn concentration mainly in shoot.The Cd15-Zn1-Si5andCd15-Zn1-Si15treatments reduced Cd concentration in the shoot and leaves compared with all Zn-Si combined treatments in both the genotypes.Xiushui 110 had ultimately lower Cd concentration of both shoot and leaf in Cd sole treatment Cd15-Zn0-Si0 than HIPJ 1,which might relate to the Cd tolerance of Xiushui 110 in reducing Cd uptake as one of the tolerance mechanisms at the later growth stage.Furthermore,Xiushui 110 had overall higher Si accumulation in all treatments than HIPJ 1,which could also relate to tolerance.
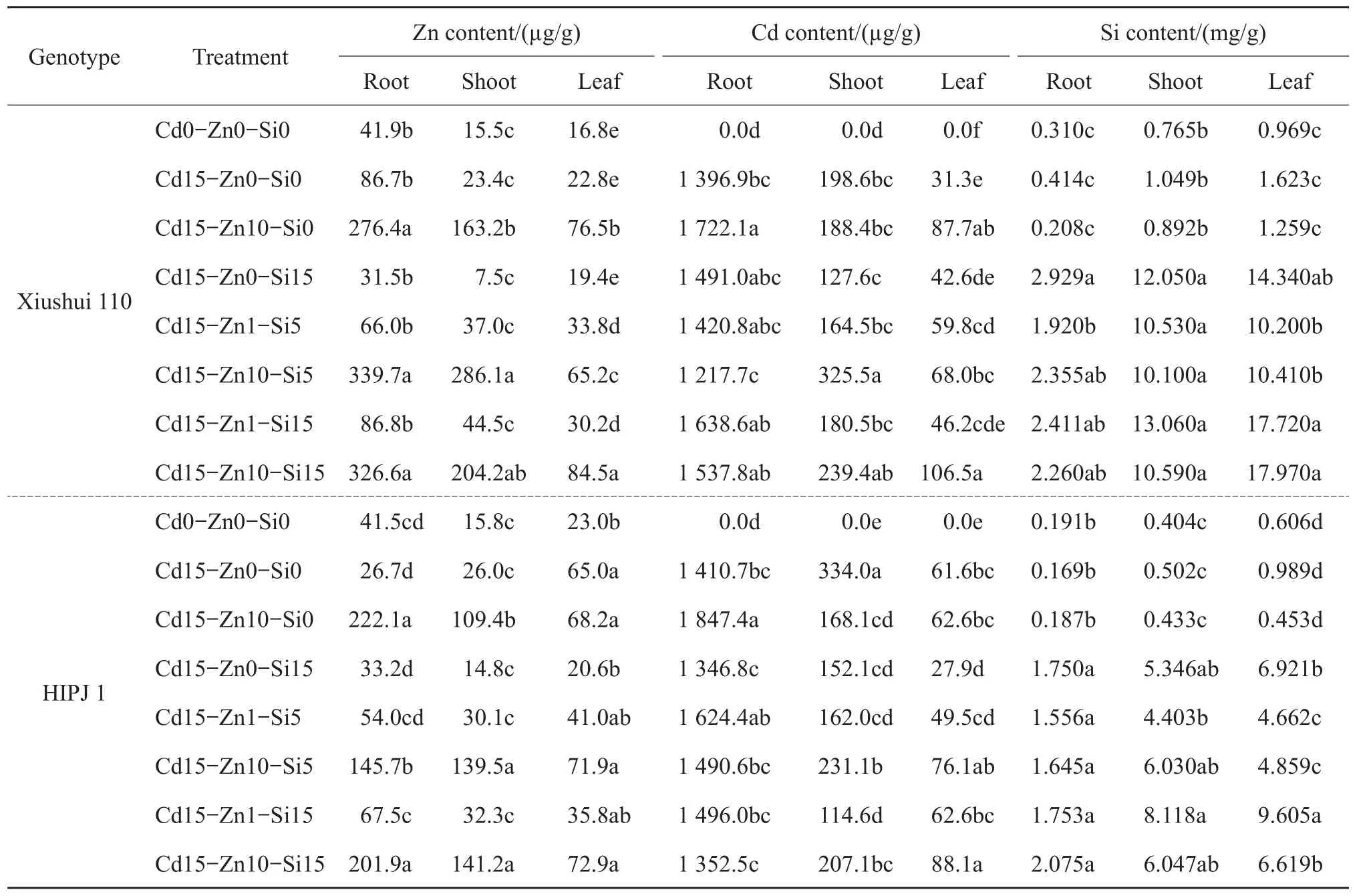
Table 4 Zn,Cd and Si concentrations of two rice genotypes,Xiushui 110 and HIPJ 1,after 90-d treatment
2.5 Effects of combination treatments on mineral elements and Cd translocation
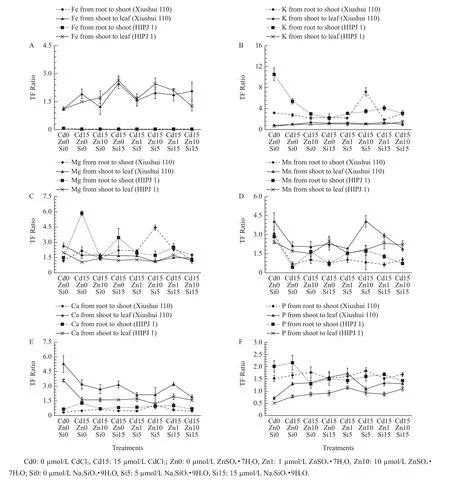
Fig.2 Elements of Fe(A),K(B),Mg(C),Mn(D),Ca(E),P(F)translocation factor ratios from root to shoot and from shoot to leaf in two rice genotypes,Xiushui 110 and HIPJ 1,after 90-d treatment
Fig.2 shows the element translocation factor(TF)ratios from root to shoot and from shoot to leaf in all treatments.Cd instigated Fe TF from the shoot to the leaf and Mg TF from the root to the shoot in both the genotypes.Alternatively,Cd ultimately reduced Mn TF from the root to the shoot and from the shoot to the leaf in both the genotypes.The same TF reduction occurred for K TF from the root to the shoot,Ca TF from the shoot to the leaf and Mg TF from the shoot to the leaf in both the genotypes.The Zn-Si effects on Fe TF influenced the uptake from the shoot to the leaf by which Si increased and Zn reduced Fe TF in Xiushui 110.Moreover,Si also increased Fe TF from the shoot to the leaf in HIPJ 1 except Cd15-Zn10-Si15 treatment.The treatment Cd15-Zn0-Si15 showed that sole Si application markedly increased the Fe TF from the shoot to the leaf than all other treatments in both the genotypes.In