PD-1/PD-L1 pathway blockade works as an effective and practical therapy for cancer immunotherapy
Long Jia, Qi Zhang, Rongxin Zhang,3
1Laboratory of Immunology and Inflammation, Department of Immunology and Research Center of Basic Medical Sciences,Key Laboratory of Immune Microenvironments and Diseases of Educational Ministry, Tianjin Medical University, Tianjin 300070, China; 2Institute of Integrative Medicines for Acute Abdominal Diseases, Tianjin Nankai Hospital, Tianjin 300100,China; 3Guangdong Province Key Laboratory for Biotechnology Drug Candidates, School of Life Sciences and Biopharmaceutics, Guangdong Pharmaceutical University, Guangzhou 510006, China
Introduction
Programmed cell death-1 (PD-1) was first discovered in 1992 by Ishida et al. as a novel member of the immunoglobulin gene super family that plays a role in programmed cell death1. Moreover in the same year, Chen et al.2found that the interaction of the B7 molecule on antigen-presenting cells with its receptors, CD28 and CTLA-4, could change antitumor immunity, which may be a useful strategy for cancer treatment. These discoveries initiated a new era for cancer immunotherapy. Since then, more immune checkpoints have been discovered and further studies have been conducted. PD-1 is one of the most useful immune checkpoints, and many drugs that target PD-1/PD-L1 pathway have been approved for clinical cancer treatment.PD-1 belongs to the CD28 family and is expressed on T lymphocytes, B lymphocytes, dendritic cells, macrophages,and natural killer cells, with a predominance on activated CD8+T cells, CD4+T cells, and B cells in peripheral tissues3-5.Programmed cell death ligand-1 (PD-L1) is the ligand of PD-1 and is expressed by antigen-presenting cells and tissue cells,including cancer cells6-8. The PD-1/PD-L1 pathway negatively regulates the immune response by inhibiting the activation and proliferation of T lymphocytes, reducing the production of cytokines, and enhancing the exhaustion of CD8+T lymphocytes5,9,10. The PD-1/PD-L1 pathway helps to mediate immune tolerance in peripheral tissues11. Moreover,for tumor cells, the PD-1/PD-L1 pathway plays an important role in dampening anti-tumor immunity12,13. Increasing number of clinical studies have indicated that the expression of PD-L1 on tumor cells is positively correlated with poor prognosis14-19. Furthermore, many studies have testified that the inhibition of PD-1/PD-L1 pathway provides a very effective tumor treatment20,21. Many drugs that target the PD-1/PD-L1 pathway have been developed, and many clinical trials have been conducted. Some of these clinical trials were so successful that the FDA approved several PD-1/PD-L1 pathway blocking drugs for clinical cancer treatment.
Clinical studies of PD-1/PD-L1 blocking drugs
So far, the FDA has approved five drugs that target the PD-1/PD-L1 pathway for cancer treatment. These five drugs are Keytruda (pembrolizumab), Opdivo (nivolumab),Bavencio (avelumab), Tecentriq (atezolizumab), and Imfinzi(durvalumab). Pembrolizumab, nivolumab, and durvalumab are PD-1 antibodies, while atezolizumab and avelumab are PD-L1 antibodies. The clinical studies that gained the FDA approval of pembrolizumab are listed in Table 1. As shown in Table 1, pembrolizumab has been approved for the treatment of seven different types of cancer. Particularly, the approval of pembrolizumab for the treatment of microsatellite instability high (MSI-H) or mismatch repair deficient (dMMR) solid tumors is the first time that the FDA has approved a drug for cancer treatment based on the marker rather than the location of cancer origin, which also reveals the extensive applicability of cancer immunotherapy.
