Ovarian cancer presenting with hypercalcemia: two cases with similar manifestations but different mechanisms
Xuegong Ma, Yingmei Wang, Xuhong Zhang, Mengting Dong, Wen Yang, Fengxia Xue
Department of Gynecology and Obstetrics, Tianjin Medical University General Hospital, Tianjin 300052, China
Introduction
The estimated yearly incidence of malignancy-associated hypercalcemia (MAHC) is 1.46%–2.74%1. MAHC is uncommon in patients with gynecological cancers, especially those with ovarian cancer, and usually indicates a poor prognosis. Causes of hypercalcemia in patients with cancer include humoral hypercalcemia of malignancy (HHM),primary or ectopic hyperparathyroidism, osteolytic hypercalcemia, such as skeletal metastasis, and vitamin D metabolism disorders in lymphomas2. Continuously high levels of serum calcium cause severe neurologic and renal complications in patients with cancer. The etiology and severity of hypercalcemia must be properly evaluated prior to therapy planning. Antihypercalcemic therapy, including saline hydration and calciuresis, may be effective, but anticancer therapy is more important and crucial for survival.
Although the manifestations of ovarian cancer-related hypercalcemia might be similar in different cases, the mechanisms, clinical features, and prognosis could be different for various etiologies. Here, we present two cases of ovarian cancer-associated hypercalcemia with distinct mechanisms. The stepwise clinical and laboratory investigations were performed to clarify the mechanism and differential diagnosis. Treatment strategies for the primary diseases based on the respective etiologies were recommended,and the clinical outcomes were unique.
Case report
Case one
A 63-year-old patient was referred to our hospital for lower abdominal distension. She complained of anorexia and constipation for half a year, and her physical examination revealed a pelvic mass with ascites. The dynamic contrastenhanced computed tomography (CT) scan and magnetic resonance (MR) images showed a bilateral ovarian mass(right, 10.5 cm; left, 4.5 cm; both cystic and solid tumor),with a large amount of ascites. The laboratory findings indicated a high serum calcium level of 15.4 mg/dL(reference range, 8.4–10.2 mg/dL). Moreover, the serum cancer antigen 125 (CA-125) level was 1080 U/mL, and the human epididymis protein (HE4) level was 180.6 pM. The calcium-related laboratory data were acquired (Table 1) and revealed a suppressed parathyroid hormone (PTH) value and hypophosphatemia. Her serum PTH-related protein(PTHrP) level, as measured using an immunoradiometric assay, was 12.2 pmol/L (normal, <1.3 pmol/L). The singlephoton emission computed tomography (SPECT) bone scan and parathyroid gland scan showed no evidence of skeletal metastasis or parathyroid gland hyperplasia (Figure 1). The patient was treated with vigorous intravenous hydration and diuresis, along with 30 mg of pamidronate by intravenous infusion. Her serum calcium level transiently decreased to 10.6 mg/dL, and she underwent a total hysterectomy with a bilateral salpingo-oophorectomy, omentectomy, peritoneal biopsies, and pelvic and para-aortic lymphadenectomy.Intraoperative findings showed bilateral ovarian tumors with mostly solid and partially cystic components, and both the omentum and rectal serosa were involved.