Xiushui 110,a highly notable sharp increase of Mg and K TFs from the root to the shoot as well as Mn TF from the shoot to the leaf occurred in the treatment Cd15-Zn10-Si5 attributed to lower Si effect,which was not observed in HIPJ 1.Zn and Si individual and combined treatments were not very effective on increasing the Ca TF from the shoot to the leaf and the Mn TF from the declined state caused by Cd.The treatment Cd15-Zn1-Si15 had the highest K TF from the root to the shoot than all other Zn-Si treatments in HIPJ 1.Cd addition caused a general increase of P TF from the root to the leaf in both the genotypes,but a substantial decrease is evident in TF from the root to the shoot by combined Zn-Si effects in HIPJ 1 alone.The TF from the root to the shoot and from the shoot to the leaf in both the genotypes was presented in Table 5.The sole Zn and Si alleviation managed to reduce Cd TF from the root to the shoot in both the genotypes.Xiushui 110 had a spearheaded increase of Cd TF from the root to the shoot in the Cd15-Zn10-Si5 treatment.Nevertheless,all Zn-Si treatments reduced Cd TF from the root to the shoot as compared with Cd sole treatment(Cd15-Zn0-Si0)in HIPJ 1.In addition,the treatment Cd15-Zn1-Si15 ultimately reduced Cd TF from the root tothe shoot as compared with all treatments under Cd stress in HIPJ 1.However,Zn-Si combined effects mainly increased the Cd TF from the shoot to the leaf in HIPJ 1.The sole Zn addition mainly stimulated Cd TF from the shoot to the leaf in both the genotypes,but sole Si addition reduced Cd TF from the shoot to the leaf.With Zn-Si combined effects,the treatments Cd15-Zn1-Si5 and Cd15-Zn1-Si15 reduced Cd TF from the root to the shoot in both the genotypes but being mainly effective on HIPJ 1.Analysis of Cd TF from the shoot to the leaf in HIPJ 1 showed the treatment Cd15-Zn1-Si5 was more effective on reducing Cd translocation in all the Zn-Si combined treatments.
