Shock Tube Study of Methyl Pentanoate Ignition at High Temperatures
LU Pengfei, GOU Yudan, HE Jiuning, LI Ping,*, ZHANG Changhua, LI Xiangyuan
1 Institute of Atomic and Molecular Physics, Sichuan University, Chengdu 610065, P. R. China.
2 College of Chemical Engineering, Sichuan University, Chengdu 610065, P. R. China.
1 Introduction
Biodiesel fuels, synthesized by the trans-esterification of various fats and oils1, have drawn much research attention due to their excellent combustion characteristics, such as low pollution and renewability2–9. Long-chain methyl esters are main compositions of biodiesel, but small esters are the intermediate species during the combustion of biodiesel and long-chain methyl esters. Experimental ignition investigations for biodiesel and long-chain methyl esters (C11–C19) have been reported abundantly10–15, but for small esters have been reported limitedly16,17. Till now, the experimental ignition result for small ester of methyl pentanoate (MPE, C6H12O2) is not available in literatures. In order to have a comprehensive understanding of combustion properties of biodiesel and long-chain methyl esters, a study on the ignition characteristics of MPE is necessary. Besides, MPE can be used as an addition to mix with other hydrocarbons to reduce the soot emission and the CO concentration during the combustion of fuels18. So,measurements of ignition delay times for MPE were conducted in present work.
For the chemical kinetic mechanism, Dievart et al.19provided a Princeton mechanism for the combustion of small methyl esters from methyl formate (MF, C2H4O2) to methyl pentanoate (MPE), and Korobeinichev et al.20developed an entire reaction mechanism base on a methyl butanoate (MB)mechanism21to simulate premixed laminar flames for MPE.These two mechanisms were used to predict ignition delay times of MPE for comparing with current measured data.
2 Experimental methods
Ignition delay times were measured behind reflected shock waves in a stainless-steel shock tube. The shock tube with an internal diameter of 10 cm was divided into two sections including a driver section of 4.0 m and a driven section of 5.0 m. Four piezoelectric pressure transducers were mounted on the driven section to determine the incident shock velocity. The CH radical emission during the ignition process was captured by a quartz optical fiber located at the same cross section as the last pressure transducer, which is 15 mm away from the end wall.Reflected shock pressure and temperature were acquired through the one-dimensional normal shock relations which using the measured initial temperature and pressure in the driven section, the measured incident shock wave velocity and the thermodynamic properties of the reactant mixtures. The uncertainties of reflected shock pressure and temperature were estimated about ±2.5% and ±1% respectively.
The ignition delay time is defined as the time interval between the arrival of the reflected shock wave determined by the jump of pressure signal and the onset of ignition indicated by the steepest rise of the CH radical emission at the side-wall observation location. An example of how to determine an ignition delay time is shown in Fig. 1. Details of the ignition delay measurement can be found in our previous parpers22,23.Considering uncertainties from reflected shock pressure and temperature, the composition of reactant mixture, and uncertainty in determining ignition delay from pressure and CH radical emission profiles, the total uncertainty in measured ignition delay time is estimated within ±20%.
3 Results and discussion
Ignition delay times of MPE/air and MPE/4%O2/Ar mixtures have been measured. Compositions of mixtures are listed in Table 1.
3.1 Ignition delay times of MPE/air
Ignition delay time data of MPE/air at ignition temperatures(T) of 1050–1350 K, ignition pressures (P) around 1.5 × 105and 16 × 105Pa, equivalence ratios of 0.5, 1 and 2 are given in Table 2 and displayed in Fig. 2. In the Fig. 2, these data have been scaled to nominal pressures of 1.5 × 105and 16 × 105Pa using common law τign∞ P−1in advance. As is distinct in the Fig. 2, the ignition delay time increases definitely with decreasing of temperature or pressure, but the effect of equivalence ratio on the ignition delay time is different at different pressures.
