Nectar secretion of RN-type cytoplasmic male sterility three lines in soybean [Glycine max (L.) Merr.]
ZHANG Jing-yong , SUN Huan, ZHAO Li-mei, ZHANG Chun-bao, YAN Hao, PENG Bao, LI Wenbin
1 Soybean Institute, Northeast Agricultural University/Key Laboratory of Soybean Biology, Ministry of Education/Key Laboratory of Soybean Biology and Breeding (Genetics), Ministry of Agriculture, Harbin 150030, P.R.China
2 Soybean Research Institute, Jilin Academy of Agricultural Sciences/National Engineering Research Center for Soybean,Changchun 130033, P.R.China
1. Introduction
Soybean is one of the most important oil crops and is the main vegetable protein source in the world. However, the yield per hectare and the genetic gain per year are lower than those of other major field crops. Use of heterosis is an effective approach to improve crop yield. Chinese scientists were the pioneers for use of heterosis in soybean and have been successful in recent years. The first soybean cytoplasmic male sterile (CMS) line and its maintainer were reported in 1993. The sterile cytoplasm came from the landrace Ru Nan Tian E Dan, which was designated as RN cytoplasm (Sunet al. 1993, 1997, 2001, 2003). Four more origins of sterile cytoplasm from China were reported later (Zhang and Dai 1997; Gaiet al. 1999; Zhao and Gai 2006; Nieet al. 2017). Based on RN CMS System, the first hybrid “HybSoy1” was released in China in 2002 (Zhaoet al.2004). This hybrid yielded 21.9% more than control cultivar in two-year uniform test. Several hybrids were released later on. However, the extensive application of these hybrids in production is still limited due to the low seed setting of female parent in seed production, resulting in prices for hybrid seeds quite higher and negatively affecting farmers’ incomes.
Soybean flowers possess anatomical adaptation characteristics of entomophilous plant species (Palmeret al. 2001). Soybean is a typical self-pollinated crop, and cultivated soybean has a natural out-crossing rate of around 0.03 to 6.32% (Caviness 1966; Carlson and Lersten 1987;Sunet al. 1992; Ahrent and Caviness 1994; Jeffery 2003).Successful production of hybrid seeds requires efficient pollen transfer from male to female plants. Insects such as honeybees and leaf cutting bees were reported to be good pollen vectors (Ortiz-Perezet al. 2008; Zhaoet al. 2009).There is a wide range of variation of out-crossing rate among nuclear male-sterile and CMS lines and significant preferential attraction of pollinators occurred in both open fields and caged plot conditions (Graybosch and Palmer 1988; Ortiz-Perezet al. 2006a, b, 2008; Zhaoet al. 2009). In addition to morphological characters such as number, size, color and openness of flowers, the quantity and quality of rewards including nectar, pollen grains, and volatile matter may play an important role in this preferential attraction (Erickson 1975,1983, 1984a). A parallel trend was found between insect number and amount of flower nectar in a day with a peak time from 12:00–13:00 in Taiyuan of China, suggesting that soybean nectar attracts insects (Daiet al. 2017). Erickson(1984b) proposed that soybean secreted nectar, and great variation of quantity (0 to 0.8 μL) and quality was found among different genotypes. Horneret al. (2003) described the fine structure of floral nectaries and development in soybean.Three stages of nectary development- preactive, active,and postactive - were identified within 24 hours. The first two stages occurred when soybean flowers did not open.Three major components of soybean aroma were identified and the production of those components varied during the day, to signal different situations of nectar secretion. However,those components were not specified by the chemical names(Robackerat al. 1988). Jaycox (1970) noted the preferential attraction and pointed out that the size of bee population visiting soybean field could be an indicator of attractiveness of different soybean varieties. For example, more bees were observed in the plot of variety “Wayne” than that of“Harosoy”. The seed-setting rate of CMS lines varied greatly among genotypes from less than 10% to over 100%, and also changed in different environments (Daiet al. 2017). This means that the seed-setting rate of CMS A-lines is an indicator of success of pollen transfer and preferential attractiveness.