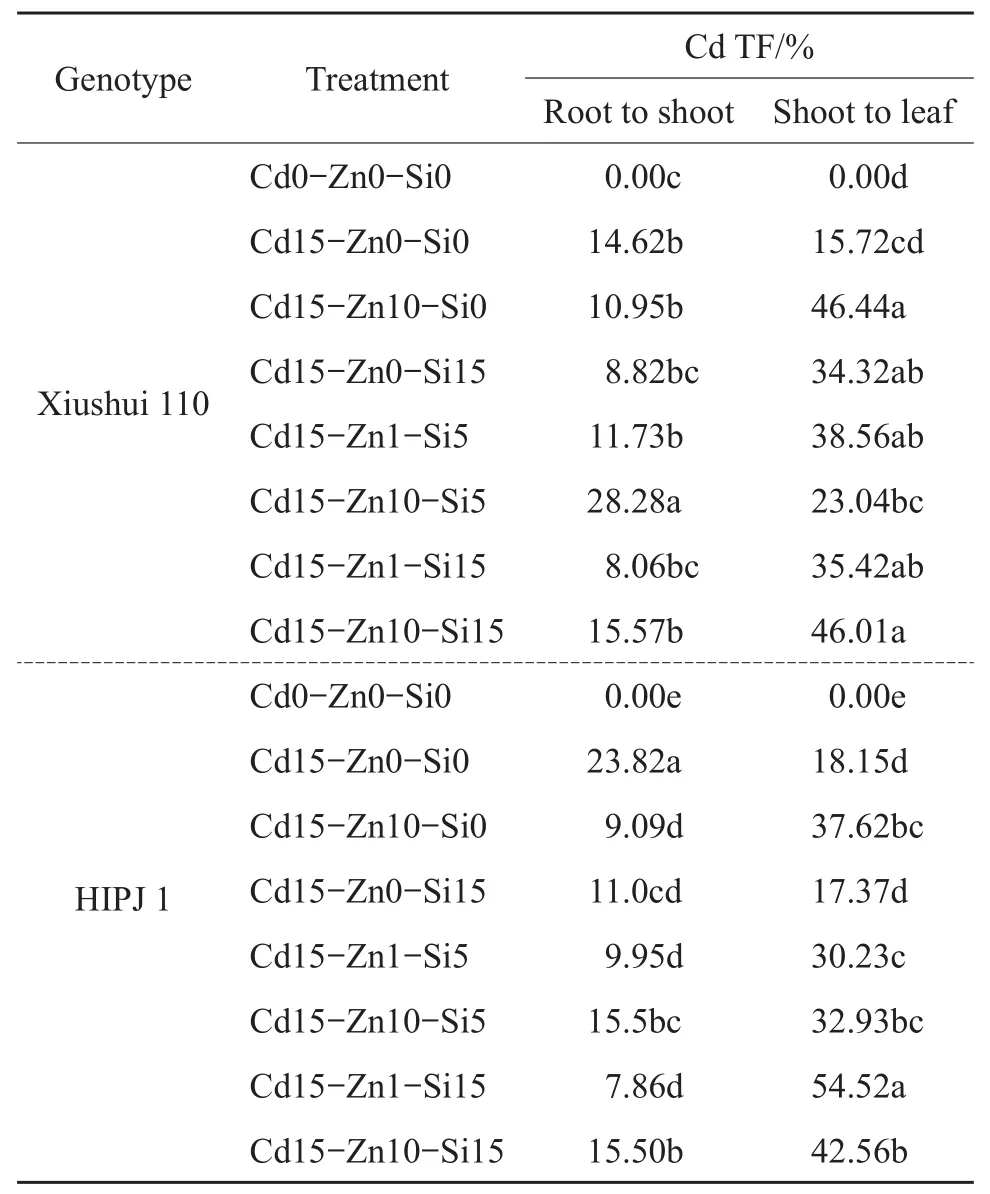
Table 5 Effect of Cd,Zn and Si treatments on Cd translocation factor(from root to shoot,from shoot to leaf)of two rice genotypes,Xiushui 110 and HIPJ 1,after 90-d treatment
3 Discussion
The present study explored Zn and Si singular and combined effects on Cd toxicity in rice plants at the 90-d treatment.Cd toxicity significantly reduced growth and photosynthetic parameters,which destabilized rice growth.This is in line with previous studies that the metabolism rates and subsequent biomass were reduced under Cd stress in rice[1]and other cereals[15]due to declined chlorophyll concentration and decreased photosynthetic rates.
The sole Zn and Si addition reduced Cd negative effects which corroborates findings of increased photosynthetic rate and biomass in rice[19,38].However,long-term exposure to 15 µmol/L Cd treatments for 90-d inevitably increased Cd uptake to aerial plantparts thusaffecting heading and greatly inhibiting grain filling.The addition of Zn ultimately increased biomass,tillering and panicle formation.Thiscould beexplained by competitivenature existing between Cd and Zn that involves synergism and antagonism between the ions due to chemical similarities[5-6,19,39].Increased rice biomass by Zn strongly suggests the dilution of Cd stress.The Zn-Si combined treatments did not significantly improve growth parameters as compared with Cd15-Zn10-Si0 treatment in both the genotypes;hence,the beneficial effects of Si on biomass increase were highly influential at vegetative stage of HIPJ 1 alone in our previous unpublished data.However,Zn-Si effects increased Pn and SPAD values in both the genotypes as compared with Cd sole treatment(Cd15-Zn0-Si0),which indicated the beneficial effects of Zn and Si on rice metabolism and stabilization of redox homeostasis in photosynthetic cell.Previous studies have reported the importance of Zn[6,19,39]and Si[40-41]for restoring chlorophyll contents and improving plant photosynthetic rate against Cd toxicity.Furthermore,Zn-Si effects increased Pn in HIPJ 1 than Cd15-Zn0-Si0,due to enhanced stomatal control stability as shown by increased Gs in both Cd15-Zn10-Si0 and Cd15-Zn0-Si15 treatments that significantly improved Gs in all Zn-Si combined treatments.This result indicates that observed differential effects in these two rice cultivars are due to their genotypic response difference,in which HIPJ 1 might utilize the Zn-Si combined effects for improved stomatic control whereas Xiushui 110 likely did notfavor combined Zn-Sieffects on Gs.Therefore,indepth research is required to understand mechanisms behind the difference in Gs response for these genotypes under Cd stress.Even though previous study reported that Si reduced Gs in rice under Cd stress[38],the present results showed Si instigated Gs only in HIPJ1,which may depictgenotypic difference while Zn stimulated Gsincrease in both the genotypes.Because the Zn-Si combined treatments mainly reduced Ci in both the genotypes as compared with control but inversely causing an increase in Tr towards control,it is likely that the plants under the combined Zn-Si treatments reduced the internal CO2concentration by utilizing CO2at a higher rate to counteract the effect of Cd induced Pn deficits and improve plantstress tolerance.This could also strongly augment the increased Tr activity in the same treatments and be a mechanism of Zn-Si-promoted plant Cd resistance.However,in accordance with NWUGO et al.[38],we found that Cd was not associated with Ci reduction in both the genotypes as shown by Cd alone treatment(Cd15-Zn0-Si0)against the mock control,which implies Cd not being highly associated with CO2diffusion and retention in leaves but likely metabolism defects associated with photosynthetic enzyme inhibition.
