The brain-derived neurotrophic factor in neuronal plasticity and neuroregeneration: new pharmacological concepts for old and new drugs
Neurotrophins: Neurotrophins are peptides or proteins that are known to regulate neuronal viability, development, and function.Beyond synaptic plasticity, neurotrophins protect neurons from apoptosis and also promote neurogenesis to recover neuronal deficit even in adulthood. For various reasons that we have highlighted previously (Habtemariam, 2016a), neuronal cells in the brain are highly susceptible to oxidative stress and hence efficient cell survival mechanisms must be maintained at all times. Many age-related and other neurodegenerative diseases (NDs) in the brain are also associated with excessive oxidative damage resulting from either high production of reactive oxygen species generated by neurotoxic agents (e.g.,amyloid beta (Aβ)) or suppressed level of antioxidant defenses. Our progress in understanding neurotrophins in recent years has now offered more insight into therapeutic options for various NDs where neuroregeneration is considered the best therapeutic approach. This include traumatic brain injury (TBI), Alzheimer’s disease (AD) and Parkinson’s disease (PD), among others. Neurotrophins that have been well-characterised to date include the nerve growth factor(NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3(NT-3), and neurotrophin-4/5 (NT-4/5). Among the neurotrophins,BDNF is the best studied with explosive number of publications appeared in the last decade to establish its functional role in the brain and targeting it to treat for many diseases. Upon binding to its receptor, a signal transduction pathway that is common to the process of cell proliferation promotion and/or inhibition of the apoptosis cascade has been shown to be activated. Not surprisingly, the common mitogen activated protein kinase (MAPK) pathway, particularly the extracellular signal-regulated kinase (ERK) pathway, that regulates cell growth and differentiation is involved. Details of the signal transduction pathway including the ERK, phosphatidylinositide 3-kinase (PI3K) and phosphoinositide phospholipase C-γ (PLCγ)pathway-mediated calcium ion mobilization and cellular events have been illustrated in a review by Numakawa et al. (2010).
Traumatic brain injury and BDNF: As a neurotrophin, BDNF can stimulate and regulate the growth of new neurons from neuronal stem cells through a process of neurogenesis. TBI resulting from a heavy blow to the head by an external force leads to direct damage to neurons or associated blood vessels, glial cells and other tissues.Beyond the immediate cellular death resulting from damage by such an external impact, secondary injury may result from the subsequent inflammatory and associated biochemical and cellular changes and/or adaptation to the primary injury. Therapeutic option for TBI is very limited but any effort in limiting the secondary damage in brain tissues as well as efforts that could increase the restoration of cellular deficits through neuroregeneration are best possible considerations.In the latter case, neurotrophins that facilitate neuronal growth or recovery from stem cells or those maintaining the viability of neurons have critical role to play (Wurzelmann et al., 2017).
BDNF and neurodegenerative diseases: Numerous studies have shown the critical role of BDNF in synaptic plasticity, memory,and cognitive functions. As a result, we have reviewed many natural products such as caffeic acid esters (e.g., salvianolic acid B) that have shown promise in AD therapy by increasing the level of BNDF(Habtemariam, 2018a). Not surprisingly, suppression of the level of BDNF is now shown to be one of the hallmarks of neurodegenerative diseases such as AD. One form of neuronal plasticity in the hippocampus is the long-term potentiation (LTP) that serves as the cellular basis of learning and memory. While BDNF is shown to modify LTP, the cortex and hippocampus, which are critical to memory-related functions, are among the brain regions where BDNF is found in abundance. BDNF also enhance synaptic transmission in the brain with the excitatory synapse (glutamatergic)appear to be stimulated, while inhibitory synapses (GABAergic) are suppressed. In addition, other classical NDs such as Huntington’s disease, PD, and amyotrophic later sclerosis are examples of pathology where neuroregeneration could be seen as the most appealing therapeutic options. Understanding the role of BDNF in neurogenesis and potential benefit in these diseases also came from evidences in recent years where the number of neurons in adult animals andin vitrohave been shown to be augmented by BDNF. The overall effect of BDNF is now believed to be mediated both through induction of cell proliferation/differentiation and cell survival mechanisms (Figure 1). An excellent insight into stem cell-based therapy options for NDs is also presented by Stonesifer et al. (2017).
