Progress in the surgery of rectal cancer
Rudolf Schiessel
Em. Chief Department of Surgery, Donauspital Vienna, 3400 Klosterneuburg, Jasmingasse 2, Austria
1. Introduction
Colorectal cancer (CRC) has been known as a major health problem in the western industrial countries for a long time. In recent years several studies report a rapid increase in the incidence of colorectal cancer in regions where colorectal cancer was not common before.- A rise in CRC incidence has been observed in Eastern Europe, China, Thailand, India, Taiwan, Singapore and Hongkong. But there are also reports from Iran, Saudi Arabia,Yemen and Egypt about an increase in the incidence of colorectal cancer[1]. The current assumption about the pathogenesis is a combination of dietary factors and the increase in the life expectancy. The western life style with a high intake of fat, meat and a low fiber diet seems to be a major factor in the development of this cancer entity. This is supported by several migration studies,where populations migrating from low incidence regions to high risk countries developed a high risk of CRC after adopting western habits.
The high incidence and the difficult diagnosis at an early stage has lead to the initiation of programs for early detection of CRC.The current recommendation in many countries is a prophylactic coloscopy at 50 years of age. This gives the opportunity to remove polyps before they become cancers.
For the established cancer without distant metastases surgery is the principal method for cure. The introduction of laparoscopic surgery has reduced the surgical trauma a great deal. The majority of colorectal resections can be facilitated without a stoma. This is dependent on the localization of the tumor. Tumors far away from the sphincter apparatus can be easily resected without a stoma,provided there are no problems prohibiting an anastomosis. A special situation are tumors in the rectum, in particular in the lower rectum. Although major progress has been made in many respects,the treatment options range from radiochemotherapy without surgery to abdominoperineal resection with a permanent stoma.
2. Current surgical concepts
The basic concept is the radical excision of the tumor together with the lymphatic drainage along the inferior mesenteric artery.Tumors of the upper part of the rectum can be resected without major difficulties from the abdominal route. The procedure is called„anterior resection“. The restoration of bowel continuity is usually facilitated with a stapling instrument that is inserted from the anus.Tumors of the lower part of the rectum are a major challenge for the surgeon. Obesity and a narrow pelvis may increase the difficulty of a radical excision. For a proper planning of surgery a preoperative diagnostic program is necessary. This should include an endoscopic biopsy with definition of the grade of differentiation, a test of anal function and a magnetic resonance investigation (MRI) of the rectum. The rectal MRI has become the standard investigation for the evaluation of tumors in the lower rectum. It gives a valuable information about the tumor concerning the depth of invasion, the distance to the sphincter and the relation to the neighboring organs in the pelvis (Figure 1,2). The result of the MRI is important for the decision whether a preoperative neoadjuvant chemoradiotherapy is necessary. It is also important for the decision, whether a sphincter salvage is possible. The systematic application of rectal MRI before surgery was first described by our group [2,3].
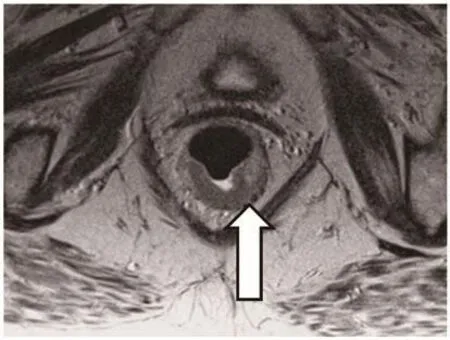
Figure 1. MRI of the rectum showing a tumour of the lower rectum.

Figure 2. MRI of the rectum. The coronal view shows a tumour close to the sphincter ani.