The effects of Cd toxicity on plant metabolism by influencing key enzyme activities,production of reactive oxygen species(ROS)and lipid peroxidation have been well documented[4,19,31,42].SOD enzymes catalyze the breakdown of excess superoxide radicals(O2·-)into H2O2and O2,while CAT enzymes counteract and decompose H2O2to H2O and O2thus reducing oxidative damage in plant cells[42].Therefore,as a defense response mechanism to elevated reactive oxygen species(ROS)production,the increased activity of these enzymes brought about by Cd toxicity is expected.In the present study,Si increased leaf SOD activity in Zn-Si treatments as compared with Zn alone treatment of suppressed SOD.It is similarly reported that Zn influenced SOD reduction in rice subjected to Cd stress and could relate to alleviated oxidative stress[19].Additionally,previous literature revealed Si was involved in SOD stimulation in rice plants grown under the presence of Cd and Cr[4,33].Increased Zn levels might reduce leaf CAT activity as shown in both the genotypes by greatly reducing CAT activity in the treatment Cd15-Zn10-Si5,which was consistent with a previous study thatZn addition caused a reduction in antioxidant enzyme levels in Cd-stressed rice[19].Besides,Zn supplementation atnon-toxic levels restored and enhanced antioxidant enzyme activities including CAT activity in tomato plantsasa mitigation response to Cd toxicity[39].Zn can therefore reduce orincrease CAT activity depending on genotypes,tolerance to heavy metals,plant tissues and most likely duration of treatment.We investigated Cd oxidative stress by MDA content as a general indicatorforlipid peroxidation[19].The sole Cd treatment caused a substantial increase in leaf MDA contents in both the genotypes and the leaf MDA levels were generally higher than the roots in all treatments,suggesting thatthe planttolerance mechanisms of reducing lipid peroxidation might be strong in root cells but much susceptible in leaf cells,because roots are the first contact with heavy metals[30].Previous studies have also confirmed Cd can increase lipid peroxidation levels in rice leaves[4,19,31].Similarly,Cd rendering MDA effects in leaves have been reported in other plants such as Brassica and cotton[36,43].It was notable that Xiushui 110 attained overall higher leaf MDA contents in all treatments than HIPJ 1,which was highly unusual for a tolerant genotype Xiushui110 and could suggestthe resilience to withstanding higher peroxidation levels.This portrays genotypic difference in response to Cd detoxification and accumulation mechanisms[42].However,sole Zn or Si application and Zn-Si combined effects such as Cd15-Zn1-Si5,Cd15-Zn1-Si15 and Cd15-Zn10-Si15 reduced leaf MDA content compared with sole Cd treatment in both the genotypes,which was expected because Zn and Si have been reported to effectively reduce MDA as a way of alleviating Cd stress in rice[19,31].