BDNF-based therapy: As with many other bioactive proteins such as insulin and cytokines (e.g., tumour necrosis factor), neurotrophins are synthesised as a large precursor protein that needs further processing by proteolytic enzymes to liberate the active/mature form. The BDNF precursor in the endoplasmic reticulum or pro-BDNF (129 amino acids more than the mature form) is processed by the Golgi apparatus and loaded into vesicles. The liberation of the mature BDNF of 118 amino acids could be a result of either intracellular enzymatic action or by proteases (e.g., metalloproteinases) in the extracellular domain (Hempstead, 2015). Both
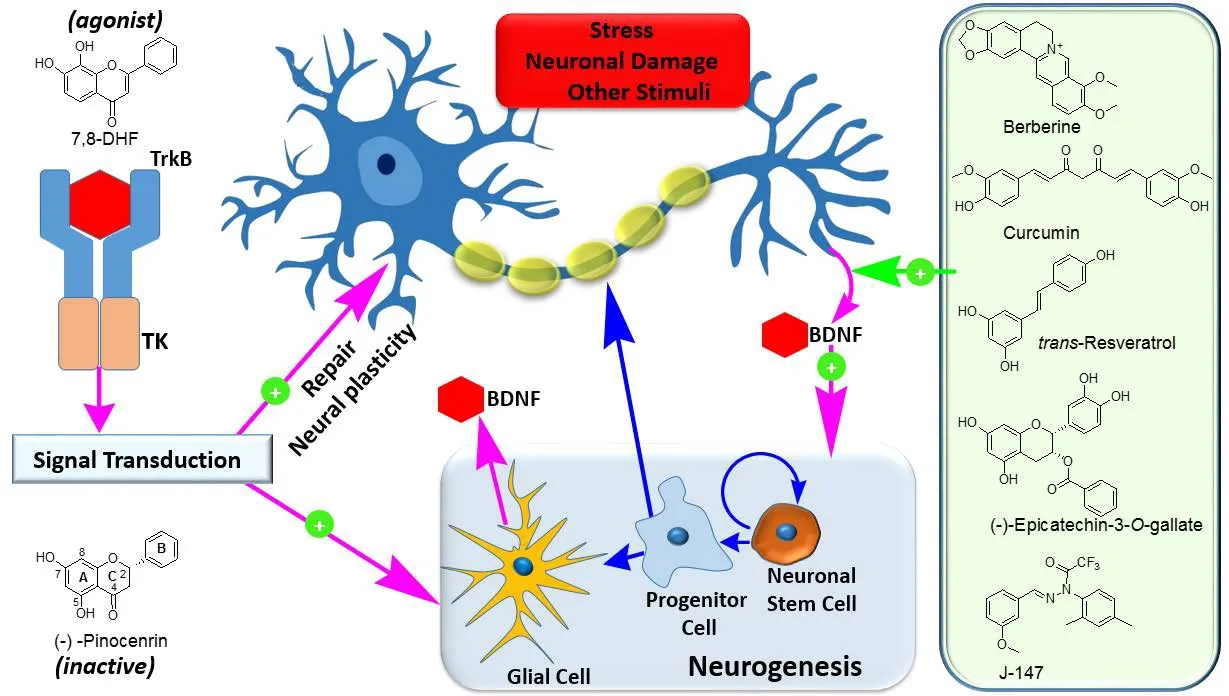
Figure 1 Targeting brain-derived neurotrophic factor (BDNF) in neuroregeneration and neuronal plasticity.