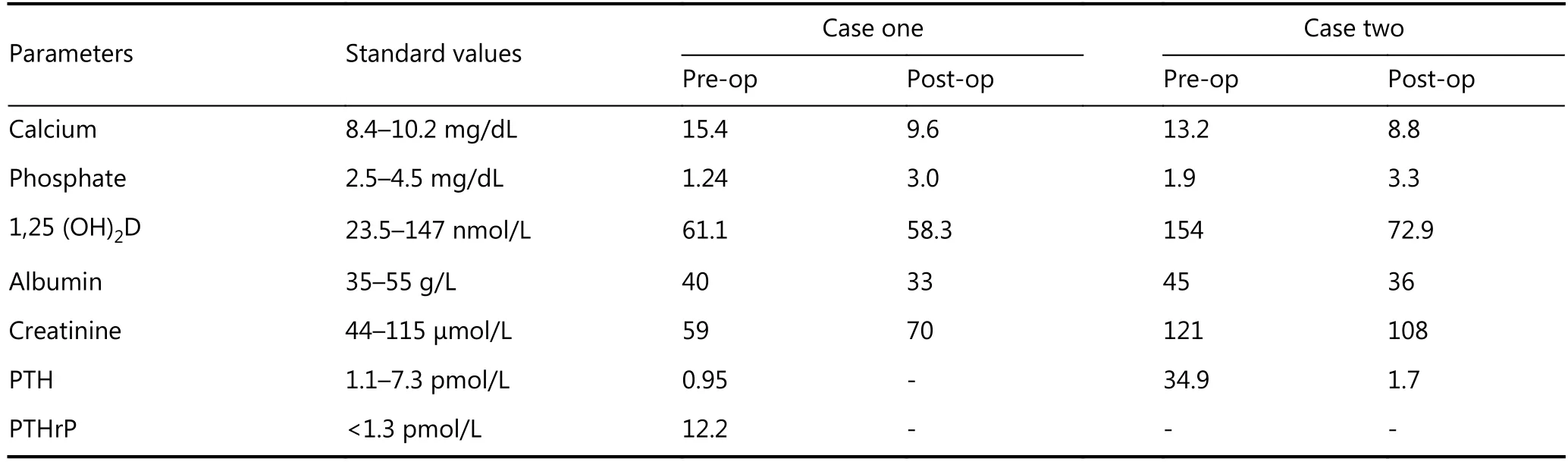
Table 1 The calcium-related laboratory data of two cases
The histopathological examination revealed clear cell adenocarcinoma with metastasis to the omentum (FIGO stage IIIC). Immunohistochemical staining was performed on the ovarian tumor tissue, which was positive for PTHrP(Figure 2A/B). Notably, the postoperative serum calcium level decreased to a normal level of 9.6 mg/dL, and the patient received six cycles of docetaxel (75 mg/m2) and carboplatin (AUC 5) chemotherapy, after which her serum CA-125 level declined to normal. Five months after the chemotherapy, the patient presented with severe abdominal distension, anorexia, and dyspnea. The CT and MR scans showed diffused metastases in the thoracic and pelvic cavities. Her serum CA-125 and calcium levels elevated to 1390 U/mL and 16.7 mg/dL, respectively. However, the patient refused any advanced chemotherapy or radiotherapy,and she turned to Chinese medicine. Two months later, she developed a coma and died within 3 days.
Case two
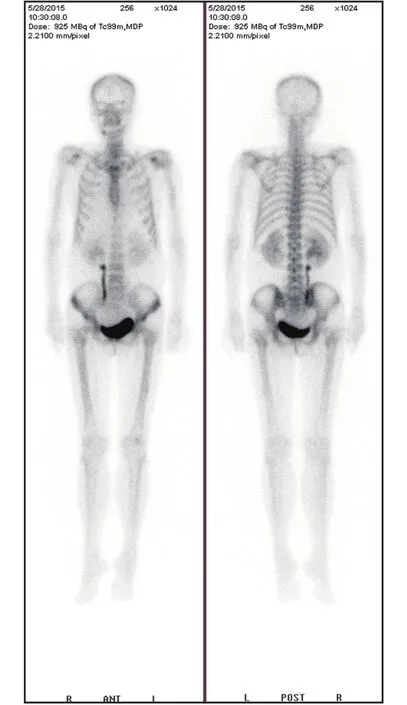
Figure 1 The single-photon emission computed tomography(SPECT) bone scan and parathyroid gland scan showing no evidence of skeletal metastasis or parathyroid gland hyperplasia in case one.