Cd has a high mobility in the environment[16,43]and plants will generally accumulate more Cd in roots than aerial parts[4,38,44].Similarily,our results also confirms high Cd levels in roots as compared with aerial parts of both the genotypes.Therefore,rice plantsare likely equipped with metaltoxicity deterrent response mechanisms to reduce uptake of metals.Inversely,plants acquired more Si in aerial parts as compared with roots as similarly have been confirmed in previous studies[32,38,45],which relates to rice enhanced ability to accumulate Si in tissues for growth and development[27,33,44]. The sole Zn application (Cd15-Zn10-Si0) had higher Cd retention in roots of both the genotypes,which supposedly improved shoot growth by limiting Cd movement to aerial parts.Zn competitive nature with Cd for the same membrane transporter channels might lessen Cd uptake and mitigate Cd oxidative stress[6,46].However,sole Si alleviation(Cd15-Zn0-Si15)was more influential in HIPJ 1 by reducing Cd concentration in shoots and leaves as compared with Cd15-Zn0-Si0 treatment,which likely mitigated Cd toxicity for sensitive genotype and prolonged rice growth in Cd environment.Previousliteratures confirm Si can reduce Cd shoot concentrations in plants such as rice,wheat and Brassica chinensis[4,32,43].Furthermore,Si can co-precipitate with metals(Cd)in endodermis cell wall thus lowering their uptake and apoplastic transport[4,47-48].Yet,previous study on maize has reported a double increase in the total amounts of Cd per plant of Cd-Si treated plants as compared with Cd treated alone even though Si improved growth parameters[49].In addition,Si failed to decrease the Cd concentration in maize leaf tissues even though net photosynthetic rate improved and Cd toxicity declined[41].Therefore,the reduction of Cd uptake by Si in plants could depend on genotypes and concentrations used, which requires further elucidation to the in planta mechanism.The mechanism behind alleviation of Cd toxicity by Zn-Si could be explained by the complex interaction between Cd and Zn-Si.The 10µmol/L Zn treatments increased Cd content in shoots of both the genotypes.However,increased plant biomass suggests that Zn diluted Cd stress in plant tissues.Furthermore,Zn1 treatments lowered Cd content but reduced biomass due to lowered Zn,showing a threshold between Cd and Zn-Si is probable to yield a balance of reduced Cd uptake and growth beneficial effects of Zn and Si at the reproductive stage of rice.
TF can reveal the ability of plants to uptake and transport metals from roots to aerial parts[37].Hence,it is imperative to examine mineral element uptake response with Cd,Zn and Si effects in rice.Cd literally reduced several elements TF in both the genotypes.Previous study has showed that increased Cd can interrupt the uptake of mineral nutrients in plants[50].Various macro-and microelements are severely affected by Cd on uptake,transportation and utilization in plants[42].In wheat,linked Cd addition to K and Ca concentrations were declined in the root and shoot[51]due to reduced TF disturbing ion homeostasis.Similarly,Cd might share some similar uptake routes with Ca affecting ion homeostasis[42],which strongly suggested the reduced Ca TF from the shoot to the leaf in the present study.Rice growth was affected because Ca plays essential roles in structure and function of plant cells by maintaining cell wall integrity and membrane structures[52].Previous study has showed that Ca is transported to plant shoots by transpiration stream[52].Therefore,the reduction in Tr caused by Cd in the present study is highly responsible for the reduced Ca translocation.Additionally,Mn has been attributed to severely reduce Cd uptake and translocation in rice mutants[46],and in thisstudy,increased Cd concentrations markedly inhibited Mn TF from the root to the shoot and the leaf in both the genotypes,which suggests the possibility of antagonistic relationship between Mn and Cd.Cd sole treatment(Cd15-Zn0-Si0)instigated Mg TF from the root to the shoot but hindered Mg TF from the shoot to leaf in both the genotypes,which is correlated to reduced Pn,showing that Mg is an essential central ion for chlorophyll structure[51].The treatment Cd15-Zn0-Si15 attained the highest Fe TF from the shoot to the leaf in all treatments of both the genotypes,which collaborates with previous study in rice that Si can induce Fe transportation to shoots[4,45].It is also imperative to note that all treatments exposed to Cd stress regardless of Zn-Si effects increased the Fe TF from the shoot to the leaf relative to the mock control,which is an indicator that Cd stress can increase Fe movement to leaves.However,Cd alleviation by sole Zn(Cd15-Zn10-Si0)in Xiushui 110 was able to reduce Fe TF from the shoot to the leaf relative to the mock control,because surplus Zn can at times suppress Fe movement and result in Fe deficiency in many cases due to similar radii between the two hydrated ions[29-30].K+can counterbalance anions in plants which can improve ion homeostasis[35].Therefore,the treatment Cd15-Zn1-Si15 with the highest K TF from the root to the shoot than all other Zn-Si treatments in HIPJ 1 requires further elucidation.The sole Si and Zn addition including combined Zn-Si effects against Cd did not ultimately increase Ca TF from the shoot to the leaf,showing a lack of association among Zn,Si and Ca leaf supply.Likewise,both Si and Zn in combined treatments did not effectively improve Mn TF,which was similar to Cd suppressed and previous studies in wheat that the application of Si failing to amend Mn uptake by roots in response to Cd toxicity[51].Alternatively,a general increase in P TF caused by Cd indicated the need of more P in Cd-stressed plants for its role in plant growth and development[35].In addition,a declined TF from the root to the shoot for P due to individual and combined Zn-Si effects in HIPJ 1 could have affected plant growth and development as a long-term effect.This shows combined Zn-Si not favoring P translocation in the long-term treatments makes it a huge deterrent for rice growth in Cd environment.