the BDNF and pro-BDNF released from the vesicles have pharmacological effects, but the more potent and specific effect of BDNF is of direct interest to therapeutic modulation in neuroregeneration. Two receptor types are known to be involved in BDNF and pro-BDNF.The p75 neurotrophin receptors mediate the low affinity binding action of pro-PDNF and result in activation of neuronal cell death mechanisms among others; while the high affinity binding of BDNF to its tropomyosin-related kinase family (Trk) receptors results in cell survival and differentiation, dendritic spine complexity, and LTP. The tropomyosin receptor kinase B (TrkB) serves as a receptor for BDNF while related TrkA and TrkC serve as receptors for NGF and NT-3, respectively. The role of BDNF via these receptors in synaptic plasticity and even increased level of TrkB receptors with neuronal activity has been well established. Beyond the correlation of physical activity/exercise and BDNF level and neuronal function(e.g., memory) in the brain, attempts have been made to use BDNF as therapy. Being a protein, the degradation of BDNF by proteolytic enzymes with half-life widely reported far less than 30 minutes coupled with its inability to pass through the blood-brain barrier (BBB)is, however, the drawback of using it as a therapeutic agent. Hence,approaches such as nanoparticles and mechanisms of delivery to the brain has been another subject area of research in recent years. The most attractive approach, both for old and novel medicines, appears to be direct receptor activationvialigands/agonists or mechanisms of increasing the BDNF level in the brain (Figure 1).
TrkB agonists: One of the most promising TrkB agonist, that gained lots of attention in recent years, is the plant-based natural product,7,8-dihydroxyflavone (7,8-DHF). Among the common plant sources of the compound are tridax daisy (Tridax procumbens L.), several species of the genusPrimula, andGodmania aesculifolia(Kunth)Standl. The simplicity of this compound is astonishing: the complete lack of oxygenation in the B-ring of the flavonoid skeleton and the common hydroxylation at C-5 position of the A-ring missing and/or rather replaced by hydroxylation at C-8 is evident (Figure 1). This compound now sourced through a synthetic route has shown promise as BDNF receptor (TrkB) agonist in potential neurogenesis as well as many other therapies. As with BDNF, 7,8-DHF triggers receptor dimerization, autophosphorylation, and signal transduction but with more sustained effect. The critical step of receptor internalization is still evident for 7,8-DHF but receptor ubiquitination and subsequent degradation is not associated with its signaling effect (see Wurzelmann et al., 2017). Hence, a more economical route of receptor cyclisation process is induced by this compound. This selective effect on the high affinity receptor coupled with a longer half-life has opened up a new avenue for the search of novel drugs acting through BDNF receptor agonistic mechanisms. Both 7,8-DHF and BDNF activate the Erk1/2 and protein kinase B (Akt) pathway to display neuroprotective effect. The incredible level of selectivity in receptor activation by 7,8-DHF was also demonstrated by the lack of effect for the related flavonoids such as pinocembrin (Figure 1). The selective effect of 7,8-DHF and how it was identified through routine screening of related compounds has been elegantly presented by Jang et al. (2010).