Recent studies have shown, that the rectal MRI can predict an unradical tumour removal when the mesorectal fascia is infiltrated[4]. This is important to know, since the surgical removal of a rectal cancer occurs always along the mesorectal fascia. That means that whenever a tumour infiltrates the fascia or comes very close to it,residual tumour will remain in the pelvis. This will be later the cause of pelvic recurrence of the tumour. Therefore a circumferential tumour free margin of 1mm is currently postulated. The prediction of lymph node involvement is at this point of low accuracy and has no major clinical role
3. Surgical techniques
The surgical techniques have been improved in three major respects including total mesorectal excision; sphincter salvage and minimal invasive surgery
3.1. Total mesorectal excision (TME)
The current standard for a radical excision of a rectal cancer does not only include a wide excision of the tumour but also a complete removal of the mesorectum up to the inferior mesenteric artery. This is important for the clearance of eventually affected lymph nodes.Incomplete lymph node removal was in the past a major cause of pelvic recurrence[5]. An average of 20 mesorectal lymph nodes should be removed.
3.2. Sphincter salvage
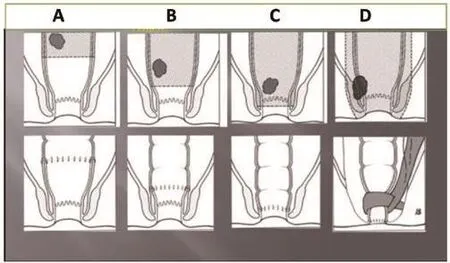
Figure 3. Sphincter salvage depends on the localization of the tumour.A: anterior resection, B:low anterior resection with coloanal anastomosis, C:intersphincteric resection with coloanal anastomosis, D: abdominoperineal resection with sphincter reconstruction by means of the gracilis muscle.
A major concern of patients with rectal cancer is the loss of continence and a permanent colostomy. In recent years the salvage rate for the anal sphincter has been increased up to 80%. Depending on the localization of the tumour different techniques are applied in order to restore continence after the TME.- After resection of a tumour in the upper part of the rectum it is usually no major problem to restore bowel continuity . This is facilitated by means of an end to end anastomosis between the remaining descending colon and the rectal stump. The anastomosis is performed with a circular stapling instrument (Figure 3A).
Tumours in the lower part of the rectum, in particular those close to the anus are more difficult to manage. It is important in such cases to exclude an infiltration of the sphincter, the vagina or prostate. This is performed by a rectal MRI. Once the sphincter is not involved, its preservation can be planned. With an intersphincteric resection even tumours close to the sphincter or even extending into the anal canal can be resected without complete removal of the sphincter. In such cases the internal sphincter is resected completely or partially [6,7].
The reconstruction of bowel continuity is facilitated by means of a coloanal anastomosis, joining the descending colon with the anal canal (Figure 3C).
Postoperative function after sphincter saving surgery can be impaired, especially in the first months after surgery. Although 60-80% of the patients have a normal continence function, several problems have been reported such as high stool frequency,fractionated defecation and incontinence. In order to minimize such problems it was suggested to restore the rectal ampulla with a reservoir. The pouch methods are of advantage in reducing the number of bowel movements in particular in the first year after surgery. The most popular technique is the J-pouch, but this is not always possible due to obesity, narrow pelvis or inadequate bowel length [8,9].
It is important to inform the patients preoperatively about possible problems and provide a good postoperative follow up in order to manage eventual functional problems. Sometimes postoperative complications such as anastomotic strictures can cause severe functional problems. Adjuvant radiotherapy increases the risk of postoperative incontinence. Postoperative chemotherapy can cause diarrhea and incontinence.
Large rectal cancers infiltrating the sphincter apparatus require an abdominoperineal resection. This operation ends up with a permanent abdominal colostomy. The life with a stoma is not easy but with time patients learn to manage this situation. The modern stoma care with one way stoma bags has improved the quality of life for these patients a great deal. For patients who cannot accept a permanent stoma a sphincter reconstruction is possible. The gracilis muscle has been used as a substitute for the anal sphincter by several institutions. It has been found that this muscle has to be stimulated with an impulse generator, like a pacemaker for the heart. Then it can build up an adequate pressure in order to warrant continence. The patient can activate the implanted generator with a remote control.The operation is called dynamic graciloplasty (Figure 4).