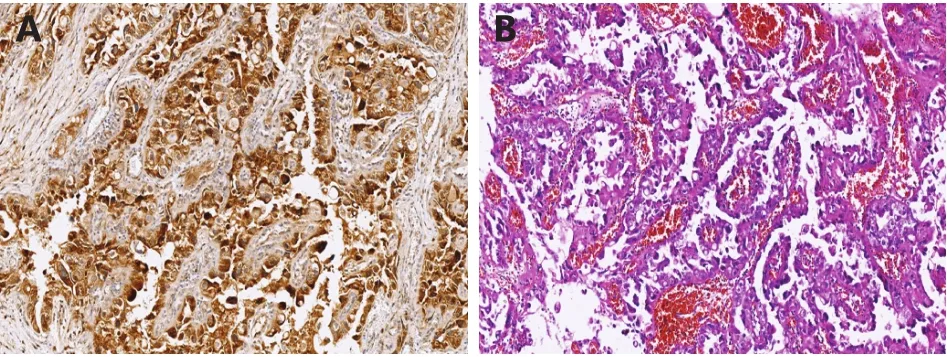
Figure 2 (A) Immunostaining of PTHrP in ovarian clear cell carcinoma (100 ×). (B) H&E staining of ovarian clear cell carcinoma (100 ×).
A 61-year-old patient was admitted to our hospital with general fatigue, constipation, and a pelvic mass, which was detected during her regular physical examination. Her vaginal ultrasound showed a 12.9 cm adnexal mass on the left side, with an 8.9 cm irregular solid component and 4 cm cyst.Her serum CA-125 level was 650 U/mL, and she had a high serum calcium level of 13.2 mg/dL. Further laboratory data indicated a low level of serum phosphate, a high level of 1,25-dihydroxyvitamin D [1,25(OH)2D], and a significantly high value of serum PTH (34.9 pmol/L; normal range, 1.1–7.3). A99mTc sestamibi-based dual-tracer scintigraphy scan revealed a left upper parathyroid gland lesion with an abnormal concentration of tracer, which was suggestive of hyperplasia or adenoma of the parathyroid gland (Figure 3). The patient was referred to the endocrinology department, where she received a left parathyroidectomy (Figure 4). Her postoperative serum calcium and PTH reduced to a normal level. After 2 months, the patient returned to the gynecologic department for surgical treatment. She underwent an optimal debulking surgery, including a total hysterectomy with bilateral salpingo-oophorectomy, omentectomy, peritoneal biopsies, and pelvic and para-aortic lymphadenectomy. Her microscopic examination showed serous adenocarcinoma,with metastasis to the omentum. The patient was staged asⅢ C and underwent six cycles of chemotherapy, which contained paclitaxel (175 mg/m2) and cisplatin (75 mg/m2).Since the primary surgery, she remained alive for 18 months,without recurrence.