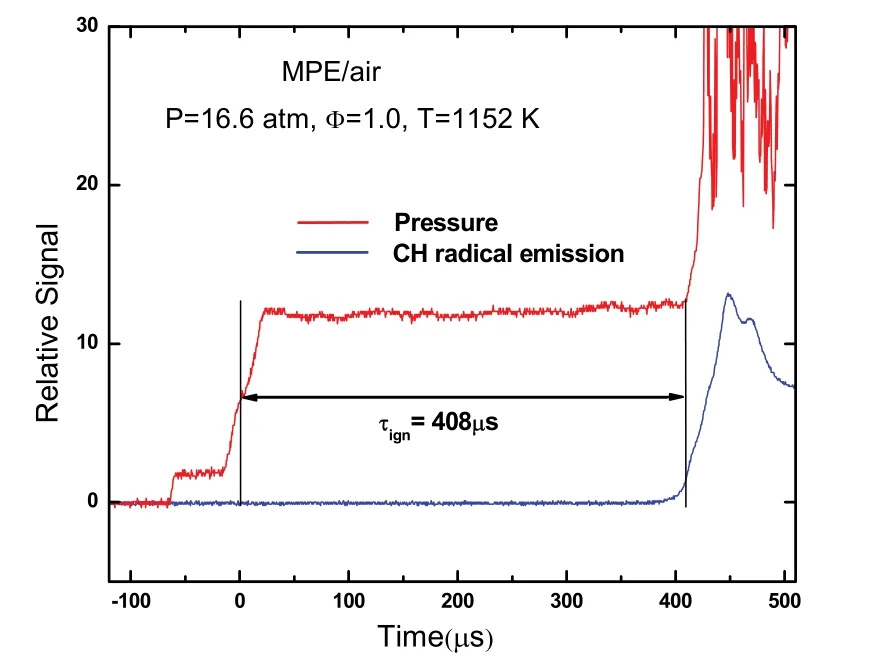
Fig. 1 Example of ignition delay time determination.

Table 1 Molar composition of mixtures.
In order to see how equivalence ratio affects ignition delay,an Arrhenius-type formula of τign= A × Фnexp(Ea/RT) was used to fit current data, where τign is the ignition delay time in microsecond, A is a constant, Ф is the equivalence ratio, n is equivalence ratio effect parameter, Eais the activation energy in kJ·mol−1, R is the universal gas constant with value of 8.314 J·mol−1·K−1, T is the reflected shock temperature in Kelvin. A single correlation for all ignition delay data is not possible,because the effects of equivalence ratio on ignition delay are not the same at 1.5 × 105and 16 × 105Pa. Two formulated results are as follow:
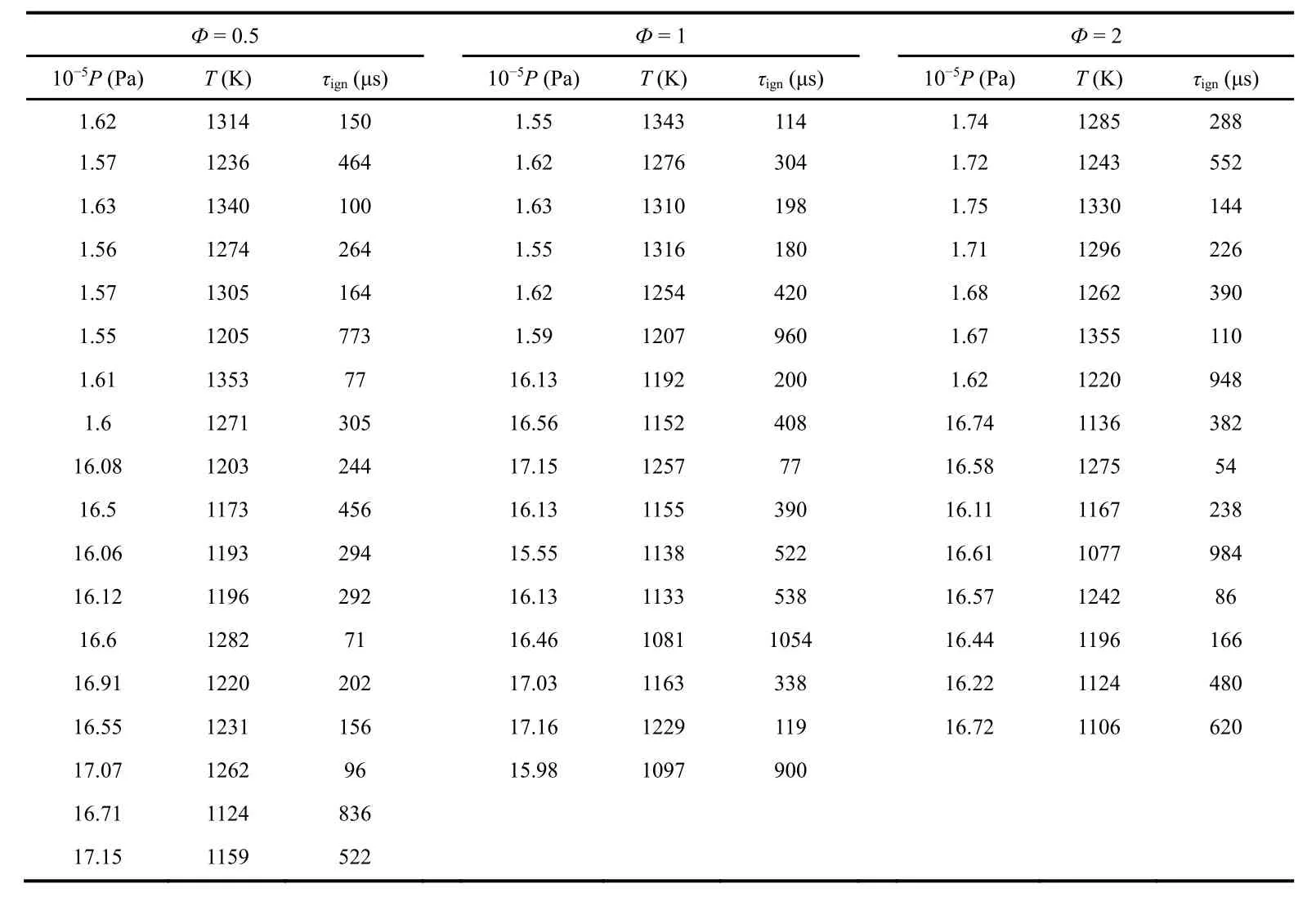
Table 2 Measured ignition delay times of MPE/air at different conditions.
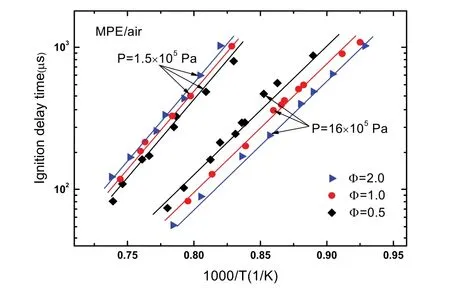
Fig. 2 Ignition delay times of MPE/air.

As shown in Eqs. (1) and (2), the effect of equivalence ratio on the ignition delay time follows τign∞ Ф+nat 1.5 × 105Pa,and on the contrary, the effect follows τign∞ Ф−nat 16 × 105Pa.The obtained equivalence ratio effect parameters indicate that ignition delay time has stronger dependence upon equivalence ratio at 16 × 105Pa. These positive and negative equivalence ratio effects are shown clearly in Fig. 2, at low pressure of 1.5 ×105Pa, ignition delay times of fuel-lean mixture are shorter than those of fuel-rich mixture, and the ignition delay times of fuel-rich mixture become shorter at 16 × 105Pa. This equivalence ratio dependence behavior is similar with other hydrocarbon fuel/air mixtures23–27. Besides, equations above show that the activation energy Eaat 1.5 × 105Pa is bigger than that at 16 × 105Pa, this means the ignition delay time is more sensitive to temperature at low pressure.