DNA mismatch repair (MMR) is a highly conserved process that plays an important role in DNA repair, meiotic and mitotic recombination, DNA-damage signaling,apoptosis, and cell-type-specific processes, such as classswitch recombination, somatic hypermutation, and tripletrepeat expansion22. When the MMR system develops a functional error or defect, this results in a specific phenotypecalled microsatellite instability (MSI), which is characterized by the insertion or deletion of short, repetitive sequences of DNA and results in mutations in cancer-related genes23.MSI-H/dMMR causes an increase of mutation-associated neoantigens, which cause more immune cells to infiltrate into tumors, trigger a greater anti-tumor immune response,and provide important targets for checkpoint blockade therapies24-27. Furthermore, the clinical trials validated the efficiency of MSI-H/dMMR as markers of PD-1/PD-L1 blocking immunotherapy, and the FDA approval is based on five such clinical trials: KEYNOTE-016 (NCT01876511, 58 patients)28, KEYNOTE-164 (NCT02460198, 61 patients)29,KEYNOTE-012 (NCT01848834, 6 patients)30, KEYNOTE-028 (NCT02054806, 5 patients), and KEYNOTE-158(NCT02628067, 19 patients)29. A total of 15 cancer types with MSI-H or dMMR were identified in the 149 patients who were enrolled across the above five clinical trials. For these 149 patients who were treated with pembrolizumab, the objective response rate (ORR) was 39.6%, and the response lasted at least six months in 78% of these patients.Accordingly, the FDA granted accelerated approval to pembrolizumab for MSI-H or dMMR solid tumors31.

Table 1 Clinical studies about pembrolizumab
The studies that allowed nivolumab to achieve FDA approval are listed in Table 2, and the studies that allowed avelumab, atezolizumab and durvalumab to acquire FDA approval are listed in Table 3. The respective indications,references, clinical trials, ORR, adverse events, survivals, and control treatments are listed in each table. As shown in Table 1 and Table 2, pembrolizumab and nivolumab had better performances and less treatment-related (TR) adverse events than the respective control treatments.
Universality and sustainability
As listed in Tables 1–3, PD-1/PD-L1 blocking drugs have been approved by the FDA for the treatment of many cancers. Additional clinical trials of PD-1/PD-L1 blocking drugs are in progress. PD-1/PD-L1 blocking therapies target the repressed immune system to re-wake the anti-tumor immune response rather than target particular molecule of cancer cells in which case cancer cells can escape the therapy by the mutation of the targeted molecule. Thus, PD-1/PD-L1 blocking therapies have a wide range of applications to many different types of cancer and consistent therapeutic effects,even after that cancer has progressed in the previous PD-1/PD-L1 blocking treatments. A large, international, phase 3 study (NCT01668784), in consistent with the results from the phase 2 study (NCT01354431), demonstrated that nivolumab for patients treated beyond RECIST progression (TBP) with nivolumab before resulted in additional clinical benefits again32,33. Tumor burden reduction was observed in patients who initially responded with nivolumab treatment and then progressed, and in patients with stable disease or progressive disease as their best overall response brfore32,33.
Can higher cut-off standards promote the ability of PD-L1 to function as an indicative marker?
While the FDA has approved the MSI-H/dMMR of solid tumors as an indication for pembrolizumab after successful clinical trials, PD-L1 still has not been approved as an indicative marker of PD-1/PD-L1 blocking therapy for pancancer treatment. This situation can be attributed to some studies indicating that PD-L1 expression levels in tumor cells or tumor infiltrating immune cells don’t correlate with the efficiency of PD-1/PD-L1 blocking therapy34,35. However, the cut-off standards of defining PD-L1 positive was relatively low in these studies (e.g. PD-L1 positive defined as > 1% of either tumor cells or immune cells staining for PD-L1). With higher PD-L1 positive thresholds, better outcomes have been seen in patients who were treated with PD-1/P-L1 blocking therapies. In the clinical trial NCT02108652, the ORR was 26% in the IC2/3 group (PD-L1≥5%), 18% in the IC1/2/3 group PD-L1≥1%), and 15% in all patients. The median overall survival was 11.4 months in the IC2/3 group, 8.8 months in the IC1/2/3, and 7.9 months across all patients36.In the clinical trial NCT02008227, the median overall survival was 12.6 months in the PD-L1 low or undetectable subgroup (≤1% of either tumor cells or immune cells staining for PD-L1), 13.2 months in the PD-L1>1%subgroup, and 20.5 months in the PD-L1 high expression subgroup (PD-L1≥50%)36. In the clinical trial NCT01693562,the ORR was 27.6% in the PD-L1 high expression subgroup(≥25% of either tumor cells or immune cells staining for PDL1) and 5.1% in the PD-L1 low expression subgroup (<25%of either tumor cells or immune cells staining for PD-L1)37.Furthermore, the studies that are listed in Tables 1–3 have indicated that PD-L1 negative patients may also benefit from PD-1/PD-L1 blocking therapies. Altogether, with more clinical studies, higher cut-off standards of the rates of PD-L1 expressing tumor cells may promote PD-L1 working as an indicative marker of pan-cancer PD-1/PD-L1 blocking treatments. Moreover, different tumors may require different PD-L1 cut-off thresholds.