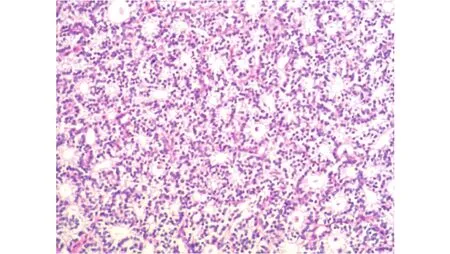
Figure 3 Parathyroid gland adenoma (H&E staining, 100 ×).
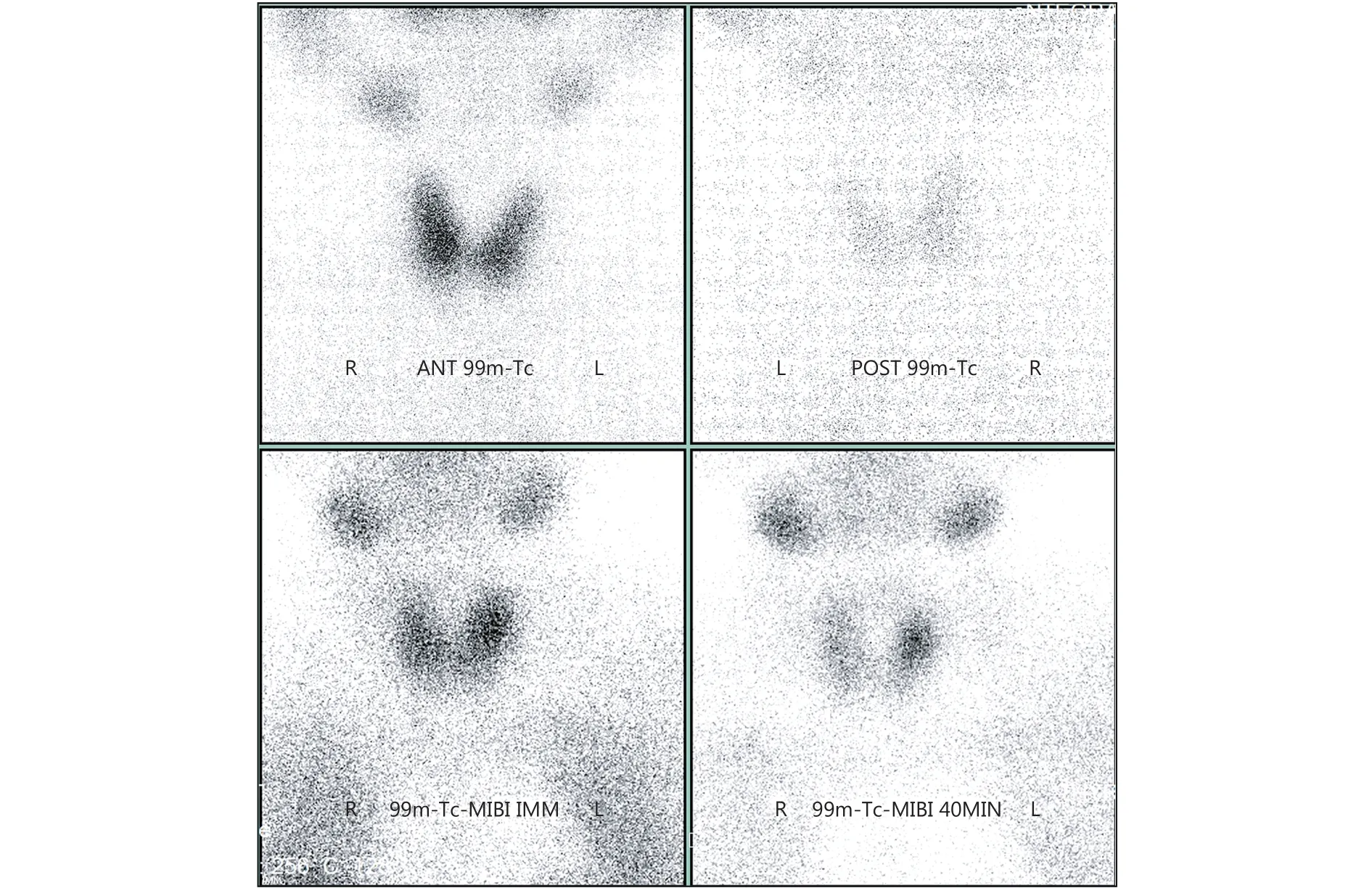
Figure 4 The emission computed tomography (ECT) scan showing left parathyroid gland lesion in case two.
Discussion
Hypercalcemia, as a metabolic disorder, is found in up to 10%–30% of patients with cancer3. MAHC usually indicates a poor prognosis, and about half of patients with MAHC die within 30 days of diagnosis4.
The most common cancers that are accompanied by hypercalcemic disorder include lung cancer, multiple myeloma, renal cell carcinoma, and breast cancer. Ovarian cancer–related hypercalcemia is rare and has only been reported in a few cases; and as such, data on the incidence of ovarian cancer–related hypercalcemia are unavailable5,6. This study reported two patients with ovarian carcinoma who presented with hypercalcemia. The underlying mechanisms that cause hypercalcemia in malignancies mainly include HHM, primary or secondary hyperparathyroidism, osteolytic hypercalcemia, and 1,25(OH)2D disorder, of which HHM that is mediated by tumor-released PTHrP is the most common cause. PTHrP is a protein that is encoded by a single-copy gene, located on the short arm of chromosome 12, is structurally similar to PTH, and is produced by cancer cells. PTHrP and PTH proteins have similar bioactive aminoterminal regions, which increase renal tubular osteoclastic bone resorption and phosphate excretion, but decrease renal calcium clearance, which results in hypercalcemia and hypophosphatemia. However, PTHrP, unlike PTH, cannot enhance 1,25(OH)2D secretion or the intestinal absorption of calcium7.