However, the biology of attractiveness of different genotypes to the pollinator and environmental factors involved have not been investigated. This paper addressed the secretion of nectar from CMS A-, B-, and R-lines with different seed-setting rates and the rhythm of nectar production.
2. Materials and methods
2.1. Plant materials
A total of 27 CMS soybean three lines were randomly selected among 463 available three lines. Nine CMS lines(A-lines): JLCMS29A, JLCMS101A, JLCMS8A, JLCMS82A,JLCMS14A, JLCMS47A, JLCMS9A, JLCMS31A, and JLCMS89A; nine corresponding maintainers (B-lines):JLCMS29B, JLCMS101B, JLCMS8B, JLCMS82B,JLCMS14B, JLCMS47B, JLCMS9B, JLCMS31B, and JLCMS89B; and nine restorers (R-lines): JLCMSR1,JLCMSR2, JLCMSR4, JLCMSR14, JLCMSR26, JLCMSR33,JLCMSR50, JLCMSR75, and JLCMSR118. All materials were developed by the Soybean Research Institute, Jilin Academy of Agricultural Sciences, China.
2.2. Design of field experiments
All experiments were conducted in the experimental fields of the Soybean Research Institute located in Gongzhuling(43°30´N, 124°48´E), Jilin Province, China during 2007–2014, due to the continuous rainfall and low temperature in whole flowering time in 2011 and 2013, the data were collected. All plant materials were grown in caged plots. The space between rows was 60 cm and each plant was 8 cm apart within a row. This experiment was designed with three replicates. Each cage measured 6.0 m×18.0 m×2.5 m, and was constructed with 1.5 mm aperture nylon mesh. The plants were covered one week before flowering.All insects in covered cages were killed by spraying insecticides at least two weeks before sampling to eliminate potential negative effects on nectar secretion.
The experiment was divided into two stages. First-stage experiments were performed in 2007 and aimed to explore basic rules of nectar secretion, including the rhythm of diurnal nectar secretion and the influence of weather on nectar secretion. Second-stage experiments were carried out from 2008 to 2014 to study the relationship between nectar secretion and the out-crossing rate of A-lines.
Stage 1: The rhythm of diurnal nectar secretion was analyzed using restorers JLCMSR118 in the summer of 2007 for three consecutive days. Nectar secretion rhythm was analyzed based on six genotypes to detect differences in diurnal variation of nectar secretion. Nectar secretion of 12 genotypes at peak secretion time was analyzed over 10 consecutive days to observe the relationship between nectar secretion and weather conditions in 2007.
Stage 2: A total of 27 CMS soybean lines including nine A-lines, nine B-lines, and nine R-lines were used to study the difference of nectar secretion among genotypes. At the same time, the out-crossing rate of nine CMS A-lines was determined.
2.3. Methods of sampling nectar
Nectar was sampled from flowers using a 1-μL capillary(manufactured by Drummond Scientific Company, USA).The nectar within each replication was collected starting with the first line, then the last line, and finally in the reverse direction to avoid artificial error. Nectar was sampled from 10 flowers in five plants with three replications. The selected flowers were chosen from the middle of the plants.Because there was no scale on the capillary, a ruler with minimum of 0.1 mm scale was used to measure the length of the nectar in the capillary. A 30× magnifier was also used to help reading scale and 0.05 mm was recorded by estimation. The inner diameterof capillary is 0.0079 inch.The length was then converted to the reading of microliter.Here 0.1 mm on the ruler equals to 0.003125 μL. To reveal the diurnal rhythm of nectar secretion, samples were taken every 30 min from 06:00 to 17:00. The samples in other experiments were collected between 07:00–08:30 during the peak of nectar secretion.
