Effect of potassium permanganate dosing position on the performance of coagulation/ultra filtration combined process☆
He Zhao ,Liang Wang *,Hongwei Zhang ,Xiaona Wu ,Bin Zhao Fei Han
1 School of Environmental Science and Technology,Tianjin University,Tianjin 300072,China
2 State Key Laboratory of Separation Membranes and Membrane Processes,Tianjin Polytechnic University,Tianjin 300387,China
3 School of Civil Engineering and Transportation,Hebei University of Technology,Tianjin 300401,China
1.Introduction
Drinking water safety is a world wide public health concern.Conventional drinking water treatment process,which comprises coagulation,filtration,and disinfection in series,is very effective for turbidity removal and pathogen inactivation[1,2].It has been commonly applied around the world for quite a long time,and guaranteed the drinking water safety for the majority[3].
Natural organic matter(NOM)is ubiquitous in surface water and introduces undelightful color and odor problems.NOM includes the micro-organisms that are the source of infection and hence require disinfection agents to be added.Moreover,NOM is also believed to be the precursor for disinfection by-products(DBPs)of chlorination[4,5],which are generally associated with potential causes for cancer and reproductive/developmental effects[6].Therefore,NOM removal before disinfection is highly desired.Unfortunately,conventional coagulation is not effective in terms of NOM removal,especially for dissolved organic carbons(DOC).Enhanced coagulation,which achieves high NOM removal by lowering solution p H or increasing coagulant doses,is proposed by the United States Environmental Protection Agency[7].However,accompanying with the improvement on NOM removal,the production of sludge is increased and the operation system is complicated.
Oxidation is a strong technique for organic pollutant degradation[8–10].It is increasingly used in drinking water treatment to deal with the conflict between stringent regulations and poor surface water quality.Besides the strong mineralization capability,oxidation can also change the properties of NOM in water.Ferrate(VI)oxidation cleaves humus into small hydrophilic fractions at p H 7,and a high dose of ferrate(VI)(20 mg·L−1)preferentially oxidized organic matters with molecular weight(MW)less than 1000[11].Ozonation destroys organic molecules with MW higher than 3000 and produced smaller ones[12].Compared with ozone and chlorine,potassium permanganate(KMn O4)is a mild oxidant[13]and can markedly avoid the production of hazardous by-products.The application of KMn O4in drinking water treatment has received great attention nowadays[14–16].Besides the oxidative degradation,NOM is also removed by adsorbing onto the in-situ produced MnO2nanoparticles as a result of KMnO4reduction[17].It was reported that KMn O4oxidation successfully removed DOC and reduced UV254from sand filter effluent due to the breakdown of strong hydrophobic organic matters,and the removal of neutral organic matters was improved due to the adsorption of intermediate Mn O2[18].In addition,KMn O4oxidation can enhance the coagulation performance.Mn O2nanoparticles produced by KMn O4reduction serves as coagulant seeds[19],and both the growth ability of flocs and the regrowth ability of broken flocs are consequently strengthened[14].
Ultra filtration(UF)has been widely used in drinking water treatment thanks to its high effluent quality,high efficiency,high integrity,and low additive addition[20–22].However,membrane fouling,which reduces the production efficiency and increases the operation cost,remains the major problem for its application[23].NOM,including polysaccharides,proteins,and humus,is recognized as the main reason for membrane fouling in drinking water treatment.Therefore,effective removal of NOM prior to UF is a pre-requisite to overcome the membrane fouling issue[24].Recent studies indicated that pre-oxidation with KMn O4significantly alleviated the UF fouling,especially for the irreversible fouling which was not removed by hydraulic backwashing.The possible mechanism involved the decline in NOM concentration,the alternation of NOM to species with less fouling capability,and the coarse and loose cake layer structure adjusted by Mn O2nanoparticles[25].Besides,KMn O4pre-oxidation also increases the membrane hydrophilicity and narrows the membrane pore size,and consequently increases the long-term stability of UF[26,27].In the KMnO4/coagulation hybrid process,cake layer with porous structure can be obtained,and the filtration resistance decreases as well as the fouling irreversibility[28].The positive effect,however,is significantly dependent on both KMn O4and coagulant concentrations[15].