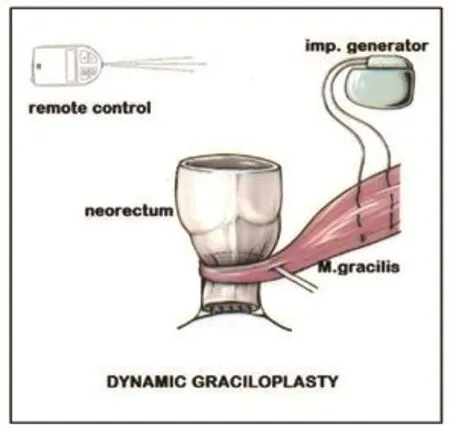
Figure 4. Dynamic graciloplasty for sphincter reconstruction after abdominoperineal rectal resection.
Although this operation has the potential of satisfaction for patients who refuse a permanent stoma, it has not received wide acceptance. Several factors make it difficult to use this method: high cost of impulse generator, technically difficult operation and the functional result depends very much on patient cooperation. Best results are achieved with highly motivated patients. The continence is in our experience good, but some patients have a difficulty with defaecation. This is explained by the complete absence of the sensoric nerves for the perception of filling in the neorectum.Therefore many patients irrigate themselves with enemas of 100-200ml in order to initiate defaecation. The majority of our patients with dynamic graciloplasty live a normal life [10].
3.3. Minimal invasive surgery
3.3.1. Laparoscopy
A major progress in abdominal surgery was the introduction of laparoscopy. The reduction of the trauma to the abdominal wall has certain advantages over the conventional technique by reducing postoperative pain, shortening the hospital stay and a better cosmetic result (Figure 5,6). Whereas laparoscopic cholecystectomy has become a standard operation, laparoscopic colorectal surgery for cancer is still not widely accepted. The primary goal of an operation for rectal cancer has to be the cure of the cancer. Now the laparoscopic technique is standardized and its oncological safety has been shown in several studies. There are some limitations such as massive adhesions after previous abdominal surgery, extensive tumors, extreme obesity etc. The operation time is usually longer than for conventional rectal resections. The outcome of patients who need to be converted from a laparoscopic operation to a conventional one is unfavourable. Newer developments in laparoscopic surgery try to reduce the number of openings into the peritineal cavity.- SILS is a technique where the operation is performed from one opening(single port technique). Another technique is the access through a natural orifice such as the vagina or anus, this is called NOTES.Since the removal of a large specimen like a rectum is not possible through a small opening, the NOSE technique was developed.- The specimen is not removed through a separated large incision in the abdominal wall, but through a natural orifice (vagina, anus). We have developed this technique for the laparoscopic intersphincteric resection. The laparosopically completely mobilized specimen is pulled through the anus, then resected and removed whitout any additional incision [11].
Figure 5. Set up for laparoscopy.
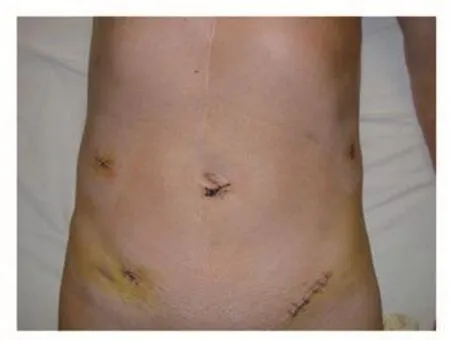
Figure 6. Patient after laparoscopic rectal resection.
The shortcomings of the laparoscopic instruments in particular in the lower pelvis are overcome by robotic instruments. A major problem is the high cost of the equipment. Robotic instruments are very small and can be moved in all three dimensions. The surgeon controls the instruments by means of joy sticks (Figure 7)[12,13].More recently some centers have published encouraging experiences,but its real advantage has to be proven. At this point it is not clear which of the new developments will be successful in the future. But it is important that all innovations will be measured on the gold standard of conventional rectal surgery in respect of complications,mortality, recurrence rate, cancer survival and functional outcome.