2.4. Evaluation of out-crossing rate
Nine pairs of A- and B-lines were planted in the row radio of 1:1 for evaluating the out-crossing rate of male sterile A-lines in the cages. The size and cover of the cage were the same as mentioned above. Row spacing was 60 cm with 8 cm between plants within a row. At the beginning of the R1 (begining bloom) period (Fehret al. 1971), honeybee hives were moved to the cages, and about 500 honeybees from each hive were used to pollinate. At the R6 (full seed)stage, 10 plants from each of A- and B-lines were sampled randomly to count pod setting. Plants near the edges of the cage were not selected. Then out-crossing rate was calculated using the following formula:
Out-crossing rate (%)=Average pod number per plant for a CMS A line/Average pod number per plant for its maintainer B line×100
2.5. Data analysis
SPSS 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, version 19.0, Armonk, NY, USA) was used for all analyses. Analysis of variance (ANOVA) for out-crossing rate and nectar secretion of different genotypes from three lines, means between genotypes, and years and types were compared based on the least significant difference (LSD)test at 0.05 probability level. Correlationsbetween nectar secretion and meteorological factors, nectar production of CMS A-lines, nectar production of CMS B-lines, and outcrossing rate of CMS A-lines were also analyzed.
3. Results
3.1. Diurnal nectar secretion rhythm
Nectar secretion of restorers JLCMS118R was examined for three consecutive days (July 12–14, 2007) to evaluate consistency of the nectar secretion rhythm (Fig. 1). The weather in those three days was sunny with relatively high temperatures. The nectar secretion pattern was highly consistent over these three days even though the starting time of nectar secretion varied slightly. The nectar secretion started at 07:00 on July 12, at 06:00 on July 13, and at 06:30 on July 14. Secretion ended at 17:00 on all three days. The nectar secretion peak occurred at 08:00–08:30. A sub-peak was found at 13:00 on July 13 and July 14, and at 14:00 on July 12. It was obvious that better weather one or two days before the samples were taken played an active role in nectary development and nectar secretion. Peak secretion time occurred soon after the initiation of nectar secretion and decreased very fast on July 12, and there was an obvious sharp peak compared to the other two days.
The diurnal nectar secretion of six genotypes is shown in Fig. 2. Flowers in most genotypes opened at 07:00 on clear days in July and sometimes opened at 06:30 in very good weather. Nectar could be detected at 06:00. For most genotypes except JLCMS82A and JLCMS82B, the quantity of nectar secretion increased and reached a peak point between 07:00–08:30 when the flowers were already opened; nectar secretion decreased gradually after that.Almost no nectar could be collected after 17:00. The nectar secretion of JLCMSR118 reached the highest level at 07:00,while JLCMSR2 had the highest level of nectar secretion at 08:30. Nectar production for JLCMS82A fluctuated: It reached the highest level at 08:30 and then decreased until 09:00. Nectar production of JLCMS82A increased and reached a higher level at 10:30. A small sub-peak was found at 13:00–13:30 for all genotypes.
3.2. The effect of meteorological factors on nectar secretion

Fig. 2 Nectar secretion of six genotypes from 06:30 to 17:00(2007).
Nectar from six genotypes was collected at peak secretion time daily from July 15 to July 24 in 2007. The sampling time was postponed to 09:00–09:30 instead of 08:00–08:30 on July 18 because of rainy weather (Table 1). Fig. 3 shows that the nectar quantity increased from July 15 and reached the highest level on July 17. Almost not any nectar was collected on July 19 and the secretion activity recovered gradually by July 20. However the nectar secretion remained at lower level over the next six days. This agreed with Erickson’s (1984a) study that soybean plants did not secrete nectar during cool weather and required three days to recover the ability to produce nectar even though subsequent flowers were open each day. It was clear that the rainfall and low temperature had a negative influence on the nectar secretion activity especially in genotypes having higher level of nectar secretion such as JLCMS 82A,JLCMS82B, JLCMSR1, and JLCMSR2.
The daily average, maximum, and minimum temperatures during July 18–22 were lower than those of days before rain. The average minimum temperature was only 14.1°C and the lowest temperature was 9.9°C on July 22 (Table 1).The minimum temperature in the early morning played an important role in the normal nectar secretion since the minimum temperature occurred just before or at the preactive and active stages of nectary development (Horneret al. 2003).