The ignition delay times of MPE (C6H12O2) are compared with the results of methyl decanoate (MD, C11H22O2) and methyl palmitate (MP, C17H34O2) here. MD, a primary component in cuphea biodiesel28, is used for surrogate of biodiesels due to its property of relative high vapor pressure. A shock tube study of ignition delay of MD has been performed by Wang et al.11. Campbell et al.12has summarized various study of MD combustion characteristics in their paper. MP, a waxy solid at room temperature, is one of five main components of biodiesel blends. A measurement of MP/air ignition delay at 10 × 105Pa has been performed by Wang et al.13. Ignition delay time results of current MPE/air, MD/air11and MP/air13are displayed in Fig. 3. All data were scaled to 16× 105Pa using relationship τign∞ P−1to account for difference in pressures. It is observed that ignition delay times of MPE are longer than those of MD and MP at the temperatures below 1200 K (1/T = 0.83 K−1) and the former is more sensitive to the temperature. When temperatures are above 1200 K, the differences of ignition delay times for three fuels are small.
3.2 Ignition delay times of MPE/4%O2/Ar
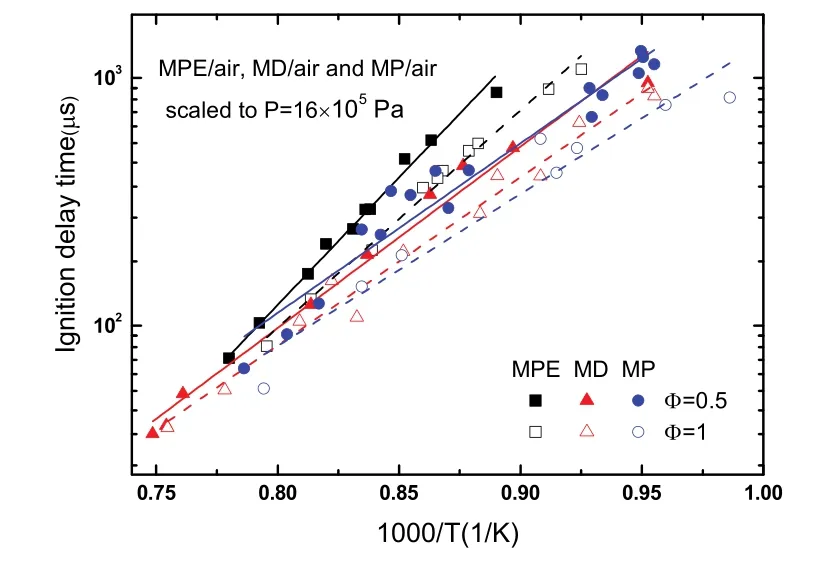
Fig. 3 Comparison of ignition delay times of MPE/air,MD/air and MP/air.
Ignition delay times of methyl laurate (MLA, C13H26O2)12and methyl oleate (MO, C19H36O2)15, both belong to methyl esters as MPE, have been measured when diluted in argon. In order to compare the ignition delay characteristic of MPE with those of them, ignition delay times of MPE in 4%O2/Ar were measured at ignition pressures of 3.5 × 105and 7 × 105Pa,ignition temperatures of 1210–1410K, and equivalence ratios of 0.75 and 1.25. These experimental conditions were chosen same as used for MLA and MO. The results obtained are given in Table 3 and shown in Fig. 4. These data are also correlated using the Arrhenius-type formula as follow:
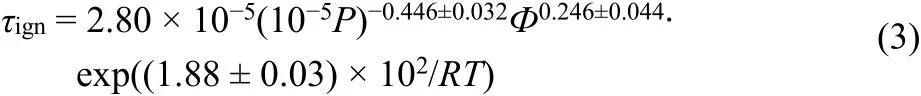

Table 3 Measured ignition delay times of MPE/4%O2/Ar at different conditions.

Fig. 4 Ignition delay times of MPE/4%O2/Ar.