Safety
As shown in Tables 1–3 and many other studies, PD-1/PDL1 blocking therapies produced a significantly lower rate ofhigh-grade TR adverse events than other immunotherapies,chemotherapies, and standard therapies38,39. This mainly be attributed to the mechanisms of the PD-1/PD-L1 pathway functioning. The PD-1/PD-L1 pathway negatively regulates the immune response mainly in peripheral tissues including the tumor microenvironments40. Moreover, as PD-1/PD-L1 blocking therapy mainly activates the inactivated, mature T cells and B cells, and prevents the inactivation of mature T cells and B cells, it mainly affects the late phase of the immune response. Thus, PD-1/PD-L1 blocking therapies produced significantly lower rate of high-grade TR adverse events. According to the studies listed in Tables 1–3, fatigue was the most common TR adverse events. Decreased appetite, asthenia, diarrhea, pneumonitis, rash, and pruritus were also common TR adverse events.

Table 2 Clinical studies about nivolumab
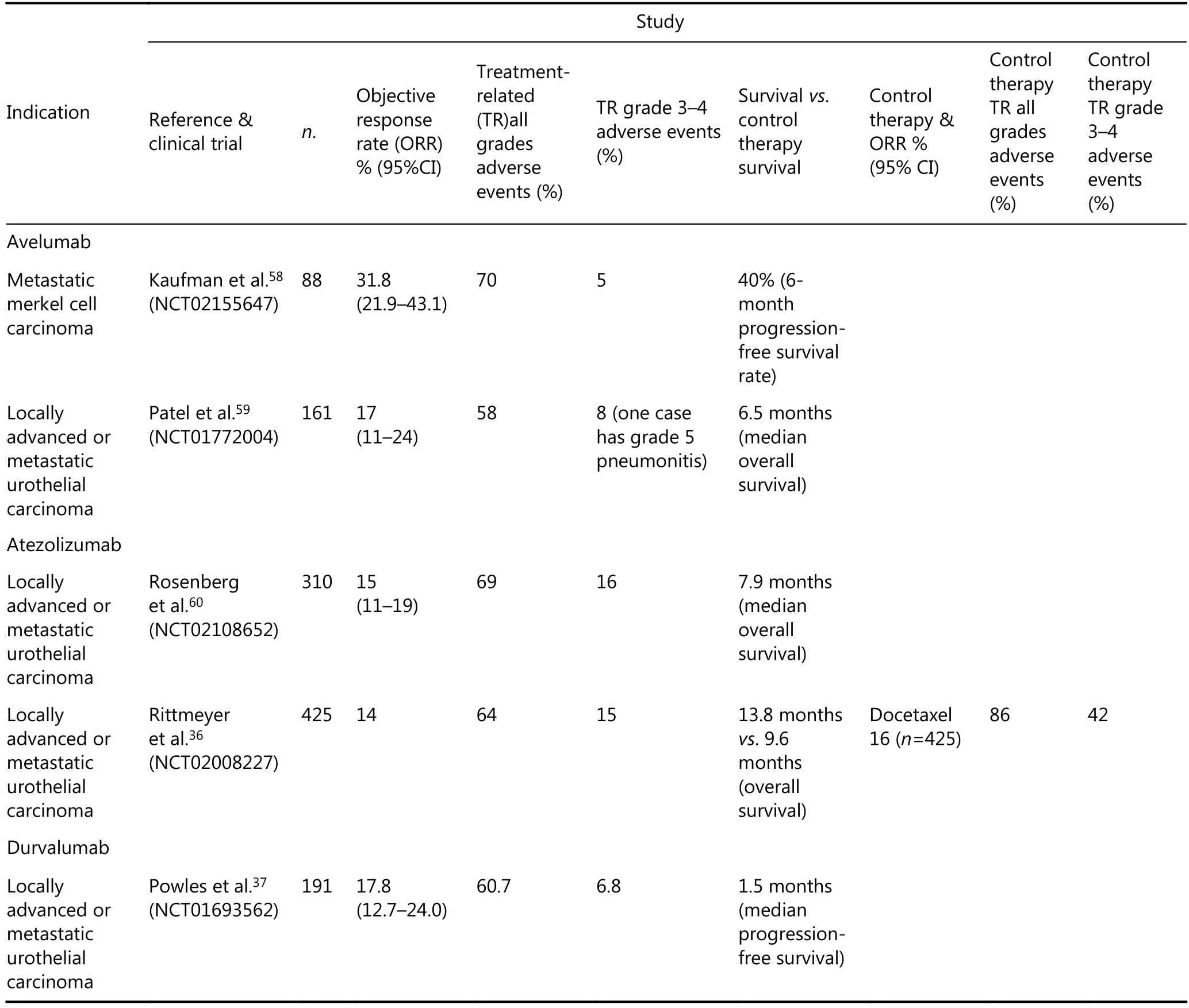
Table 3 Clinical studies about avelumab, atezolizumab and durvalumab
Combination therapies
Besides working as monotherapy, PD-1/PD-L1 blocking therapies can also be used in combination with other antitumor therapies, and some of these combination therapies have had successful and inspiring effects. In the clinical trial NCT02039674, pembrolizumab was combined with chemotherapy for the treatment of non-small cell lung cancer(NSCLC). The ORR was 55% in the combination group and 29% in the chemotherapy alone group41. The incidences of grade 3 or