Nectar production was positively correlated with the lowest temperature on the day that samples were collected(Table 2). It was also significantly correlated with the average, lowest, and highest temperatures on the previous day. Nectar production was not correlated with air humidity on the sampling days.
3.3. Nectar production among genotypes
Nectar secretion of 27 genotypes was measured during flowering stage from 2008 to 2014 (except in 2011 and 2013). Large variation in nectar production was foundamong genotypes. Genotypes-tested were divided into three groups A-, B-, and R-lines. Within the same group,nectar production differed significantly among genotypes(Table 3). For A-lines, the highest nectar production was 0.137 μL in JLCMS101A and the lowest was 0.047 μL in JLCMS9A. The nectar production in the B-lines ranged from 0.053 μL (JLCMS9B) to 0.126 μL (JLCMS101B). The highest nectar production was 0.152 μL (JLCMR1) and the lowest nectar production was 0.81 μL (JLCMSR33)in R-lines. There were significant differences in nectar production among the three groups. Nectar production in R-lines was significantly higher than that of A- and B-lines.No difference was detected between A- and B-lines. Nectar production in the three lines within the same group also varied in different years. The highest nectar production was observed in 2012 and the lowest in 2008 for all groups.

Table 1 Meteorological factors during July 9–24, 2007

Fig. 3 Variation of nectar production of 12 genotypes from July 15 to 24 in 2007.

Table 2 Correlation coefficients among nectar production of three lines and meteorological factors1)
3.4. Out-crossing rate among A-lines
significant differences of out-crossing rate were found among A-lines (Table 4). The highest out-crossing rate was 80.3% in JLCMS29 A and the lowest out-crossing rate was 7% in JLCMS89A. This difference provides an opportunity for breeders to select high out-crossing rate line or parents in breeding programs. The out-crossing rate of A-lines also varied in different years.
3.5. Correlation between out-crossing rate and nectar production
The results of correlation analysis are shown in Table 5.The out-crossing rate of A-lines was significantly positively correlated (P<0.01) with nectar production in A- and B-lines, and the correlation coefficients were 0.683 and 0.553, respectively. The nectar production in A-lines was also significantly positively correlated (P<0.01) with the nectar production in B-lines, and the correlation coefficient was 0.729. Nectar is one of the most important foods and attractors for pollinators. Lines having high quantities of nectar might attract more pollinators leading to a higher out-crossing rate. Taking nectar production of a line as an indicator to select high out-crossing A- and B-lines in the early stage of breeding may greatly improve selection efficiency.

Table 3 Nectar production of three lines in soybean in different years
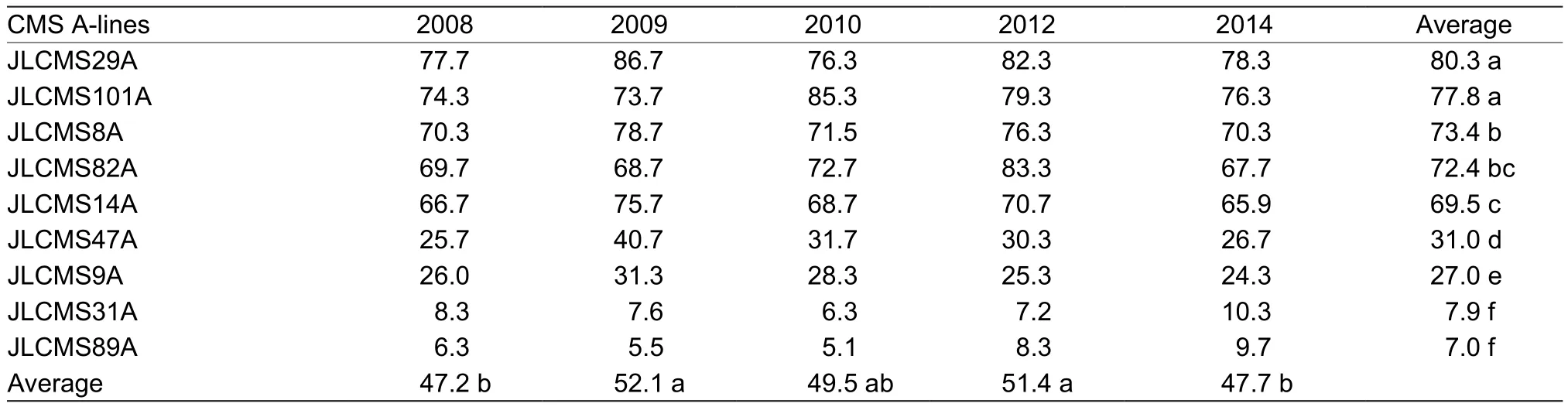
Table 4 Out-crossing rate of cytoplasmic male sterile (CMS) A-lines in cages from 2008 to 2014 (%)
4. Discussion
4.1. Favorable factors to nectar secretion and pollination
The relatively low seed-setting rate of female parents is one of the bottlenecks for commercialization of hybrid soybean.Several factors such as environmental conditions, species,and number of pollinators, and attractiveness of parents to pollinators may greatly influence the yield of hybrid seeds production (Sunet al. 2003; Zhaoet al. 2010). Rainy and cold weather have been shown to be unfavorable for pollen transfer, and Erickson (1984a) indicated that temperatures above 22–24°C were required to ensure soybean nectar production.