It was forthcoming to have reduced Cd TF from the root to the shoot in both the genotypes due to individual Zn and Si mitigation effects(Cd15-Zn10-Si0 and Cd15-Zn0-Si15)against Cd15-Zn0-Si0.The immediate assumption being the antagonistic effects existing between Cd and Zn lowered Cd uptake frequency,and Si suppressed Cd uptake by direct or indirect mechanisms.This suggests Zn might have a big role in modulation of Cd from root to shoot transfer[1].According to previous study,supplemental Si is also linked to a reduction in Cd from root to shoot transport in Brassica chinensis L.[43]and rice[27].In the present study,when increased exogenous Zn in the presence of Cd,lowered Si supplementation greatly affected Cd alleviation as it amplified Cd TF from the root to the shoot and increased toxicity in the tolerant genotype.This is why Xiushui 110 increased Cd TF from the root to the shoot in Cd15-Zn10-Si5 treatment linked to low Si and the concurrent surges in Mg TF from the root to the shoot,P TF from the root to the shoot and Mn TF from the shoot to the leaf as response mechanisms to alleviate the stress.The reduced translocation of most mineral elements subdued the growth of the sensitive genotype HIPJ 1 even though there was reduced Cd TF from the root to the shoot in the treatments Cd15-Zn1-Si5 and Cd15-Zn1-Si15.This may suggest that the Cd TF from the root to the shoot varies highly depending on genotype and interactive effects of Cd,Zn and Si,but still requires more elucidation to the mechanisms involved.However,the present study also showed that Zn-Si effects in the treatments mainly suppressed Cd TF from the root to the shoot and increased Cd TF from the shoot to the leaf as compared with Cd sole treatment in HIPJ 1 but not in Xiushui 110.This shows that there are different physiological mechanisms that plants used to discriminate between metals thereby affecting translocation,which depicts genotypic difference between Xiushui 110 and HIPJ 1 in suppressing Cd TF.A previous study also showed durum wheat plants having different patterns of translocation and discrimination between Cd and Zn by physiological mechanisms[22].In the same way,it is possible that the two rice genotypes can have different physiological mechanisms that discriminate utilization between Zn and Si against Cd giving rise to different Cd TF patterns.
4 Conclusions
The results suggest that sole Zn alleviation treatments can effectively reduce Cd toxicity in both the genotypes by increasing plant biomass because of increasedSPAD andPn;however,ZnandSi combined treatments can reduce Cd translocation from the root to the shoot in HIPJ1.It is possible that the mechanisms ofsensitive genotype include utilizing Zn-Si combined association to restrict Cd from root to shoot compared with tolerant genotype,but the reduction in translocation of most mineral elements could have reduced rice growth.The effect of Zn-Si combination treatments on Cd from root to shoot translocation is not uniform in both the genotypes,suggesting the observed effects can be due to genotypic differences and/or the differences in concentrations of Zn and Si used in the combination treatments.Furthermore,Zn-Si does not effectively improve elements such as Ca and Mg TF from the shoot to the leaf,Mn TF from the root to the leaf,and K TF from the root to the shoot under Cd stress;neither does P TF from the root to the shoot in HIPJ 1.Therefore,Zn and Si could have utilized other mechanism to improve Cd resistance of plants.We also observed that Zn-Si mediates the reduction of lipid peroxidation and ROS stress by reducing MDA contents and increasing antioxidant enzyme activities.These findings can assist further researches on sole and combined nutrientassociationsagainstCd toxicity in crops/other plants.
AcknowledgementThe authors gratefully acknowledge Dr.OUYANG Younan of China National Rice Research Institute(CNRRI)for providing us the rice germplasm(seeds)used in experiments.
:
[1] HASSAN M J,ZHU Z J,AHMAD B,et al.Influence of cadmium toxicity on rice genotypes as affected by zinc,sulfur and nitrogen fertilizers. Caspian Journal of Environmental Sciences,2006,4(1):1-8.
[2] BOLAN N S,MAKINO T,Kunhikrishnan A,et al.Cadmium contamination and its risk management in rice ecosystems//Advances in Agronomy.Ohio,USA:Academic Press,2013,119:183-273.
[3] XIE L H,TANG S Q,WEI X J,et al.The cadmium and lead content of the grain produced by leading Chinese rice cultivars.Food Chemistry,2017,217:217-224.
[4] WANG S H,WANG FY,Gao S C.Foliar application with nanosilicon alleviates Cd toxicity in rice seedlings.Environmental Science and Pollution Research,2015,22(4):2837-2845.
[5] MA J,ZHANG X Q,WANG L J.Synergistic effects between[Si-hemicellulose matrix]ligands and Zn ions in inhibiting Cd ion uptake in rice(Oryza sativa)cells.Planta,2017,245(5):965-976.
[6] WU J T,DUMAT C,LU H P,et al.Synergistic improvement of crop physiological status by combination of cadmium immobilization and micronutrient fertilization.Environmental Science and Pollution Research,2016,23(7):6661-6670.
[7] SHAMSI I H,WEI K,JILANI G,et al.Interactions of cadmium and aluminum toxicity in their effect on growth and physiologicalparametersin soybean.Journalof Zhejiang University:SCIENCE B,2007,8(3):181-188.
[8] ZHU Y G,ZHAO Z Q,LI H Y,et al.Effect of zinccadmium interactions on the uptake of zinc and cadmium by winter wheat(Triticum aestivum)grown in pot culture.Bulletin of Environmental Contamination and Toxicology,2003,71(6):1289-1296.