KMn O4pre-oxidation as well as its assistance to coagulation has been testified to be effective for UF fouling mitigation,but little in formation can be obtained on the combination of these two positive approaches.In this study,a novel mode of KMn O4dosing was developed based on the conventional coagulation/UF process,i.e.,KMnO4wasadded into both upstream and downstream of coagulation at the same time.The KMnO4dosage proportion between these two positions was investigated for lake water treatment in terms of effluent quality and membrane fouling.The underlying mechanism was discussed based on the alteration of NOM characteristics and the fouling layer morphology.The results may provide some insight into the application of KMn O4oxidation for UF system.

Table 1 Quality of raw water
2.Materials and Methods
2.1.Feed water and membrane module
Raw water was collected from an artificial lake in Tianjin Polytechnic University,China.Its properties are shown in Table 1.
Polyvinylidene fluoride(PVDF)hollowfiber membrane was provided by Tianjin Motimo Membrane Co.,Ltd.,China.The average membrane pore size was 0.02 μm.The internal and external diameters of membrane fiber were 0.7 mm and 1.2 mm,respectively.Submerged U-shape membrane module composed of 24 fibers was homemade with an effective area of 0.036 m2.Before use,new membrane module was soaked in a 0.1%NaClO solution for 2 h,and then washed with deionized water.
2.2.KMnO4 pre-oxidation enhanced coagulation
Jar test was conducted for oxidation using KMnO4and coagulation using polyaluminium chloride(PACl).KMn O4stock solution(2500 mg·L−1)was first introduced into the rawlake water,and its effective concentration was investigated in between 0 mg·L−1to 1.0 mg·L−1.The contact time of oxidation was 20 min at a rotation speed of 40 r·min−1.PACl stock solution(2000 mg·L−1as Al2O3)was subsequently introduced into the pre-oxidated lake water,and its effective concentration was at 30 mg·L−1.The rotation speed of coagulation was programmed at 200 r·min−1for 1 min,and then 40 r·min−1for 20 min.After settling for 30 min,the supernatant was used as the in fluent for UF.
2.3.Ultra filtration

Fig.1.Schematic diagram of the experimental ultra filtration set-up.
Experimental UF set-up is shown in Fig.1.Supernatant of the pretreated lake water was pumped into the membrane tank(6 L).On/off of the pump was controlled by the level gauge.Filtration was conducted using a suction pump at a constant flux of 50 L·m−2·h−1.Mercury manometer was used to on-line monitor the trans membrane pressure(TMP).When the two-position dosing mode was investigated,KMnO4stock solution(2500 mg·L−1)was also added into the supernatant of the pre-treated lake water besides directly into the raw water.The effective KMnO4concentration in the supernatant was investigated in between 0 mg·L−1to 0.5 mg·L−1.KMn O4dosage in the raw water decreased as that in the supernatant increased,and the total effective KMn O4dosage in the two-position dosing mode was kept at 0.5 mg·L−1.Each filtration test ran for 2 h without backwash.New membrane module was used for each test.
2.4.Molecular weight
MW distribution of dissolved NOM in lake water before and after KMn O4oxidation was analyzed using asymmetric regenerate cellulose ultra filtration membranes(Ultracel,Millipore).Water samples were first filtered through 0.45 μm membrane.Permeates were then filtered through Ultracel membranes with nominal molecular weight cut-off(MWCO)of 1000,3000,10000,30000,and 100000,respectively.NOM MW was divided into six intervals,namely <1000,1000–3000,3000–10000,10000–30000,30000–100000,and >100000.Concentration difference of DOC in permeates was used to evaluate the NOM amount in each MW interval.
2.5.Hydrophobicity and hydrophilicity
The hydrophobicity and hydrophilicity of dissolved NOM in lake water before and after KMn O4oxidation was analyzed using the combined Amberlite XAD-4 and XAD-8 resin procedure[29].Five fractions namely hydrophobic basic fractions(HoB),hydrophobic neutral fractions(HoN),hydrophobic acid fractions(HoA),hydrophilic fractions(HiM),and weak hydrophobic acid fractions(wHoA)were quantitatively analyzed based on DOC values.Water samples were filtered through 0.45 μm membrane.Permeates were fed into XAD-8 column,where HoN and HoB fractions were adsorbed.The eluate was then acidified with HCl to p H 2.The HoB fraction retained in XAD-8 was eluted using a0.2 mol·L−1H3PO4solution.The acidified eluate passed through the XAD-8 again to retain the HoA fraction.The eluate was then fed into the XAD-4 column.The wHoAfraction was retained in XAD-4,while the HiM fraction remained in the eluate.
2.6.Membrane fouling resistance
The overall filtration resistance(R,m−1)was calculated according to Darcy's law(Eq.(1)):

where J is the permeate flux,L·m−2·h−1;ΔP is the trans membrane pressure,Pa;μ is the viscosity of water,Pa·s.