Clearly continuous high temperature and lower rainfall are important for improving nectar secretion and maintaining a high out-crossing rate. This information provides a guidelinefor the selection of hybrid seed production regions. In areas where there is less rainfall and higher temperatures,especially enough high daily average temperatures during the whole flowering period, these sites will be the ideal places for hybrid seed production. However, great differences of seed setting rate of CMS female parents exist at the same place because of preferential visitation by pollinators. Female and male parents should be balanced in regard to attractiveness to pollinators, because nectar and pollen grains are rewards for pollinators. Sterile pollen grains in CMS lines are not as attractive to pollinators and more nectar production in CMS lines is required to maintain attractiveness to pollinators. The present study showed that the yield of hybrid seeds in female parent JLCMS9A of HybSoy1 was relatively low, and the nectar production of JLCMS9A was only one third of male parent JLCMSR1. It is possible that pollinators visit male parents continuously for both nectar and pollen grains and seldom visit female parents. This leads to a low seed setting rate in female parents since pollination is only achieved when pollinators alternate between visits to male and female plants. Higher nectar production in both female and male parents is better than unbalanced nectar production between male and female parents; however, more studies needs to be done to verify this assumption.

Table 5 Correlation coefficients among out-crossing rate of A-lines, nectar production of A-lines, and nectar production of B-lines in soybean
4.2. Reasons for R-lines had higher nectar production
The present study also found that R-lines had higher nectar production compared to A- and B-lines. One of the possible reasons might be the less diversified origin of R-lines. The restoration sources used in our breeding program mainly came from a foreign country while the maintainers primarily came from domestic genetic resources. There is a possibility that different selections in foreign breeding programs unconsciously favored the performance of nectar secretion or that varieties with higher nectar production were used as the basic genetic background in foreign breeding.
4.3. Breeding methods for high out-crossing rate A-lines
Because of the significant positive correlation between nectar production of B-lines and out-crossing rate of A-lines, the measurement of nectar may be used to select A-lines with a high out-crossing rate by selecting B-lines with high nectar production. However the sampling operation of nectar is time-consuming work and it is difficult to handle a large number of breeding lines in a limited time. Further improvement of sampling method needs to be explored.
Volatile matter secreted from flowers was considered to be another factor for pollinator attraction (Erickson 1975,1983, 1984a; Horner 1998). Minimal data about the role of aroma in soybean pollen transfer are available so far,and detailed studies in this field are required to obtain these data. Higher and stable hybrid seed production is a complicated process and environmental, entomological,and genotypic factors should all be considered to produce low-cost hybrid seeds.