[9] KIRKHAM M B.Cadmium in plants on polluted soils:Effects of soil factors,hyperaccumulation,and amendments.Geoderma,2006,137(1/2):19-32.
[10]SONG W E,CHEN S B,LIU J F,et al.Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by speciessensitivity distribution.Journal of Integrative Agriculture,2015,14(9):1845-1854.
[11]LIU C H,CHAO Y Y,KAO C H.Effect of potassium deficiency on antioxidant status and cadmium toxicity in rice seedlings.Botanical Studies,2013,54(1):2.
[12]ZHAO F J,MA Y B,ZHU Y G,et al.Soil contamination in China:Current status and mitigation strategies.Environmental Science&Technology,2015,49(2):750-759.
[13]BASHIR K,ISHIMARU Y,NISHIZAWA N K,et al.Molecular mechanisms of zinc uptake and translocation in rice.Plant and Soil,2012,361(1/2):189-201.
[14]EIDE D J.Zinc transporters and the cellular trafficking of zinc.Biochimica et Biophysica Acta(BBA)-Molecular Cell Research,2006,1763(7):711-722.
[15]PALMGREN M G,CLEMENS S,WILLIAMS L E,et al.Zinc biofortification of cereals:Problems and solutions.Trends in Plant Science,2008,13(9):464-473.
[16]SANAEIOSTOVAR A,KHOSHGOFTARMANESH A H,SHARIATMADARI H,et al.Effects of zinc activity in nutrient solution on uptake,translocation,and root export of cadmium and zinc in three wheat genotypes with different zinc efficiencies.Soil Science and Plant Nutrition,2011,57(5):681-690.
[17]RICACHENEVSKY F K,MENGUER P K,SPEROTTO R A,et al.Got to hide your Zn away:Molecular control of Zn accumulation and biotechnologicalapplications.Plant Science,2015,236:1-17.
[18]VIDAKOVIĆ-CIFREK Ž,TKALEC M, ŠIKIĆ S,et al.Growth and photosynthetic responses of Lemna minor L.exposed to cadmium in combination with zinc or copper.Archives of Industrial Hygiene and Toxicology,2015,66(2):141-152.
[19]HASSAN M J,ZHANG G P,WU F N,et al.Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice.Journal of Plant Nutrition and Soil Science,2005,168(2):255-261.
[20]WU F B,ZHANG G P.Alleviation of cadmium-toxicity by application of zinc and ascorbic acid in barley.Journal of Plant Nutrition,2002,25(12):2745-2761.
[21]CHOUDHARY M,BAILEYT L D,GRANTL C A,et al.Effect of Zn on the concentration of Cd and Zn in plant tissue of two durum wheat lines.Canadian Journal of Plant Science,1995,75(2):445-448.
[22]HART J J,WELCH R M,NORVELL W A,et al.Zinc effects on cadmium accumulation and partitioning in nearisogenic lines of durum wheat that differ in grain cadmium concentration.New Phytologist,2005,167(2):391-401.
[23]SAIFULLAH,SARWAR N,BIBI S,et al.Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat(Triticum aestivum L.).Environmental Earth Sciences,2014,71(4):1663-1672.
[24]TKALEC M,ŠTEFANIĆ P P,CVJETKO P,et al.The effects of cadmium-zinc interactions on biochemical responses in tobacco seedlings and adult plants.PLoS One,2014,9(1):e87582.
[25]YONEYAMA T,ISHIKAWA S,FUJIMAKI S,et al.Route and regulation of zinc,cadmium,and iron transport in rice plants(Oryza sativa L.)during vegetative growth and grain filling:Metal transporters,metal speciation,grain Cd reduction and Zn and Fe biofortification.International Journal of Molecular Sciences,2015,16(8):19111-19129.
[26]TAKAHASHI R,ISHIMARU Y,SHIMO H,et al.The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice.Plant Cell and Environment,2012,35(11):1948-1957.
[27]MEHARG C,MEHARG A A.Silicon,the silver bullet for mitigating biotic and abiotic stress,and improving grain quality,in rice?Environmental and Experimental Botany,2015,120:8-17.
[28]SONG A,LI P,FAN F L,et al.The effect of silicon on photosynthesis and expression of its relevant genes in rice(Oryza sativa L.)under high-zinc stress.PLoS One,2014,9(11):e113782.
[29]MEHRABANJOUBANI P,ABDOLZADEH A,SADEGHIPOUR H R,et al.Impacts of silicon nutrition on growth and nutrient status of rice plants grown under varying zinc regimes.Theoretical and Experimental Plant Physiology,2015,27(1):19-29.
[30]SADDIQUE M A B,ALI Z,KHAN A S,et al.Inoculation with the endophyte Piriformospora indica significantly affects mechanisms involved in osmotic stress in rice.Rice,2018,11:34.https://doi.org/10.1186/s12284-018-0226-1.