The aforementioned biological features of PTHrP help in distinguishing HHM and other causes of hypercalcemia in cancer8. In case one, the analysis of calcium-related laboratory data indicated a low level of serum PTH and phosphate, and a normal level of 1,25(OH)2D. SPECT showed no evidence of osseous metastasis or parathyroid gland adenoma, indicating that in this case, HHM might be the potential mechanism for the hypercalcemia that was associated with the ovarian cancer. Therefore, the serum PTHrP level was measured, and immunostaining for PTHrP in the clear cell ovarian cancer tissue was performed, to verify the result.
Ovarian cancers have been shown to be associated with HHM predominantly with clear cell subtype and small-cell carcinomas(Table 2)9. Clear cell carcinoma is characterized by the presence of clear cells, upon hematoxylin and eosin staining for rich cytoplasmic glycogen content, and highgrade nuclei with mitotic hyperactivity, which indicate the aggressive nature of this histological subtype. A 44-year-old patient with clear cell ovarian cancer (stage IC) who presented with hypercalcemia was reported in 201210. Clearcell carcinoma has been proven to be the dominant histological subtype, accounting for 38% of ovarian cancer–associated hypercalcemia cases. Further research has revealed that PTHrP seemed to promote tumor growth and metastasis, leading to tumor progression and poor outcome in patients with cancer11,12. Although a comprehensive treatment, which included optimized surgery combined with chemotherapy and antihypercalcemic therapy, was performed in case one, the patient died within 7 months since her primary treatment.
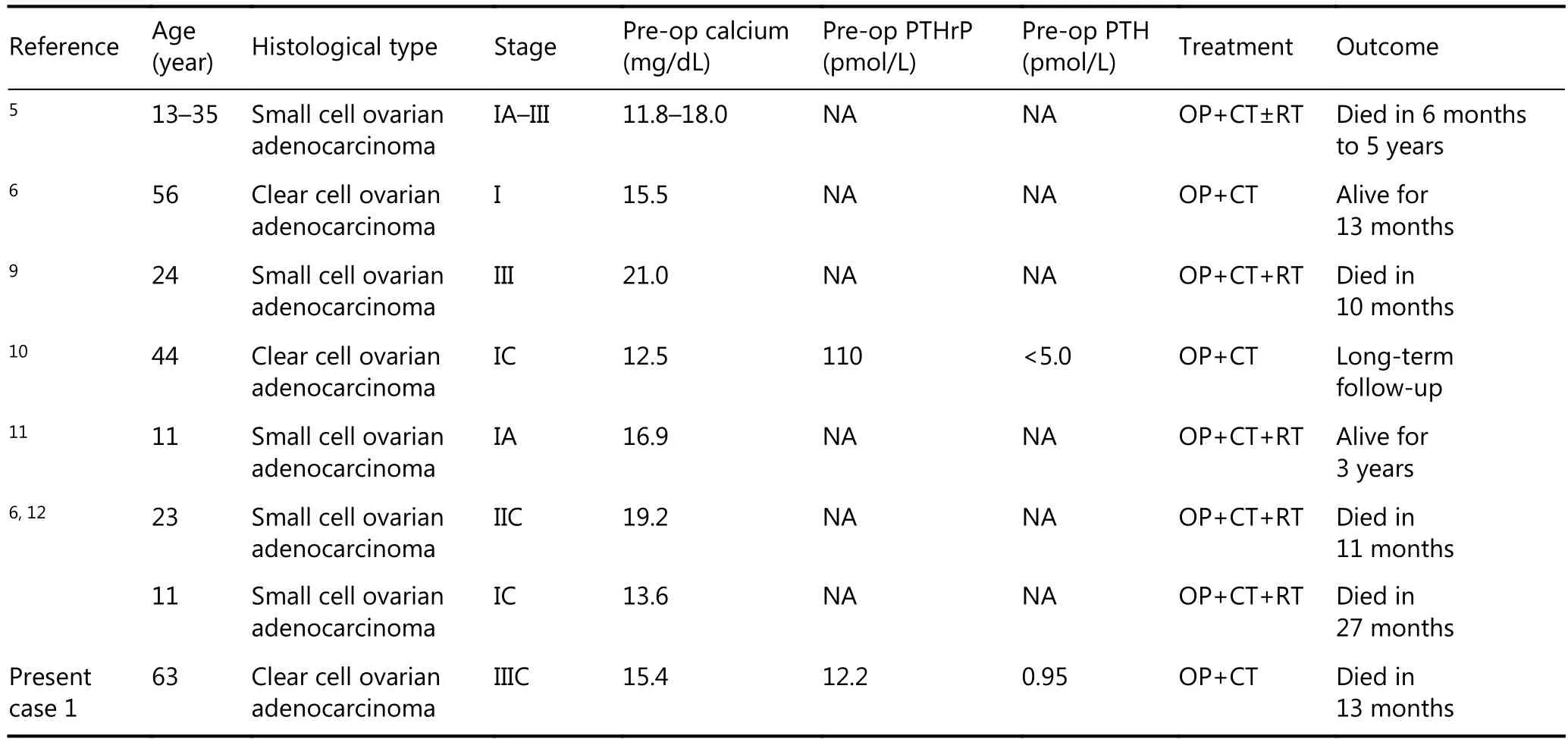
Table 2 Case reports of ovarian cancer associated hypercalcemia
Notably, a positive association has been found between the severity of hypercalcemia and the progression of ovarian cancer. The association between total serum calcium levels and ovarian cancer mortality was examined in the Third National Health and Nutrition Survey, which found that the risk of fatal ovarian cancer increased by 52% for each 0.1 mmol/L increase in total serum calcium levels13. The serum calcium level changed with the primary surgery in case one,and following the recurrence of the disease, the coma was suspected to be associated with severe hypercalcemia. This observation is similar to a case reported by the University of Texas MD Anderson Cancer Center in 200814.