The meaning of τign, P, Ф, R and T and their units in Eq. (3)are same as in Eqs. (1) and (2). As shown in Fig. 4, the increase of temperature or pressure, or decrease of equivalence ratio will cause the decrease of ignition delay time, and ignition delay times have the same sensitivity to temperature at each tested condition, this behavior is same as that in ignition delay of methyl acetate (MA)/4%O2/Ar studied by Zhang et al.17.Comparing to Eqs. (1) and (2), the activation energy of Eq. (3)is between 2.11 × 102and 1.73 × 102kJ·mol−1, this indicates that the sensitivity of MPE/4%O2/Ar ignition on temperature is between those of MPE/air at pressures of 1.5 × 105Pa and 16 ×105Pa. And the equivalence ratio effect parameter of Eq. (3) is close to that in the Eq. (1), indicating that MPE/4%O2/Ar has the similar ignition dependence on equivalence ratio with MPE/air at low pressure of 1.5 × 105Pa.
Fig. 5 gives the ignition delay times of MPE/4%O2/Ar and MPE/air with the equivalence ratio of 1 and the pressure of 1.5 × 105Pa. The data for MPE/4%O2/Ar have been scaled to 1.5 × 105Pa using the Eq.(3). At this equivalence ratio, the fuel concentration is 0.5% for MPE/4%O2/Ar, much less than the concentration of 2.56% for MPE/air, so the ignition delay time of MPE/4%O2/Ar is longer than that of MPE/air. This follows the law that a reduction in fuel concentration results in an increase in ignition delay time17, and diluted gases do not affect the ignition delay time29.
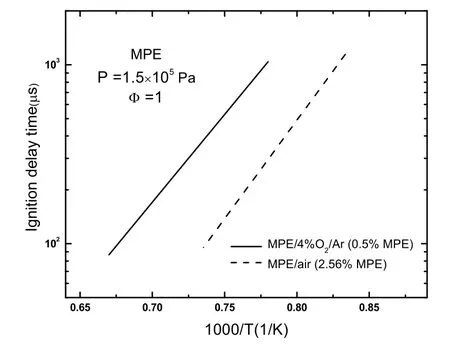
Fig. 5 Comparison between ignition delay times of MPE/4%O2/Ar and MPE/air.
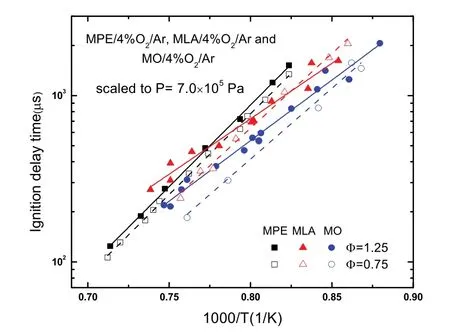
Fig. 6 Comparison of ignition delay times of MPE/4%O2/Ar,MD/4%O2/Ar and MP/4%O2/Ar.
Fig. 6 compares present ignition delay times of MPE/4%O2/Ar with those of MLA/4%O2/Ar12and MO/4%O2/Ar15. The results show that the ignition delay time of MPE is more sensitive to temperature, and MPE ignition delay times are longer than those of MLA and MO when temperatures below 1280 K (1/T = 0.78 K−1). The same property is found in the ignition delay of MPE/air, as shown in Fig. 3. So for the methyl esters fuels, ignition delay times of a small ester are not longer than those of long-chain methyl esters at relatively high temperatures.
3.3 Comparison with model predictions
Dievart et al.19provided a combustion mechanism(Princeton mechanism) for small methyl esters from methyl formate (MF) to methyl pentanoate (MPE). Korobeinichev et al.20developed a detailed chemical kinetic mechanism to predict the premixed laminar flames of methyl pentanoate(MPE) and methyl hexanoate (MHX). Two mechanisms were used to predict the ignition delay times of MPE utilizing the CHEMKIN software at constant-volume, adiabatic and homogeneous conditions. The prediction results are displayed in Fig. 7 as lines.
As shown in Fig. 7, two mechanisms can qualitatively predict the ignition delay times of MPE, but from quantitative point of view, Korobeinichev mechanism20exceedingly underestimates the measured ignition delay data, and Princeton mechanism19overestimates the current data at most conditions.Only at 16 × 105Pa and temperatures below 1200 K, simulated results of Princeton mechanism19for MPE/air agree well with current data at three equivalence ratios. In general, the predictions of Princeton mechanism19are relatively close to present experimental data. The comparison result indicates that the kinetic mechanisms for MPE need refinement, and current work provides experimental data for this refinement.