[31]TRIPATHI D K,SINGH V P,KUMAR D,et al.Rice seedlings under cadmium stress:Effect of silicon on growth,cadmium uptake,oxidative stress,antioxidant capacity and root and leaf structures.Chemistry and Ecology,2012,28(3):281-291.
[32]RIZWAN M,MEUNIER J D,MICHE H,et al.Effect of silicon on reducing cadmium toxicity in durum wheat(Triticum turgidum L.cv.Claudio W.)grown in a soil with aged contamination.Journal of Hazardous Materials,2012,209:326-334.
[33]ZENG F R,ZHAO F S,QIU B Y,et al.Alleviation of chromium toxicity by silicon addition in rice plants.Agricultural Sciences in China,2011,10(8):1188-1196.
[34]TRIPATHI D K,SINGH V P,PRASAD S M,et al.Siliconmediated alleviation of Cr(Ⅵ)toxicity in wheat seedlings as evidenced by chlorophyll florescence,laser induced breakdown spectroscopy and anatomical changes.Ecotoxicology and Environmental Safety,2015,113:133-144.
[35]ZVOBGO G,HU H L,SHANG S H,et al.The effects of phosphate on arsenic uptake and toxicity alleviation in tobacco genotypes with differing arsenic tolerances.Environmental Toxicology and Chemistry,2015,34(1):45-52.
[36]FAROOQ M A,ALI S,HAMEED A,et al.Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis,antioxidant enzymes;suppressed cadmium uptake and oxidative stress in cotton.Ecotoxicology and Environmental Safety,2013,96:242-249.
[37]WANG S H,WANG F Y,GAO S C,et al.Heavy metal accumulation in different rice cultivars as influenced by foliar application of nano-silicon.Water,Air,and Soil Pollution,2016,227(7):228.
[38]NWUGO C C,HUERTA A J.Effects of silicon nutrition on cadmium uptake,growth and photosynthesis of rice plants exposed to low-level cadmium.Plant and Soil,2008,311(1/2):73-86.
[39]CHERIF J,MEDIOUNI C,AMMAR W B,et al.Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solarium lycopersicum).Journal of Environmental Sciences,2011,23(5):837-844.
[40]FAROOQ M A,DETTERBECK A,CLEMENS S,et al.Silicon-induced reversibility of cadmium toxicity in rice.Journal of Experimental Botany,2016,67(11):3573-3585.
[41]VACULÍK M,PAVLOVIČ A,LUX A,et al.Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize.Ecotoxicology and Environmental Safety,2015,120:66-73.
[42]WANG F J,WANG M,LIU Z P,et al.Different responses of low grain-Cd-accumulating and high grain-Cd-accumulating rice cultivars to Cd stress.Plant Physiology and Biochemistry,2015,96:261-269.
[43]SONG A,LI Z J,ZHANG J,et al.Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L.is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity.Journal of Hazardous Materials,2009,172(1):74-83.
[44]ZHANG C C,WANG L J,NIE Q,et al.Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice(Oryza sativa L.).Environmental and Experimental Botany,2008,62(3):300-307.
[45]FU Y Q,SHEN H,WU D M,et al.Silicon-mediated amelioration of Fe2+toxicity in rice(Oryza Sativa L.)roots.Pedosphere,2012,22(6):795-802.
[46]HE J Y,REN Y F,WANG F J,et al.Characterization of cadmium uptake and translocation in a cadmium-sensitive mutant of rice(Oryza Sativa L.ssp.japonica).Archives of Environmental Contamination and Toxicology,2009,57(2):299-306.
[47]YE J,YAN C L,LIU J C,et al.Effects of silicon on the distribution of cadmium compartmentation in root tips of Kandelia obovata(S.,L.)Yong.Environmental Pollution,2012,162:369-373.
[48]DA CUNHA K P V,DO NASCIMENTO C W A.Silicon effects on metal tolerance and structural changes in Maize(Zea mays L.)grown on a cadmium and zinc enriched soil.Water,Air,and Soil Pollution,2009,197(1/2/3/4):323-330.
[49]VACULÍK M,LUX A,LUXOVÁ M,et al.Silicon mitigates cadmium inhibitory effects in young maize plants.Environmental and Experimental Botany,2009,67(1):52-58.
[50]WEI K,SHAMSI I H,ZHANG G P.Synergistic interaction of NaCl and Cd on growth and photosynthetic parameters in soybean genotypes differing in salinity tolerance.Journal of Zhejiang University:SCIENCE B,2007,8(4):266-271.
[51]RIZWAN M,MEUNIER J D,DAVIDIAN J C,et al.Silicon alleviates Cd stress of wheat seedlings(Triticum turgidum L.cv.Claudio)grown in hydroponics.Environmental Science and Pollution Research,2016,23(2):1414-1427.
[52]KUMAR A,SINGH U M,MANOHAR M,et al.Calcium transportfromsourcetosink:Understandingthemechanism(s)of acquisition,translocation,and accumulation for crop biofortification.Acta Physiologiae Plantarum,2015,37(1):1722.