Enhancing BDNF production: To date, numerous natural products that belong to various structural groups (Elufioye et al., 2017) and specifically the monoterpenes (Habtemariam, 2018b), flavonoids(Habtemariam, 2016a), and other polyphenols (Habtemariam,2016b) have shown their promise as therapy for NDs. Most of these compounds are classical examples of old drugs or compounds that are active principles of many traditional medicines. Even though multiple mechanisms of action ranging from general antioxidant and antiinflammatory mechanisms to specific action on the brain are involved, effects on neurotrophins are now emerging as a further mechanism of action. Good examples are curcumin, resveratrol, (-)-epigallocatechin-3-gallate (EGCG), and berberine (Figure 1). Under various experimental conditions, these compounds have been shown to increase the BDNF level under neurodegenerative experimental models bothin vitroandin vivo. Since these compounds are active in modulation of gene expression and signal transduction pathways associated with cell growth and differentiation, their effect on BDNF production could be a result of multiple mechanisms. The effect of curcumin in ameliorating the spatial memory deficit induced by Aβ, for example, is known to be mediated through activation of the cAMP response element-binding protein (CREB) and ERK signalling. Efforts in finding novel derivatives of the above natural products with a better efficacy profile is also ongoing. For example, Chen et al.(2011) have identified a range of novel curcumin derivatives that led to the identification of J-147 (Figure 1) as a potent anti-AD agent.Hence, there seems to be sufficient scientific justification and plenty of enthusiasm to find novel compounds with increased bioavailability and efficacy in neurogenesis through neurotrophin production or agonist-like action. Due to the diverse effect of BDNF in neuronal cells and its downregulations in pathologies such as depressive and other psychological disorders, agents that upregulate its production or function also have diverse therapeutic applications.
Conclusion: BDNF plays a critical role in maintaining normal neuronal functions, their survival under normal and pathological conditions, and promoting neurogenesis in the adult brain subjected to NDs. Understanding the mechanism of BDNF production, secretion, and receptor-mediated function in the past decade has opened up a an opportunity to discover new drugs as well as explaining the mechanisms of old potential therapies of natural origin. Future research in this direction will provide more insight into the application of neurogenesis-based therapy and/or stem-cell therapy in NDs.
Solomon Habtemariam*
Herbal Analysis Services UK & Pharmacognosy Research Laboratories, University of Greenwich, Chatham-Maritime,Kent, UK
*Correspondence to:Solomon Habtemariam, Ph.D.,
s.habtemariam@herbalanalysis.co.uk.
orcid:0000-0001-6743-2244 (Solomon Habtemariam)
Accepted:2018-04-16
doi:10.4103/1673-5374.233438
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers: Carla Lucini, Universita degli Studi di Napoli Federico II, Italy; Cristy Phillips, Arkansas State University, USA; Jonathan M. Borkum, University of Maine, USA.
Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C,Akaishi T, Abe K, Maher P, Schubert D (2011) A novel neurotrophic drug for cognitive enhancement and Alzheimer’s disease. PLoS One 6:e27865.Elufioye TO, Berida TI, Habtemariam S (2017) Plants-derived neuroprotective agents: cutting the cycle of cell death through multiple mechanisms.Evid Based Complement Alternat Med 2017:3574012.
Habtemariam S (2016a) Rutin as a natural therapy for Alzheimer’s disease:insights into its mechanisms of action. Curr Med Chem 23:860-873.
Habtemariam S (2016b) The therapeutic potential of Rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. Evid Based Complement Alternat Med 2016:2680409.
Habtemariam S (2018a) Molecular pharmacology of rosmarinic and salvianolic acids: potential seeds for Alzheimer’s and vascular dementia drugs.Int J Mol Sci 19:E458.
Habtemariam S (2018b) Iridoids and other monoterpenes in the Alzheimer’s brain: recent development and future prospects. Molecules 23:E117.Hempstead BL (2015) Brain-derived neurotrophic factor: three ligands,many actions. Trans Am Clin Climatol Assoc 126:9-19.
Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K (2010) A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A 107:2687-2692.
Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H(2010) BDNF function and intracellular signaling in neurons. Histol Histopathol 25:237-258.
Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV(2017) Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 158:94-131.
Wurzelmann M, Romeika J, Sun D (2017) Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen Res 12:7-12.
- 中国神经再生研究(英文版)的其它文章
- Role of brain-derived neurotrophic factor during the regenerative response after traumatic brain injury in adult zebrafish
- Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases
- How random is the random forest ? Random forest algorithm on the service of structural imaging biomarkers for Alzheimer’s disease: from Alzheimer’s disease neuroimaging initiative (ADNI) database
- Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury
- INVITED REVIEW
- Role of presynaptic calcium stores for neural network dysfunction in Alzheimer’s disease