According to the resistance-in-series model,the overall filtration resistance was the summary of intrinsic membrane resistance(Rm,m−1),physically reversible fouling resistance(Rrev,m−1),and physically irreversible fouling resistance(Rirr,m−1).Rmwas determined by the pure water flux of new membrane.Physical cleaning wasconducted by backwash using permeate and assisted with aeration at 10 L·min−1.The filtration resistance after backwash was determined by pure water flux,which was the summary of Rmand Rirr.Experiments of each condition were replicated three times,and the average values were reported.
2.7.Analytical methods
Ultraviolet absorbance at 254 nm(UV254)was determined by an ultraviolet/visible spectrometer(Cary 60,Agilent).DOC was determined by a TOC analyzer(TOC-VCPH,Shimadzu).SUVA was the ratio of UV254/DOC.Turbidity was determined by a HACH 2100N turbidimeter.p H value was determined by a p H meter(SensION™156,Hach).Excitation-emission matrix(EEM) fluorescence spectroscopy was obtained using a luminescence spectrometer(Fluorolog 3–21,Jobin Yvon).The excitation wavelengths were increased from 260 to 500 nm,while the emission wavelengths were increased from 260 to 550 nm.The step value was 5 nm.The surface morphology of membrane samples was analyzed using a field emission scanning electron microscopy(FE–SEM)(S-4800,Hitachi).Before analysis,all membrane samples were dried at 40°C for more than 12 h in a vacuum oven.
3.Results and Discussion
3.1.NOM removal by KMnO4 addition prior to coagulation
KMnO4pre-oxidation enhanced NOM removalin thecoagulation/UF process significantly(Fig.2).Compared to the control test without KMnO4,DOC removal increased by around 100%at a KMnO4dosage of 0.25 mg·L−1.However,further increase in KMn O4dosage barely changed the NOM removal.KMn O4oxidation prior to coagulation also markedly decreased the SUVA value from 2.26 L·mg−1·m−1to 1.60 L·mg−1·m−1.High SUVA value of raw lake water indicated the presence of organic molecules containing abundant unsaturated functional groups with strong ultraviolet absorption,such as humic substances[30].These organic matters were regarded as DBPs precursors,and contributed greatly to the harmful chlorination DBPs[31,32].KMn O4was prone to attack the unsaturated functional groups with rich electron density,and oxidized them into oxygenousgroups.Asaresult,the SUVA value decreased significantly after KMn O4addition.

Fig.2.DOC removal in coagulation/UF process with KMn O4 addition prior to coagulation.
Humic substances in water samples were qualitatively and quantitatively analyzed based on EEM images(Fig.3).The strong peak within the Ex.values of 320–360 nm and Em.values of 420–460 nm in Fig.3,namely Peak C,was assigned to the humic substances originating from terraneous organisms[33,34].This result was in accordance with the property of the artificial lake water,in which organic matters mainly originated from the leachate of the soil.The addition of KMn O4decreased the overall fluorescence absorption significantly,and the intensity of Peak C decreased by around 10%.
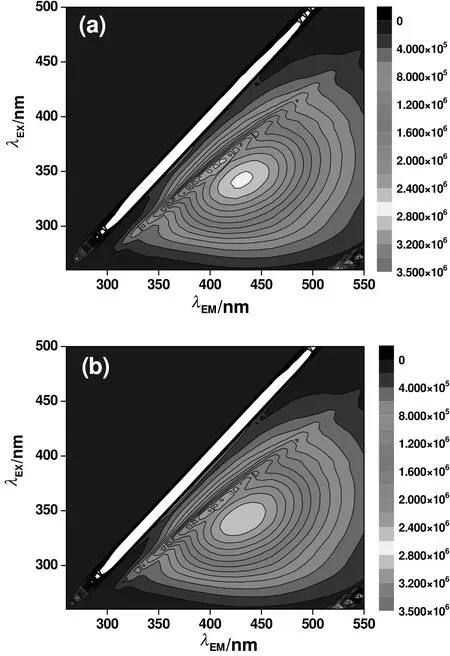
Fig.3.EEM images of raw lake water(a)and water after KMnO4 oxidation(0.5 mg·L−1)(b).