3.4 Sensitivity analysis
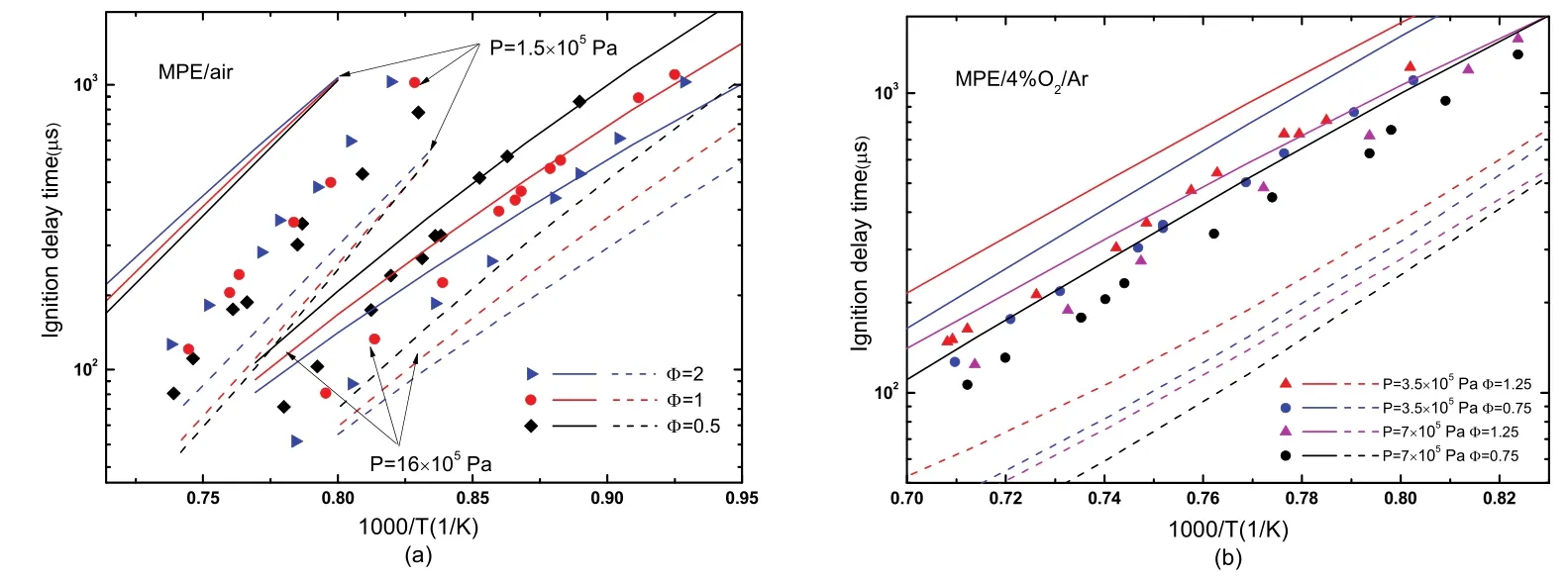
Fig. 7 Comparison between current data and predictions of mechanism s.

Fig. 8 The results of sensitivity analysis for MPE ignition at 1280 K using the Princeton mechanism 19.
Sensitivity analysis was carried out using Princeton mechanism19to assess key reactions that play the important role during the ignition process of MPE. A percent change,100 × [τign(2ki) − τign(ki)]/τign(ki), is used to define the sensitivity coefficient of the i th reaction to ignition delay times30, where τ is the predicted ignition delay time and kistands for the rate constant of i th reaction. A negative sensitivity coefficient implies a promoting effect for overall reactivity, and vice versa.The analysis results are shown in Fig. 8.