PTH-mediated hypercalcemia is another common cause,in addition to PTHrP-induced HHM. Although ectopic hyperparathyroidism caused by tumor cells is extremely rare,primary hyperparathyroidism, due to parathyroid adenoma or hyperplasia, occurs more frequently15. Related research has shown that 8 cases of 133 patients with cancerrelated hypercalcemia were caused by primary hyperparathyroidism16. PTH-induced hypercalcemia was found to contributed to 3.3% of MAHCs, among which 0.5%were caused by tertiary hyperparathyroidism17. PTH,produced by the parathyroid glands, can increase renal calcium absorption but decrease renal phosphorus absorption, and stimulate the conversion of 25-hydroxy vitamin D into 1,25(OH)2D, resulting in an increased serum calcium level, a decreased serum phosphorus level, and a normal or high level of 1,25(OH)2D18. Therefore, serum 1,25(OH)2D levels should be routinely evaluated during differential diagnosis between PTHrP-induced HHM and hypercalcemia caused by hyperparathyroidism. On the other hand, Tc-99m sestamibi-based parathyroid imaging has been most widely used and is certified to be the most sensitive imaging method for detecting the preoperative localization of a parathyroid adenoma19. A subsequent dual-tracer scintigraphy scan, or the combination of SPECT of the parathyroid gland and histological evidence of parathyroid adenoma or hyperplasia after parathyroidectomy, could confirm the diagnosis20.
Conclusions
In summary, the present study reported two cases of ovarian cancer accompanied with hypercalcemia. The patients showed similar clinical presentations, but different laboratory findings and clinical outcomes due to distinct mechanism.PTHrP-induced HHM is the most common cause of cancerrelated hypercalcemia and usually indicates poor prognosis for cancerous diseases. The evaluation of the serum level of PTHrP and immunostaining are recommended. Laboratory measurements of PTH and 1,25(OH)2D, and scintigraphy scans of the parathyroid gland, are useful in differential diagnosis. An appropriate therapy should be considered based on the different etiologies. Anti-hypercalcemic infusion is helpful, and the treatment for primary diseases such as anti-tumor therapy is more essential.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81402141) and General Project of Science and Technology of Tianjin (Grant No.15JCYBJC26600).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
1.Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016; 12:426-32.
2.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005; 352: 373-9.
3.Santarpia L, Koch CA, Sarlis NJ. Hypercalcemia in cancer patients:pathobiology and management. Horm Metab Res. 2010; 42:153-64.
4.Ralston SH, Gallacher SJ, Patel U, Campbell J, Boyle IT. Cancerassociated hypercalcemia: morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990; 112:499-504.