The effect of KMn O4oxidation on MW distribution of dissolved NOM is shown in Fig.4.MW of dissolved NOM in raw lake water was less than 100000,and small molecules with MW less than 1000 accounted for 43%of the entire NOM(as DOC).Oxidation with 0.5 mg·L−1KMn O4significantly changed the MW distribution of NOM.The complex organic matters in 30000–100000 decreased by 85%,most of which were destroyed into smaller molecules with simple structures.As a result,the organic matters of 10000–30000 markedly increased by around 4.7 times.The breakdown of macromolecular organic matters to form substances of lower MW due to KMn O4oxidation was also reported by Lin et al.[18,25].For smaller molecules with MW less than 10000,KMn O4oxidized them in an easier and more complete way,such as converting them into CO2and H2O.Their average removal efficiency was 10%–50%.It should also be noted that large molecules with MW higher than 100000 appeared after KMnO4oxidation.This was probably because of the dissolution and degradation of undissolved organic matters in raw lake water due to the KMn O4oxidation.
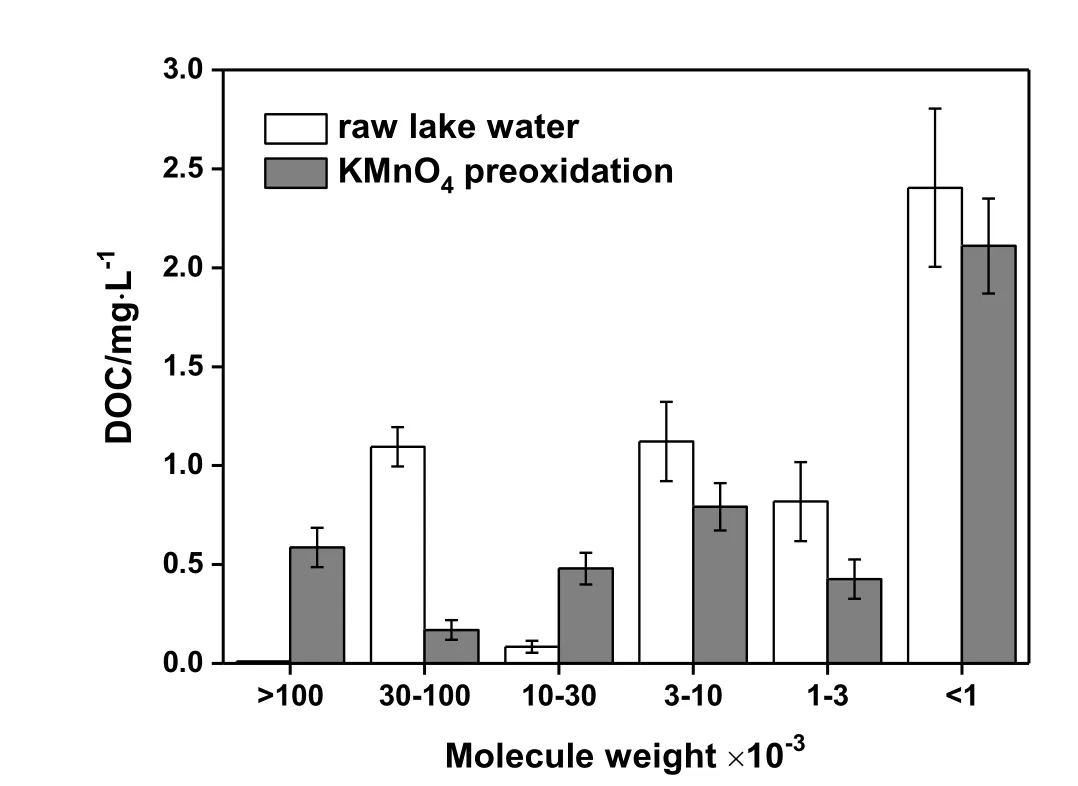
Fig.4.Effect of KMnO4 oxidation(0.5 mg·L−1)on molecular weight distribution of NOM.
The characteristics of hydrophilic and hydrophobic fractions of dissolved NOM were quantitatively analyzed to investigate the mechanism of KMn O4oxidation on NOM properties(Fig.5).The strong hydrophobic fractions of dissolved NOM in raw lake water,namely HoB,HoN,and HoA,accounted for around 57%of the total DOC,which were almost twice as HiM.The amount of HoN was less than that of HoA,but more than that of HoB.After KMn O4oxidation,HoN markedly decreased by 64%.HoA decreased by 17%,and HoB increased by 41%.It seemed that HoN and HoA might have been converted to HoB during the KMn O4oxidation.The total hydrophobic fractions decreased by 28%.According to the EEM results(Fig.3),it was reasonable to presume that the hydrophobic fractions in raw water were the reason for the fluorescence absorption.HiM was generally the NOM species of small molecules with strong polar functional groups,which was difficult to be removed by coagulation.Our results indicated that 9%of HiM was removed after the KMn O4addition.Overall,the NOM removal efficiency was 18%as a result of the KMnO4oxidation.