5. Conclusion
Soybean nectar secretion had diurnal variation. Secretion initiated at about 06:00 for most materials and reached a peak at 07:00–08:30 after flower opened, then the nectar secretion decreased gradually. A sub-peak appeared at about 13:00, while the nectar could not be detected at 17:00.
Nectar secretion was greatly influenced by the weather conditions. Rainy weather and low temperatures inhibited nectar secretion. The amount of nectar secretion increased gradually over time during periods of high temperature and no rainfall for several days.
There were obvious variations of nectar amount among different genotypes tested. There was no significant difference in nectar secretion between A- and B-lines. A-and B-lines with higher out-crossing rates secreted more nectar. The amount of nectar secretion of A- and B-lines were significant positively correlated with the out-crossing rate of A-lines.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFD0101500,2016YFD0101502), and the Agricultural Science and Technology Innovation Project of Jilin Province, China(CXGC2017Z004).
Ahrent D K, Caviness C E. 1994. Natural cross pollination of twelve soybean cultivars in Arkansas.Crop Science, 34,376–378.
Carlson J B, Lersten N R. 1987. Reproductive morphology. In:Boerma H R, Specht J E, eds.,Soybeans:Improvement,Production,and Uses. 2nd ed. Agronomy Monograph No.16. American Society of Agronomy/Crop Science Society of America/Soil Science Society of America, Madison, WI.pp. 95–134.
Caviness C E. 1966. Estimates of natural cross pollination in Jackson soybeans in Arkansas.Crop Science, 6, 211–212.
Dai J Y, Zhang R J, Wei B G, Nie Z X, Xing G N, Zhao T J,Yang S P, Gai J Y. 2017. Key biological factors related to outcrossing-productivity of cytoplasmic-nuclear male-sterile lines in soybean [Glycine max(L.) Merr.].Euphytica, 213,1–18.
Erickson E H. 1975. Variability of floral characteristics influences honey bee visitation to soybean blossoms.Crop Science,15, 767–771.
Erickson E H. 1983. Soybean for bees and beekeeping.Apiacta,8, 1–7.
Erickson E H. 1984a. Soybean floral economy and insect pollination.Soybean Gentics Newsletter, 11, 152–162.
Erickson E H. 1984b. Soybean pollination and honey production- A research progress report.American Bee Journal, 124,775–779.
Fehr W R, Caviness C E, Burmood D T, Pennington J S. 1971.Stage of development descriptions for soybeans,Glycine max(L.) Merrill.Crop Science, 11, 929–931.
Gai J Y, Ding D R, Cui Z L, Qiu J X. 1999. Development and performance of the cytoplasmic-nuclear male sterile line NJCMS1A of soybean.Scientia Agricultura Sinica, 32,23–27. (in Chinese)
Graybosch R A, Palmer R G. 1988. Male sterility in soybean- An overview.American Journal of Botany, 75, 144–156.
Horner H T. 1998. Floral nectaries in soybean: Engineering a system to improving cross-pollination for commercial hybrid seed production.Iowa Academic ScienceAbstract, 12.
Horner H T, Healy R A, Cervantis-Martinez T, Palmer R G.2003. Floral nectar fine structure and development inGlycine maxL. (Fabaceae).International Journal of Plant Sciences,164, 675–690.
Jaycox E R. 1970. Ecological relationships between honeybees and soybeans. III. The honeybee factors.Amercian Bee Journal, 110, 383–385.
Jeffery D R, Thomas C K, Craig A A, Robert L P. 2003. Soybean natural cross-pollination rates under field conditions.Environmental Biosafety Research, 2, 133–138.
Nie Z X, Zhao T J, Yang S P, Gai J Y. 2017. Development of a cytoplasmic male-sterile line NJCMS4A for hybrid soybean production.Plant Breeding, 136, 516–525.
Ortiz-Perez E, Horner H T, Hanlin S J, Palmer R G. 2006a.Evaluation of insect-mediated seed set among soybean lines segregating for male sterility at thems6locus.Field Crops Research, 97, 353–362.