It can be seen from Fig. 8 that the chain-branching reaction H + O2 = O + OH and the reaction CH3 + HO2 =CH3O + OH play the main promoting effect on ignition of MPE/air and MPE/4%O2/Ar. For the inhibiting effect, the chain termination reaction CH3+ HO2= CH4+ O2plays the main role for MPE/air and MPE/4%O2/Ar ignition. The most promoting reaction H + O2= O + OH here is also the most promoting reaction in the high temperature ignition of alkanes22,23. The sensitivity analysis result of Zhang et al.17indicates that the reaction H + O2= O + OH also plays the most promoting effect on the ignition of MA, it may conclude that the most promoting reaction at high temperature ignition of methyl esters is H + O2= O + OH.
4 Conclusions
Ignition delay times of methyl pentanoate (MPE) diluted in air and in argon have been measured at high temperatures respectively. To our knowledge, ignition delay times of MPE are first reported here. Results show that an increase of temperature or pressure results in a decrease of ignition delay time definitely, but the effect of equivalence ratio on ignition delay is complex. For MPE/air, the ignition delay time follows τign∞ Ф+nat 1.5 × 105Pa and τign∞ Ф−nat 16 × 105Pa. And for MPE/4%O2/Ar, it follows τign∞ Ф+nat both 3.5 × 105and 7 ×105Pa. At present experimental condition, the ignition delay times of MPE/air are shorter than those of MPE/4%O2/Ar,because the concentration of MPE/air is much greater than that of MPE/4%O2/Ar. Compare to long-chain methyl esters,ignition delay times of MPE are more sensitive to temperature and longer than those of long-chain methyl esters only at relatively low temperatures (below 1200 K for MPE/air and below 1280 K for MPE/4%O2/Ar). Two chemical kinetic mechanisms used cannot well predict the measured ignition delay times of MPE, a further refinement of the mechanisms is required. Sensitivity analysis shows that the most promoting reaction for the high temperature ignition of MPE is H + O2=O + OH, which is same as that in the high temperature ignition of alkanes. Current study results are contributed to understand ignition characteristics of MPE, and are valuable for refining chemical kinetic mechanisms of MPE.
(1) Shao, P.; He, J. Z.; Sun, P. L.; Jiang, S. T. Biosyst. Eng. 2009, 102,285. doi: 10.1016/j.biosystemseng.2008.11.014
(2) Sarathy, S.; Thomson, M.; Pitz, W.; Lu, T. Proc. Combust. Inst. 2011,33, 399. doi: 10.1016/j.proci.2010.06.058
(3) Dayma, G.; Sarathy, S.; Togbé, C.; Yeung, C.; Thomson, M.; Dagaut,P. Proc. Combust. Inst. 2011, 33, 1037.doi: 10.1016/j.proci.2010.05.024
(4) Togbe, C.; Dayma, G.; Mze-Ahmed, A.; Dagaut, P. Energy Fuels 2010,24, 3906. doi: 10.1021/ef100484q
(5) Glaude, P. A.; Herbinet, O.; Bax, S.; Biet, J.; Warth, V.; Battin-Leclerc,F. Combust. Flame 2010, 157, 2035.doi: 10.1016/j.combustflame.2010.03.012
(6) HadjAli, K.; Crochet, M.; Vanhove, G.; Ribaucour, M.; Minetti, R.Proc. Combust. Inst. 2009, 32, 239. doi: 10.1016/j.proci.2008.09.002
(7) Haylett, D. R.; Davidson, D. F.; Hanson, R. K. Combust. Flame 2012,159, 552. doi: 10.1016/j.combustflame.2011.08.021