Fig.5.Effect of KMn O4 oxidation(0.5 mg·L−1)on the hydrophobicity and hydrophilicity of NOM.
3.2.KMnO4 dosing position on NOM removal
KMn O4pre-oxidation can enhance the coagulation performance in terms of NOM removal.Moreover,KMn O4itself can remove some NOM species,which are not able to be effectively removed by coagulation,through the mechanism of oxidation and adsorption.Since NOM is also key inducement for membrane fouling,especially for the irreversible fouling,significant reduction of NOM concentration prior to UF is highly desired[35,36].
In order to further remove NOM before UF,a two-position dosing mode was proposed for KMn O4addition in this study.A total dosage of 0.5 mg·L−1KMn O4was divided into two portions.One was added prior to coagulation,while the other was added in the supernatant after coagulation,i.e.,prior to UF.Compared with the pre-oxidation prior to coagulation,the addition of partial KMn O4after coagulation obtained a comparable NOM removal efficiency(Fig.6).The maximum NOM removal was 44%with half of KMn O4delivered upstream of coagulation and half downstream.It seemed that KMn O4dosing position did not affect its function in terms of NOM removal.The SUVA value slightly decreased when KMn O4was added in the two position dosing mode(Fig.6).It should be noted that KMn O4addition after coagulation introduced MnO2nanoparticles into the UF in fluent,and the turbidity consequently increased.Therefore,KMn O4oxidation was usually used as pre-treatment technique in the enhanced coagulation process[37].UF can effectively retain the Mn O2nanoparticles,which made the addition of KMn O4after coagulation an alternative dosing position in the coagulation/UF combined process.However,DOC and SUVA results indicated that the novel two-position dosing mode did not contribute to the extra NOM removal significantly.

Fig.6.Effect of KMn O4 dosing position on NOM removal(total KMn O4 dosage of 0.5 mg·L−1).
3.3.KMnO4 dosing position on membrane fouling

Fig.7.Effect of KMnO4 dosing position on trans membrane pressure pro file.
The effect of KMn O4dosing position on UF fouling was investigated in the coagulation/UF process(Fig.7).0.5 mg·L−1KMn O4was added prior to coagulation,after coagulation,or in a split-mode(0.25 mg·L−1+0.25 mg·L−1).With the filtration goingon,TMP linearly increased,indicating the occurrence of membrane fouling.NOM in raw water was one of the main reasons for fouling.In the control test without KMn O4,TMP increased to 1.9 times its initial value.The hydrophobic fraction of NOM had astrong affinity with the hydrophobic PVDF membrane,and firmly attached or adsorbed on the membrane surface and pores.Large molecules were prone to block the pores and increase the resistance of filtration[38].It has been shown in Figs.4 and 5 that KMn O4oxidized large hydrophobic molecules and converted them into smaller ones with higher hydrophilicity.Therefore,when KMn O4was added in whichever position investigated,the increase in TMP was significantly mitigated.Compared with the KMn O4addition prior to coagulation,KMn O4addition after coagulation exhibited a better performance in membrane fouling alleviation,and the increase rate in TMP decreased by around 27%.This was mainly attributed to the presence of Mn O2nanoparticles in the in fluent of UF.These Mn O2nanoparticles adsorbed small organic molecules,and prevented them from entering into membrane pores.Therefore,the narrowing and blocking of membrane pores caused by NOM were alleviated.More importantly,when Mn O2nanoparticles were retained by UF membrane,they adjusted the fouling cake layer to a loose structure,and the filtration resistance consequently decreased[39].The best fouling control performance was obtained in the proposed two-position KMn O4dosing mode.The increase rate in TMP decreased by at least 57%compared to either one-position dosing mode(KMn O4addition prior to or after coagulation).Therefore,it can be concluded that the two-position KMn O4dosing mode combined the advantages of KMn O4use prior to coagulation and prior to UF in terms of membrane fouling control.On the one hand,KMn O4pre-oxidation enhanced the NOM removal by coagulation,and facilitated the NOM conversion to less fouling species.On the other hand,the production of Mn O2nanoparticles rearranged the structure of the fouling layer besides its adsorption of NOM.