Figure 7. Set up for robotic surgery.
3.3.2. Other forms of minimal invasive surgery
Small rectal tumours can be resected transanally by different techniques such as simple transanal excision, transanal endoscopic microsurgery (TEM), transanal endoscopic operation (TEO) and with the use of a coloscope as endoscopic mucosal resection(EMR). These techniques are very useful for flat benign lesions. For infiltrating cancer even full thickness excisions leave a certain risk of local recurrence and untreated lyph node metastasis.
4. Adjuvant treatment
Adjuvant treatment of rectal cancer has been shown to be effective in reducing local recurrence and prolonging survival as well (14).Currently the preoperative chemoradiation for advanced T3-T4 tumours is used in different forms.- Radiation is either used as a long course regimen for 6-8 weeks with 50 Gy or as as a short course with 25 Gy over 5 days. The chemotherapy in chemoradiotherapy regimens consists usually of a combination of 5-fluorouracil and leucovorin. Other substances have been tested such as capecitabine,which is an oral alternative to 5-fluorouracil. Oxaliplatin has been shown to increase toxicity of radiotherapy in several studies and is therefore not recommended. The new epidermal growth factor receptor-targeted monoclonal antibodies cetuximab and panitumumab are currently under investigation.
The good response to preoperative chemoradiation has lead to the idea of nonoperative treatment in selected cases with low rectal cancer. This has been advocated by Habr-Gama (15) and requires a strict follow up program after chemoradiation confirming a complete local tumour control. This protocol is not widely accepted since this“watch and wait regimen” has the drawback that the response to the chemoradition is not predictible.- Nonresponders will come very late to curative surgery. Therefore a nonooperative treatment of rectal cancer outside clinical studies is at this point not recommended.
5. Discussion
Several innovations have improved the management of rectal cancer. The therapeutic approach has changed from strict rules to an individualized program for each patient. In an earlier study we asked patients with rectal cancer before surgery about what they expected from the operation. The answers were very clear: the first priority was the cure from cancer, very close to that came the wish to have no permanent stoma. Other parameters such as cosmetic result, normal bowel habits etc. had only a low priority [16]. The cure of cancer has therefore to have the highest priority also for the surgeon. We have also to take in account that social an religious factors have a big impact on the quality of life after surgery for rectal cancer [17]. It has been shown that the acceptance of a stoma is very low in southern Europe and in islamic countries [18,19].
But more surgery is not always better surgery.- This had to be learned from the Miles dogma that each rectal cancer independent of its localization should undergo abdominoperineal exstirpation[20].A rule that discouraged the development of sphincter saving surgery for about 50 years. The other extreme was the idea of nonoperative treatment of low rectal cancers.- Encouraged from the downstaging effects of neoadjuvant chemoradiotherapy, a watch and wait policy was advocated.- The final results have shown, that the local recurrence rate after a complete response is as high as 31% [21].Therefore this concept cannot longer considered as useful.
In order to minimize the surgical trauma laparoscopic surgery has been introduced. The difficulties in a narrow pelvis might be obviated by the use of a robot system. This is already established in the surgery of the prostate. Clear advantages in rectal surgery are not evident at this point in comparison to standard laparoscopic surgery[13]. The high cost of a robotic system is doubtless a major handicap.
Declare of interest statement
We declare that we have no conflict of interest.
[1] Bisheshari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Constatini R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways and opportunities for prevention.World J Gastroenterol2014;20:6055-6072.
[2] Urban M, Rosen H, Hölbling N, Feil W, Hochwarther G, Hruby W, et al.MR imaging for the planning of sphincter-saving surgery for tumors of the lower third of the rectum: use of intravenous and endorectal contrast materials.Radiology2000 ;214:503-508.
[3] Holzer B, Urban M, Hölbling N, Feil W, Novi G, Hruby W, Rosen H,Schiessel R. Magnetic resonance imaging predicts sphincter invasion of low rectal cancer and influences selection of operation.Surgery2003;133:656-61.