Ortiz-Perez E, Horner H T, Hanlin S J, Palmer R G. 2006b.Insect-mediated seed-set evaluation of 21 soybean lines segregating for male sterility at 10 different loci.Euphytica,152, 351–360.
Ortiz-Perez E, Hunt W T, Horner W H, Davis R G. 2008. Insectmediated cross-pollination in soybean [Glycine max(L.)Merrill]: II. Phenotypic recurrent selection.Euphytica, 162,269–280.
Palmer R G, Gai J Y, Sun H, Burton J W. 2001. Production and evaluation of hybrid soybean.Plant Breed Reviews,21, 263–308.
Robacker D C, Meese B J D, Erickson E C. 1988. Floral aroma:How far will plants go to attract pollinator?American Institute of Biological Science, 38, 390–398.
SPSS Version 19.0. 2010.IBM Corp. Released 2010. IBM SPSS Statistics for Windows. version 19.0. Armonk, NY,USA.
Sun H, Zhao L M, Huang M. 1993. Studies on cytoplasmicnuclear male sterile soybean.Chinese Science Bulletin,38, 1535–1536. (in Chinese)
Sun H, Zhao L M, Huang M. 1997. Cytoplasmic-nuclear male sterile soybean line from interspecific crosses betweenG.maxandG.soja. In:Proceedings of International Soybean Research Conference V. Banpot Napompethedited by Banpot Napompeth, Chiang Mai, Thailand. pp. 99–102.
Sun H, Zhao L M, Huang M. 2001.Cytoplasmic-Genetic Male Sterile Soybean and Method for Producing Hybrid Soybean.United State Patent, No. US 6,320,098B1, 2001-11-20.
Sun H, Zhao L M, Wang S M, Wang Y Q, Li J P. 2003. Research progress on the use of heterosis in soybean.Chinese Journal of Oil Crop Sciences, 25, 92–96, 100. (in Chinese)
Sun Z Q, Tian P Z, Wang J, Yan R H. 1992. Estimation of the frequency of natural cross pollination in soybeam.Chinese Journal of Oil Crop Scineces, 14, 13–17. (in Chinese)
Zhang L, Dai O H. 1997. Selection and breeding of nucleocytoplasmic male sterile line W931A in soybean.Scientia Agricultura Sinica, 30, 90–91. (in Chinese)
Zhao L M, Peng B, Zhang W L, Zhang L F, Zhang J Y, Li J P,Li M H, Sun H. 2010. Establishment of technology system for hybrid soybean seed production.Soybean Sciences,29, 707–711. (in Chinese)
Zhao L M, Sun H, Peng B, Li J P, Wang S M, Li M H, Zhang W L, Zhang J Y, Wang Y Q. 2009. Pollinator effects on genotypically distinct soybean cytoplasmic male sterile lines.Crop Science, 49, 2080–2086.
Zhao L M, Sun H, Wang S M, Wang Y Q, Huang M, Li J P. 2004.Breeding of hybrid soybean “HybSoy1”.Chinese Journal of Oil Crop Scineces, 26, 15–17. (in Chinese)
Zhao T J, Gai J Y. 2006. Discovery of new male-sterile cytoplasm sources and development of a new cytoplasmicnuclear male-sterile line NJCMS3A in soybean.Eupytica,152, 387–396.
 Journal of Integrative Agriculture2018年5期
Journal of Integrative Agriculture2018年5期
- Journal of Integrative Agriculture的其它文章
- Detection and characterization of an isolate of Tomato mottle mosaic virus infecting tomato in China
- Genes encoding heat shock proteins in the endoparasitoid wasp,Cotesia chilonis, and their expression in response to temperatures
- Molecular mechanisms controlling seed set in cereal crop species under stress and non-stress conditions
- Rapid semi-quantification of triacylglycerols, phosphatidylcholines,and free fatty acids in the rice bran of one grain
- lnfluence of different nitrogen application on flour properties,gluten properties by HPLC and end-use quality of Korean wheat
- Evaluation indices of sour flavor for apple fruit and grading standards