The SEM images in Fig.8 clearly show that the membrane pores are markedly covered by foulants after 2 h of filtration.Therefore,the filtration resistance increased.However,fouling layer morphology exhibited significant differences among different operation modes.When KMn O4was added prior to coagulation,the fouling layer was mainly composed by organic macromolecules remaining after coagulation and presented a gel-like structure[Fig.8(b)].Such a structure was resulted from the direct attachment of organic compounds onto membrane surface as well as the blockage of membrane pores,and was considered to greatly contribute to the filtration resistance.When KMn O4was added after coagulation,the in-situ formed Mn O2adsorbed these small organic compounds and adjusted the fouling layer to a much looser structure[Fig.8(c)and(d)].Therefore,the fouling resistance decreased.The mitigation of membrane fouling due to the presence of intermediate product Mn O2was also demonstrated by Lin et al.[18].The amount of KMn O4used after coagulation in the two-position dosing mode was less than that in the one-position dosing mode(0.25 mg·L−1vs.0.5 mg·L−1).As a result,less Mn O2was accumulated on the membrane surface and the fouling layer became looser and opener[Fig.8(d)].
Fig.9 shows the components of fouling resistance in the systems of different operation modes by the end of the two-hour filtration.Clearly,fouling resistance contributed significantly to the overall filtration resistance.The use of KMn O4decreased both reversible and irreversible resistances.However,when KMn O4was added after coagulation,the in-situ produced Mn O2nanoparticles contacted directly with the membrane surface,which successfully prevented the foulants from entering into the membrane pores and made the backwash more effective.As a result,the irreversible resistance significantly decreased by 30%.When the two-position dosing mode with the same total KMn O4concentration was applied,both the reversible and irreversible resistances further decreased compared to those in the one-position dosing mode by 10%and 35%,respectively.The simple two-position KMnO4dosing mode exhibited synergic effects on membrane fouling control,especially for irreversible resistance,and was a better alternative way to apply KMn O4in conventional coagulation/UF combined process.In addition,when 0.5 mg·L−1KMn O4was evenly dosed upstream and downstream of coagulation,Mn concentration in the effluent was around 0.03 mg·L−1,which was complied with the drinking water standards recommended by US Environmental Protection Agency(0.05 mg·L−1)[40].

Fig.8.SEM images of new membrane(a)and used membranes in coagulation/UF process with KMnO4 addition prior to coagulation(b),after coagulation(c),and in the two-position dosing mode(d).

Fig.9.Effect of KMn O4 dosing position on the components of fouling resistance.
4.Conclusions
KMn O4oxidation was able to alter the characteristics of NOM in lake water.The hydrophobic organic matters,such as HoN and HoA,decreased after KMn O4addition,and NOM species with MW between 30000 and 100000 kDa also decreased.Compared to coagulation without KMnO4,KMnO4addition markedly improved the DOC removal.Even though the dosing position,either in raw water prior to coagulation or in the supernatant after coagulation,did not seem to affect the DOC concentration in the UF permeate,it significantly affected the membrane fouling.The proposed two-position dosing mode,namely KMnO4addition both prior to and after coagulation,was much more effective in terms of membrane fouling alleviation and fouling irreversibility reduction.The in-situ produced MnO2managed to form loose cake layer on membrane surface,and consequently improved the performance of filtration and backwash.The two-position dosing mode was a simple and effective way to apply KMnO4in the conventional coagulation/UF combined process in order to improve the effluent quality and mitigate membrane fouling.
[1]K.Zhang,G.Achari,R.Sadiq,C.H.Langford,M.H.Dore,An integrated performance assessment framework for water treatment plants,Water Res.46(6)(2012)1673–1683.
[2]A.Matilainen,M.Vepsalainen,M.Sillanpaa,Natural organic matter removal by coagulation during drinking water treatment:A review,Adv.Colloid Interf.Sci.159(2)(2010)189–197.
[3]K.A.Reynolds,K.D.Mena,C.P.Gerba,Risk of waterborne illness via drinking water in the United States,Rev.Environ.Contam.Toxicol.192(2008)117–158.
[4]P.C.Singer,Humic substances as precursors for potentially harmful disinfection byproducts,Water Sci.Technol.40(9)(1999)25–30.
[5]E.E.Chang,P.C.Chiang,Y.W.Ko,W.H.Lan,Characteristics of organic precursors and their relationship with disinfection by-products,Chemosphere 44(5)(2001)1231–1236.