[4] Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al.Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Study Group. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence; 5-year follow-up results of the MERCURY study.J Clin Oncol2014;32:34-43.
[5] Heald RJ, Husband EM, Ryall RDH. The mesorectum in rectal cancer surgery. The clue to pelvic recurrence?Br J Surg1982; 69:613-616.
[6] Schiessel R, Karner-Hanusch J, Herbst F, Teleky B, Wunderlich M:Intersphincteric resection for low rectal tumours.Br J Surg1994;81:1376-1378.
[7] Schiessel R, Novi G, Holzer B, Rosen HR, Renner K, Hölbling N,Feil W, Urban M. Technique and long term results of intersphincteric resection for low rectal cancer.Dis Colon Rectum2005;48:1858-1865.
[8] Lazorthes F, Fages P, Chiotasso P, Lemozy J, Bloom F. Resection of the rectum with construction of a colonic reservoir and coloanal anastomosis for carcinoma of the rectum.Br J Surg1986;731:36-38
[9] Parc R. Tiret E, Frileux P, Moskowski, Loygue J. Resection and coloanal anastomosis with colonic reservoir for rectal carcinoma.BrJ Surg1986;73:139-141.
[10] Rosen HR, Urbarz C, Novi G, Zöch G, Schiessel R. Long-term results of modified graciloplasty for sphincter replacement after rectal excision.Colorectal Dis2002; 4:266-269.
[11] Metzger P. The laparoscopic technique of intersphincteric rectum resection. In:Intersphincteric resection for low rectal tumours. Schiessel R,Metzger P (eds). New York: Springer Wien; 2012, p. 85-97.
[12] Kim Seon Hahn, Shin Jae Won. Robot- assisted intersphincteric resection. In:Intersphincteric resection for low rectal tumors. New York:Springer Wien; 2012,159-163.
[13] Jayne D. Effect of robotic assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer. The ROLARR randomized clinical trial.JAMA2017; 318:1569-1580.
[14] Kye BH, Cho HM: Overview of radiation therapy for treating rectal cancer.Ann Coloproctol2014;30:165-174.
[15] Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization.Dis Colon Rectum2010;53:1692-1698.
[16] Holzer B, Gyasi A, Schiessel R, Rosen HR. Patients expectations of colorectal surgery for cancer.Colorectal Dis2006;8:186-191.
[17] Renner K, Rosen HR, Novi G, Hölbling N, Schiessel R. Quality of life after surgery for rectal cancer.Dis Colon Rectum1999;42:1160-1163.
[18] Holzer B, Rosen HR, Matzel K, Christiansen J, Kuzu M, Hussein A,Richter P, Lehur P, Oeresland T and the study group for QOL in rectal cancer: Do geographic and educational factors influence the quality of life (QOL) in rectal cancer patients with a permanent colostomy?Dis Colon Rectum2005;48:2009-2016.
[19] Kuzu MA, Topcu Ö, Ucar K et al,: Effect of sphincter sacrificing surgery for rectal carcinoma on quality of life in muslim patients.Dis Colon Rectum2002;45:1362-1366.
[20] Miles WE: A method of performing abdomino-perineal excision for carcinoma of the rectum and the terminal portion of the pelvic colon.Lancet1908;2:1812-1813.
[21] Habr-Gama A, Gama-Rodriguez J, Sao Juliao GP, Proscurshim J,Sabbagh C, Lynn PB, et al. Local recurrence after complete response an watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control.Int J Oncol Biol Phys2014;88:822-828.
 Journal of Acute Disease2018年1期
Journal of Acute Disease2018年1期
- Journal of Acute Disease的其它文章
- A case report of acute pulmonary vein stenosis
- Acute intra-operative brain swelling managed effectively with emergency basal cisternostomy: A case report
- Diminazene aceturate modified nanocomposite for improved efficacy in acute trypanosome infection
- Role of first day levels and subsequent trends of serum proteins in acute burns
- The laparoscopic approach in emergency surgery: A review of the literature
- Acute myocardial infarction and Yin Yang imbalance