worse TR adverse events were similar between the two groups (39% in the pembrolizumab plus chemotherapy group and 26% in the chemotherapy alone group)41. Based on these clinical trial results, the FDA approved pembrolizumab in combination with pemetrexed and carboplatin as the first-line treatment of patients with metastatic non-squamous NSCLC. In the clinical trial(NCT01024231), nivolumab in combination with ipilimumab resulted in an objective response in that 53% of patients, and all with tumor reductions of 80% or more;however grade 3 or 4 TR adverse events occurred in 53% of the patients42. In the clinical trial (NCT01927419), the ORR and median progression free survival (PFS) in the nivolumab and ipilimumab combined group were 61% and 8.9 months respectively, while the ORR and median PFS were 11% and 4.7 months respectively in the ipilimumab monotherapy group43. In this trial, the median tumor volume was a 68.1%decrease in the combination group and a 5.5% increase in the ipilimumab monotherapy group43. However, the TR grade 3-4 adverse events was 54% in the combination group, versus 24% in the ipilimumab monotherapy group43. Based on these results, the FDA approved nivolumab and ipilimumab combination therapy for unresectable or metastatic melanomas. By now, the FDA only approved the above two PD-1/PD-L1 blocking therapies related combination therapies. Due to the great potential of combination therapies, more and more combination therapy clinical trials are currently in process. With better efficiency, the FDA may approve more combination therapies in the future.