[6]V.Uyak,I.Koyuncu,I.Oktem,M.Cakmakci,I.Toroz,Removal of trihalomethanes from drinking water by nano filtration membranes,J.Hazard.Mater.152(2)(2008)789–794.
[7]J.G.Jacangelo,J.DeMarco,D.M.Owen,S.J.Randtke,Selected processes for removing NOM:An overview:Natural organic matter,J.Am.Water Work Assoc.87(1)(1995)64–77.
[8]B.V.Petrusevski,A.N.Van Breemen,G.Alaerts,Effect of permanganate pretreatment and coagulation with dual coagulants on algae removal in direct filtration,Aqua 45(6)(1996)316–326.
[9]J.D.Plummer,J.K.Edzwald,Effect of ozone on algae as precursors for trihalomethane and haloacetic acid production,Environ.Sci.Technol.35(18)(2001)3661–3668.
[10]J.D.Plummer,J.K.Edzwald,Effects of chlorine and ozone on algal cell properties and removal of algae by coagulation,J.Water Supply Res.Technol.AQUA 51(6)(2002)307–318.
[11]Y.L.Song,Y.Deng,C.I.Jung,Mitigation and degradation of natural organic matters(NOMs)during ferrate(VI)application for drinking water treatment,Chemosphere 146(2016)145–153.
[12]D.An,J.X.Song,W.Gao,G.G.Chen,N.Y.Gao,Molecular weight distribution for NOM in different drinking water treatment processes,Desalin.Water Treat.5(1–3)(2009)267–274.
[13]H.G.Peterson,S.E.Hrudey,I.A.Cantin,T.R.Perley,S.L.Kenefick,Physiological toxicity,cell membrane damage and the release of dissolved organic carbon and geosmin by Aphanizomenon Flos-Aquae after exposure to water treatment chemicals,Water Res.29(6)(1995)1515–1523.
[14]W.Z.Yu,J.Gregory,T.Liu,Y.L.Yang,M.Sun,G.B.Li,Effect of enhanced coagulation by KMn O4 on the fouling of ultra filtration membranes,Water Sci.Technol.64(7)(2011)1497–1502.
[15]J.Y.Tian,M.Ernst,F.Cui,M.Jekel,KMnO4pre-oxidation combined with FeCl3coagulation for UF membrane fouling control,Desalination 320(2013)40–48.
[16]P.Phatai,J.Wittayakun,W.H.Chen,C.M.Futalan,C.C.Kan,Removal of manganese(II)and iron(II)from synthetic groundwater using potassium permanganate,Desalin.Water Treat.52(31–33)(2014)5942–5951.
[17]H.J.Liu,R.P.Liu,C.Tian,H.Jiang,X.L.Liu,R.Zhang,J.H.Qu,Removal of natural organic matter for controlling disinfection by-products formation by enhanced coagulation:A case study,Sep.Purif.Technol.84(2012)41–45.
[18]T.Lin,S.Pan,W.Chen,S.Bin,Role of pre-oxidation,using potassium permanganate,for mitigating membrane fouling by natural organic matter in an ultra filtration system,Chem.Eng.J.223(2013)487–496.
[19]C.Tian,F.Liu,Y.Bai,R.Liu,H.Chen,B.Wang,J.Qu,Comparison of Fe-Mn enhanced coagulation and O-3-BAC for removing natural organic matter from source waters:A case study,Desalin.Water Treat.57(20)(2016)9101–9114.
[20]S.J.Xia,X.Li,R.P.Liu,G.B.Li,Pilot study of drinking water production with ultra filtration of water from the Songhuajiang River(China),Desalination 179(1–3)(2005)369–374.
[21]K.Glucina,A.Alvarez,J.M.Laine,Assessment of an integrated membrane system for surface water treatment,Desalination 132(1–3)(2000)73–82.
[22]I.Sutzkover-Gutman,D.Hasson,R.Semiat,Humic acid removal by deep-bed filtration and by UF membranes,Desalin.Water Treat.31(1–3)(2011)42–53.
[23]S.Vulf,I.Sutzkover-Gutman,D.Hasson,R.Semiat,Effect of the concentration polarization on the fouling driving force of UF membranes,Desalin.Water Treat.31(1–3)(2011)54–58.
[24]W.Gao,H.Liang,J.Ma,M.Han,Z.L.Chen,Z.S.Han,G.B.Li,Membrane fouling control in ultra filtration technology for drinking water production:A review,Desalination 272(1–3)(2011)1–8.