5.Dickersin GR, Kline IW, Scully RE. Small cell carcinoma of the ovary with hypercalcemia: a report of eleven cases. Cancer. 1982;49: 188-97.
6.Sawada M, Uehara T. A case of ovarian cancer associated with hypercalcemia. Jpn J Clin Oncol. 2008; 38: 719
7.Wysolmerski JJ. Parathyroid hormone-related protein: an update. J Clin Endocrinol Metab. 2012; 97: 2947-56.
8.Mirrakhimov AE. Hypercalcemia of malignancy: an update on pathogenesis and management. N Am J Med Sci. 2015; 7: 483-93.
9.Kascak P, Zamecnik M, Bystricky B. Small cell carcinoma of the ovary (Hypercalcemic Type): malignant rhabdoid tumor. Case Rep Oncol. 2016; 9: 305-11.
10.Lewin S, Dezube D, Guddati AK, Mittal K, Muggia F, Klein P.Paraneoplastic hypercalcemia in clear cell ovarian adenocarcinoma.Ecancermedicalscience. 2012; 6: 271
11.Martinez-Borges AR, Petty JK, Hurt G, Stribling JT, Press JZ,Castellino SM. Familial small cell carcinoma of the ovary. Pediatr Blood Cancer. 2009; 53: 1334-6.
12.McDonald JM, Karabakhtsian RG, Pierce HH, Iocono JA,DeSimone CP, Bayliff SL, et al. Small cell carcinoma of the ovary of hypercalcemic type: a case report. J Pediatr Surg. 2012; 47: 588-92.
13.Schwartz GG, Skinner HG. Prospective studies of total and ionized serum calcium in relation to incident and fatal ovarian cancer.Gynecol Oncol. 2013; 129: 169-72.
14.Delgado-Guay MO, Yennurajalingam S, Bruera E. Delirium with severe symptom expression related to hypercalcemia in a patient with advanced cancer: an interdisciplinary approach to treatment. J Pain Symptom Manage. 2008; 36: 442-9.
15.Da Silva Gomes L, Kulak CAM, Da Rocha Lemos Costa TM,Vasconcelos EC, De Carvalho M, Borba VZC. Association of primary hyperparathyroidism and humoral hypercalcemia of malignancy in a patient with clear cell renal carcinoma. Arch Endocrinol Metab. 2015; 59: 84-8.
16.Stewart AF. Hyperparathyroidism, humoral hypercalcemia of malignancy, and the anabolic actions of parathyroid hormone and parathyroid hormone-related protein on the skeleton. J Bone Miner Res. 2002; 17: 758-62.
17.Szymanski JJ, Otrock ZK, Patel KK, Scott MG. Incidence of humoral hypercalcemia of malignancy among hypercalcemic patients with cancer. Clin Chim Acta. 2016; 453: 190-3.
18.Sternlicht H, Glezerman IG. Hypercalcemia of malignancy and new treatment options. Ther Clin Risk Manag. 2015; 11: 1779-88.
19.Meng ZW, Tan J, Zhang MF, Dong F, Jia Q, Zhang FH. Tc-99m pertechnetate/sestamibi imaging in a case of recurrent parathyroid carcinoma with metabolic bone disorder. Clin Nucl Med. 2009; 34:479-82.
20.Zhou WB, Chen M. A case report of mediastinal ectopic parathyroid adenoma presented as parathyroid crisis localized by SPECT/CT. Medicine. 2016; 95: e5157
 Cancer Biology & Medicine2018年2期
Cancer Biology & Medicine2018年2期
- Cancer Biology & Medicine的其它文章
- Primary resistance to crizotinib treatment in a non-small cell lung cancer patient with an EML4-ALK rearrangement: a case report
- Ultrasound features of extranodal extension in the metastatic cervical lymph nodes of papillary thyroid cancer: a case-control study
- Diagnostic value of whole-body MRI with diffusion-weighted sequence for detection of peritoneal metastases in colorectal malignancy
- Clinical significance of miRNA - 106a in non-small cell lung cancer patients who received cisplatin combined with gemcitabine chemotherapy
- Enterolactone modulates the ERK/NF-κB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB-231 to revert the TGF-β-induced epithelial–mesenchymal transition
- Progress in non-invasive detection of liver fibrosis