Future prospective
Over the past several years, cancer immunotherapy has advanced greatly and some have achieved the FDA approval for cancer treatment. Moreover, the combination therapies that include PD-1/PD-L1 blocking therapies have shown great potencies and efficacies. In the future, combination therapies may become mainstream therapy for cancer treatments. Meanwhile, TR adverse events, including immune-related adverse events, have emerged with the development of cancer immunotherapies, and require more attention and solution. Furthermore, with more clinical studies and higher PD-L1 expression cut-off rates, PD-L1 may also be an indicative marker for pan-cancer treatment with PD-1/PD-L1 blocking therapies.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
1.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily,upon programmed cell death. EMBO J. 1992; 11: 3887-95.
2.Chen LP, Ashe S, Brady WA, Hellström I, Hellström KE, Ledbetter JA, et al. Costimulation of antitumor immunity by the B7 counter receptor for the T lymphocyte molecules CD28 and CTLA-4. Cell.1992; 71: 1093-102.
3.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubat T, Yagita H,et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996; 8: 765-72.
4.Iwai Y, Okazaki T, Nishimura H, Kawasaki A, Yagita H, Honjo T.Microanatomical localization of PD-1 in human tonsils. Immunol Lett. 2002; 83: 215-20.
5.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000; 192: 1027-34.
6.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N,et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002; 84: 57-62.
7.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med.2003; 198: 39-50.
8.Takada K, Toyokawa G, Shoji F, Okamoto T, Maehara Y. The significance of the PD-L1 expression in non-small-cell lung cancer:trenchant double swords as predictive and prognostic markers. Clin Lung Cancer. 2017; 19: 120-9.
9.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ.Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27: 111-22.
10.Mishra AK, Kadoishi T, Wang XG, Driver E, Chen ZG, Wang XJ,et al. Squamous cell carcinomas escape immune surveillance via inducing chronic activation and exhaustion of CD8+T cells co-expressing PD-1 and LAG-3 inhibitory receptors. Oncotarget.2016; 7: 81341-56.
11.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA,et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006; 203: 883-95.
12.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N.Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade.Proc Natl Acad Sci USA. 2002; 99: 12293-7.
13.Taube JM, Anders RA, Young GD, Xu HY, Sharma R, McMiller TL,et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4: 127ra37
14.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong HD,Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004; 101: 17174-9.
15.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S.Overexpression of B7-H1 (PD-L1) significantly associates withtumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007; 56: 1173-82.
16.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al.Clinical significance of programmed Death-1 Ligand-1 and programmed Death-1 Ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005; 11: 2947-53.
17.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al.Clinical significance and therapeutic potential of the programmed Death-1 Ligand/programmed Death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007; 13: 2151-7.
18.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y,Yamaguchi K, et al. Programmed cell Death 1 Ligand 1 and tumorinfiltrating CD8+T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007; 104: 3360-5.
19.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006; 8: 190-8.
20.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res.2013; 73: 6900-12.
21.Somasundaram A, Socinski MA, Villaruz LC. Immune checkpoint blockade in lung cancer. Discov Med. 2016; 22: 55-65.
22.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006; 7: 335-46.
23.Richman S. Deficient mismatch repair: read all about it (review).Int J Oncol. 2015; 47: 1189-202.
24.Gubin MM, Zhang XL, Schuster H, Caron E, Ward JP, Noguchi T,et al. Checkpoint blockade cancer immunotherapy targets tumourspecific mutant antigens. Nature. 2014; 515: 577-81.
25.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015; 348: 69-74.
26.Ward JP, Gubin MM, Schreiber RD. The role of neoantigens in naturally occurring and therapeutically induced immune responses to cancer. Adv Immunol. 2016; 130: 25-74.
27.Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R,et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016; 15: 857-65.
28.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK,et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017; 357: 409-13.
29.Diaz L, Marabelle A, Kim TW, Geva R, Van Cutsem E, André T,et al. 386p Efficacy of pembrolizumab in phase 2 KEYNOTE-164 and KEYNOTE-158 studies of microsatellite instability high cancers. Ann Oncol. 2017; 28: mdx367.020
30.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al.Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial.Lancet Oncol. 2016; 17: 956-65.