[25]T.Lin,L.Li,W.Chen,S.L.Pan,Effect and mechanism of preoxidation using potassium permanganate in an ultra filtration membrane system,Desalination 286(2012)379–388.
[26]T.Lin,S.Pan,W.Chen,C.Yu,Effect of potassium permanganate pre-oxidation on fouling and pore size of ultra filtration membrane for drinking water treatment,Desalin.Water Treat.50(1–3)(2012)254–263.
[27]Z.Wang,T.Lin,W.Chen,Effect on membrane fouling and intrinsic characteristics of UF subjected to potassium permanganate pre-oxidation,Desalin.Water Treat.57(29)(2016)13404–13414.
[28]W.Z.Yu,N.Graham,H.J.Liu,H.Li,J.H.Qu,Membrane fouling by Fe-humic cake layers in nano-scale:Effect of in-situ formed Fe in-situ formed Fe(III)coagulant,J.Membr.Sci.431(2013)47–54.
[29]E.M.Thurman,R.L.Malcolm,Preparative isolation of aquatic humic substances,Environ.Sci.Technol.15(4)(1981)463–466.
[30]J.K.Edzwald,Coagulation in drinking water treatment:Particles,organics and coagulants,Water Sci.Technol.27(11)(1993)21–35.
[31]C.Volk,K.Bell,E.Ibrahim,D.Verges,G.Amy,M.Lechevallier,Impact of enhanced and optimized coagulation on removal of organic matter and its biodegradable fraction in drinking water,Water Res.34(12)(2000)3247–3257.
[32]J.K.Edzwald,J.E.Tobiason,Enhanced coagulation:US requirements and a broader view,Water Sci.Technol.40(9)(1999)63–70.
[33]P.G.Coble,Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy,Mar.Chem.51(4)(1996)325–346.
[34]K.Sazawa,M.Tachi,T.Wakimoto,T.Kawakami,N.Hata,S.Taguchi,H.Kuramitz,The evaluation for alterations of DOM components from upstream to downstream flowof rivers in Toyama(Japan)using three-dimensional excitation-emission matrix fluorescence spectroscopy,Int.J.Environ.Res.Public Health 8(5)(2011)1655–1670.
[35]A.W.Zularisam,A.Ahmad,M.Sakinah,A.F.Ismail,T.Matsuura,Role of natural organic matter(NOM),colloidal particles,and solution chemistry on ultra filtration performance,Sep.Purif.Technol.78(2)(2011)189–200.
[36]A.S.Al-Amoudi,Factors affecting natural organic matter(NOM)and scaling fouling in NF membranes:A review,Desalination 259(1–3)(2010)1–10.
[37]J.J.Chen,H.H.Yeh,I.C.Tseng,Effect of ozone and permanganate on algae coagulation removal— Pilot and bench scale tests,Chemosphere 74(6)(2009)840–846.
[38]K.J.Howe,M.M.Clark,Fouling of micro filtration and ultra filtration membranes by natural waters,Environ.Sci.Technol.36(16)(2002)3571–3576.
[39]Z.Lu,T.Lin,W.Chen,X.B.Zhang,Influence of KMnO4preoxidation on ultra filtration performance and membrane material characteristics,J.Membr.Sci.486(2015)49–58.
[40]United States Environmental Protection Agency,Drinking Water Contaminants–Standards and Regulations,Secondary Drinking Water Standards:Guidance for Nuisance Chemicals,Available from:https://www.epa.gov/d wstandardsregulations/secondary-drinking-water-standards-guidance-nuisance-chemicals(accessed on 15th March,2017).
 Chinese Journal of Chemical Engineering2018年1期
Chinese Journal of Chemical Engineering2018年1期
- Chinese Journal of Chemical Engineering的其它文章
- Preparation of water-soluble magnetic nanoparticles with controllable silica coating☆
- Bi-/multi-modal pore formation of PLGA/hydroxyapatite composite scaffolds by heterogeneous nucleation in supercritical CO2 foaming☆
- Comparative experimental study on reactive crystallization of Ni(OH)2 in an airlift-loop reactor and a stirred reactor☆
- The green hydrolysis technology of hemicellulose in corncob by the repeated use of hydrolysate☆
- Determination of 4-nonylphenol and 4-tert-octylphenol compounds in various types of wastewater and their removal rates in different treatment processes in nine wastewater treatment plants of Iran
- Lipid enhancement in microalgae by temporal phase separation:Use of indigenous sources of nutrients