(8) Herbinet, O.; Pitz, W. J.; Westbrook, C. K. Combust. Flame 2010, 157,893. doi: 10.1016/j.combustflame.2009.10.013
(9) Westbrook, C. K.; Naik, C. V.; Herbinet, O.; Pitz, W. J.; Mehl, M.;Sarathy, S. M.; Curran, H. J. Combust. Flame 2011, 158, 742.doi: 10.1016/j.combustflame.2010.10.020
(10) Nguyen, V. H.; Dinh, L. T. Biosyst. Eng. 2015, 134, 1.doi: 10.1016/j.biosystemseng.2015.03.009
(11) Wang, W. J.; Oehlschlaeger, M. A. Combust. Flame 2012, 159, 476.doi: 10.1016/j.combustflame.2011.07.019
(12) Campbell, M. F.; Davidson, D. F.; Hanson, R. K. Fuel 2014, 126, 271.doi: 10.1016/j.fuel.2014.02.050
(13) Wang, W. J.; Gowdagiri, S.; Oehlschlaeger, M. A. Combust. Flame 2014, 161, 3014. doi: 10.1016/j.combustflame.2014.06.009
(14) Campbell, M. F.; Davidson, D. F.; Hanson, R. K. Fuel 2016, 164, 151.doi: 10.1016/j.fuel.2015.09.078
(15) Campbell, M. F.; Davidson, D. F.; Hanson, R. K.; Westbrook. C. K.Proc. Combust. Inst. 2013, 34, 419.doi: 10.1016/j.combustflame.2014.12.015
(16) Akih-Kumgeh, B.; Bergthorson, J. M. Combust. Flame 2011, 158,1037. doi: 10.1016/j.combustflame.2010.10.021
(17) Zhang, Z. H.; Hu, E. J.; Peng, C.; Meng, X.; Chen,Y. Z.; Huang, Z. H.Energy Fuels 2015, 29, 2719. doi: 10.1021/acs.energyfuels.5b00316
(18) Dmitriev, A. M.; Knyazkov, D. A.; Bolshova, T. A.; Shmakov, A. G.;Korobeinichev, O. P. Combust. Flame 2015, 162, 1964.doi: 10.1016/j.combustflame.2014.12.015
(19) Dievart, P.; Won, S. E.; Gong, J.; Dooley, S.; Ju, Y. Proc. Combust.Inst. 2013, 34, 821. doi: 10.1016/j.proci.2012.06.180
(20) Korobeinichev, O. P.; Gerasimov, I. E.; Knyazkov, D. A.; Shmakov,A. G.; Bolshova, T. A.; Hansen, N.; Westbrook, C. K.; Dayma, G.;Yang, B. Z. Phys. Chem. 2015, 229, 759.doi: 10.1515/zpch-2014-0596
(21) Yang, B.; Westbrook, C. K.; Cool, T. A.; Hansen, N.;Kohse-Hoinghaus, K. Phys. Chem. Chem. Phys. 2011, 13, 6901.doi: 10.1039/c0cp02065f
(22) He, J. N.; Yong, K. L.; Zhang, W. F.; Li, P.; Zhang, C. H.; Li, X. Y.Energy Fuels 2016, 30, 8886. doi: 10.1021/acs.energyfuels.6b01122
(23) Yong, K. L.; He, J. N.; Zhang, W. F.; Xian, L. Y.; Zhang, C. H.; Li, P.;Li, X. Y. Fuel 2017, 188, 567. doi: 10.1016/j.fuel.2016.09.054
(24) He, J. N.; Li. Y. L.; Zhang. C. H.; Li. P.; Li. X. Y. Acta Phys. -Chim.Sin. 2015, 31, 836. [何九宁, 李友亮, 张昌华, 李萍, 李象远. 物理化学学报, 2015, 31, 836.] doi: 10.3866/PKU.WHXB201503121
(25) Darcy, D.; Tobin, C. J.; Yasunaga, K. Combust. Flame 2012, 159,2219. doi: 10.1016/j.combustflame.2012.02.009
(26) Shen, H. P. S.; Vanderover, J.; Oehlschlaeger, M. A. Proc.Combust. Inst. 2009, 32, 165. doi: 10.1016/j.proci.2008.05.004
(27) Zhu, Y.; Davidson, D. F.; Hanson, R. K. Combust. Flame 2014, 161,371. doi: 10.1016/j.proci.2008.05.004
(28) Knothe, G.; Cermak, S. C.; Evangelista, R. L. Energy Fuels 2009, 23,1743. doi: 10.1021/ef800958t
(29) Akih-Kumgeh, B.; Bergthorson, J. M. Energy Fuels 2010, 24, 2439.doi: 10.1021/ef901489k
(30) Kumar, K.; Mittal, G.; Sung, C. J.; Law C. K. Combust. Flame 2008, 153, 343. doi:10.1016/j.combustflame.2007.11.012