31.U.S. FOOD & DRUG. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication.https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm. 2017.
32.Escudier B, Motzer RJ, Sharma P, Wagstaff J, Plimack ER,Hammers HJ, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in checkmate 025. Eur Urol. 2017; 72: 368-76.
33.George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol.2016; 2: 1179-86.
34.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE,Poddubskaya E, et al. nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373: 1627-39.
35.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A,Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015; 33: 4015-22.
36.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, et al. atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3,open-label, multicentre randomised controlled trial. Lancet. 2017;389: 255-65.
37.Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017; 3: e172411
38.Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017; 24: 26
39.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL,Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13: 473-86.
40.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12: 252-64.
41.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA,Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-smallcell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016; 17: 1497-508.
42.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA,Lesokhin AM, et al. nivolumab plus Ipilimumab in advanced melanoma. N Engl J Med. 2013; 369: 122-33.
43.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K,McDermott D, et al. nivolumab and Ipilimumab versus Ipilimumab in untreated melanoma. N Engl J Med. 2015; 372:2006-17.
44.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al.pembrolizumab versus Ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017; 390: 1853-62.
45.Reck M, Rodríguez-Abreu D, Robinson AG, Hui RN, Csőszi T,Fülöp A, et al. pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375: 1823-33.
46.Mehra R, Seiwert TY, Mahipal A, Weiss J, Berger R, Eder JP, et al.Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC): pooled analyses after long-term follow-up in KEYNOTE-012. J Clin Oncol. 2016;34: 6012
47.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P,et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35: 2125-32.
48.Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T,et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer(KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017; 18: 1483-92.
49.Fuchs CS, DoiT, Jang RWJ, Muro K, Satoh T, Machado M, et al.KEYNOTE-059 cohort 1: efficacy and safety of pembrolizumab(pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol. 2017; 35: 4003
50.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B,et al. nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment(checkmate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015; 16: 375-84.
51.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM,Cowey CL, et al. Adjuvant nivolumab versus Ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377: 1824-35.
52.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ,Srinivas S, et al. nivolumab versus everolimus in advanced renalcell carcinoma. N Engl J Med. 2015; 373: 1803-13.
53.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016; 17: 1283-94.
54.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD,Licitra L, et al. nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016; 375: 1856-67.
55.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J,et al. nivolumab in metastatic urothelial carcinoma after platinum therapy (Checkmate 275): a multicentre, single-arm, phase 2 trial.Lancet Oncol. 2017; 18: 312-22.
56.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C,et al. nivolumab in patients with advanced hepatocellular carcinoma (Checkmate 040): an open-label, non-comparative,phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492-502.
57.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer(Checkmate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017; 18: 1182-91.
58.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, et al. avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group,open-label, phase 2 trial. Lancet Oncol. 2016; 17: 1374-85.
59.Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, et al. avelumab in metastatic urothelial carcinoma after platinum failure (javelin solid tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2017;19: 51-64.
60.Rosenberg JE, Hoffman-Censits J, Powles T, Van Der Heijden MS,Balar AV, Necchi A, et al. atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a singlearm, multicentre, phase 2 trial. Lancet. 2016; 387: 1909-20.
 Cancer Biology & Medicine2018年2期
Cancer Biology & Medicine2018年2期
- Cancer Biology & Medicine的其它文章
- Ovarian cancer presenting with hypercalcemia: two cases with similar manifestations but different mechanisms
- Primary resistance to crizotinib treatment in a non-small cell lung cancer patient with an EML4-ALK rearrangement: a case report
- Ultrasound features of extranodal extension in the metastatic cervical lymph nodes of papillary thyroid cancer: a case-control study
- Diagnostic value of whole-body MRI with diffusion-weighted sequence for detection of peritoneal metastases in colorectal malignancy
- Clinical significance of miRNA - 106a in non-small cell lung cancer patients who received cisplatin combined with gemcitabine chemotherapy
- Enterolactone modulates the ERK/NF-κB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB-231 to revert the TGF-β-induced epithelial–mesenchymal transition
