Pt/Zr O2-Al2O3催化剂催化氧化C3H 6和CO性能
杜君臣 马江丽 王凤军 杨冬霞*,,3 郑婷婷 赵云昆*,
0 Introduction
With the birth of three-way catalyst in 1970s,its contribution to automobile exhaust CO,HC and NOxpurification has been seen widely[1].In order to improve human′s living environment and meet the stringent environmental regulations,researchers have always been devoted to the improvement of catalyst performance[2-7].With the implementation of ultra-low and even zero emission regulation,pure single-TWC catalyst is difficult to meet the requirement.It has been agreed that with the installation of close coupled catalyst at engine manifold,the HC and CO emitted during the cold start can be lowered[8].
Due to the large surface area,good adsorption performance,moderate chemical activity and low cost,Al2O3is widely used as the automobile exhaust gas purification catalyst substrate[9]. However, due to thermal or hydrothermal shock,if the Al2O3carrier is directly used in the close coupled catalyst,its phase and specific area is liable to change and specific area will decrease rapidly,thus inactivating the catalyst[10].Researchers have been devoted to improving the thermal stability of Al2O3since 1940s.The results show that the modification or doping of La,Ce,Pr,Zr,P,Si,Ba,Sr or other elements can delay the high temperature sintering and phase transition of Al2O3material[11-13].ZrO2has the characteristics of acidity,basicity,redox,and is easy to produce oxygen vacancies.However,it also represents the characteristics of small specific surface area and porosity,low mechanical strength,expensive price,and is liable to agglomerate under high temperatures,which will limit its application as the carrier[14].By understanding the characteristics of Al2O3and ZrO2,ZrO2-Al2O3composite oxides with large specific surface area and good heatresistance were synthesized.When using ZrO2-Al2O3as carrier,catalyst always shows excellent catalytic activity and selectivity in ethanol reforming,NO reduction,CH4oxidation and other reactions[15-19].
Studies have showed that Pt and Pd have excellent activity for HC and CO oxidation[20-21].However,the main work is focusing on the oxidation of HC and CO,Pd and Pd-Rh close coupled catalyst research[22-26],while little focuses on Pt-based catalysts because of the high price in the past.With the continued decline of Pt price in recent years,the study of Pt based oxidation catalysts has shown great significance.In order to obtain high efficiency close coupled catalyst,Pt/Al2O3,Pt/ZrO2-Al2O3and Pt/ZrO2serial catalysts were prepared,and characterized by XRD,N2adsorption-desorption,H2-TPR,CO pulse adsorption and the oxidization system of C3H6&CO was also studied.The effect of catalyst phase structure,specific surface area and particle size on the catalytic activity of the catalyst were investigated.
1 Experimental
1.1 Materials
All the chemicals were of analytical grade.Pt(NO3)2solution(62.02 g·L-1)came from Kunming Institute of Precious Metals.ZrO(NO3)2·2H2O,Al(NO3)3·2H2O,NH3·H2O(25%)were purchased from Tianjin Guangfu Fine Chemical Research Institute.
1.2 Carrier and catalyst preparation
The ZrO2-Al2O3composite supports with different mass ratio were prepared by co-precipitation method.NH3·H2O solution was added in the mixed ZrO(NO3)2solution(0.5 mol·L-1)and Al(NO3)3solution(0.5 mol·L-1)at room temperature until pH=9,then the obtained precipitate was vigorously stirred for 4 h before being stayed overnight.After being washed several times with distilled water,the mixed sol was dried at 100℃for 12 h,and then calcined at 750℃for 4 h in air.Pure Al2O3and ZrO2supports were also prepared in a similar method.The ZrO2(x)-Al2O3(where massfraction of ZrO2,x=0,0.2,0.4,0.6,0.8 and 1)composite was denoted as Zr(x)-Al.The six supports were treated with an aqueous solution of Pt(NO3)2to obtain a 1.5%(w/w)loading of Pt by an isovolume impregnation.After being dried and calcined at 550℃for 4 h in air,serial catalysts were marked as Pt/Al,Pt/Zr(0.2)-Al,Pt/Zr(0.4)-Al,Pt/Zr(0.6)-Al,Pt/Zr(0.8)-Al,Pt/Zr.
1.2 Catalytic evaluation
The catalytic properties were evaluated using a continuous flow fixed-bed reactor.The catalysts were sieved through a 40-mesh and a 60-mesh sieve before testing.0.3 g of the catalyst was evaluated in 1%CO+0.3%C3H6+5%O2+93.7%N2(V/V)with a space velocity of 18 000 mL·h-1·g-1.The purified exhausts were dehydrated by desiccant magnesium chloride first and then measured in Agilent 7890A on-line gas chromatography analyzer.The products were detected by TCD and FID in the analyzer.
1.3 Characterization of catalyst
The X-ray powder diffraction patterns of the catalysts were characterized by Rigaku D/max 2000 powder diffractometer,which was operated at 40 kV and 100 mA using Cu Kα (λ=0.154 06 nm)radiation.Inten-sities of the diffraction peaks were recorded in the 2θrange of 10°~90°with a step size of 0.02°,and the scanning speed was 10°·min-1.
The N2adsoption-desorption was carried out at-196℃on Quanachrome NOVA2000e physisoption apparatus.The specific surface area was calculated using BET method,and pore size and volume were determined using BJH model.
H2-TPR was measured on Quanachrome CHEMBET 3000 chemical adsorption instrument.100 mg of catalyst was loaded into a U-shaped tube,heated at a rate of 10℃·min-1to 300℃,and kept at 300℃for 1 hour with the Ar gas stream(flow rate 45 mL·min-1),and cooled to the ambient temperature afterwards.H2-TPR was performed in 10.2%(V/V)H2/Ar mixture gas(flow rate 75 mL·min-1)with increasing temperature up to 800℃ at a rate of 10℃·min-1.The H2consumption was measured with a TCD detector.
CO in-situ diffuse reflectance infrared flourier transform spectroscopy (DRIFT)was performed on a near-infrared FT-IR spectrometer manufactured by Themo Nicolet.The spectra were scanned 32 times at a resolution of 4 cm-1.Before the adsorption test,catalyst was reduced,followed by N2purging of the pipeline and background signal collection.Afterwards,pure CO gas was introduced for adsorption until a saturated status was reached,then feed gas was switched to N2to purge tube residual and signal attributed to CO chemisorbed on the catalyst was recorded.
The dispersion of Pt over the catalyst was determined by CO pulsed adsorption method with CHEMBET 3000 chemical adsorption instrument.The sample was first reduced at 450℃with H2/He for 2 h at a gas flow rate of 45 mL·min-1.And then the feed gas was switched to pure He gas to purge the residual H2on the surface or absorbed.When the reaction furnace temperature was cooled to 80℃,temperature was kept constant for 30 min.After pulse injecting the high purity CO,CO adsorption signals were recorded with a TCD until no change could be observed in the adsorption peaks.The metal dispersion was calculated by assuming a CO to surface metal atom ratio of 1∶1.
2 Results and discussion
2.1 Catalytic activity evaluation
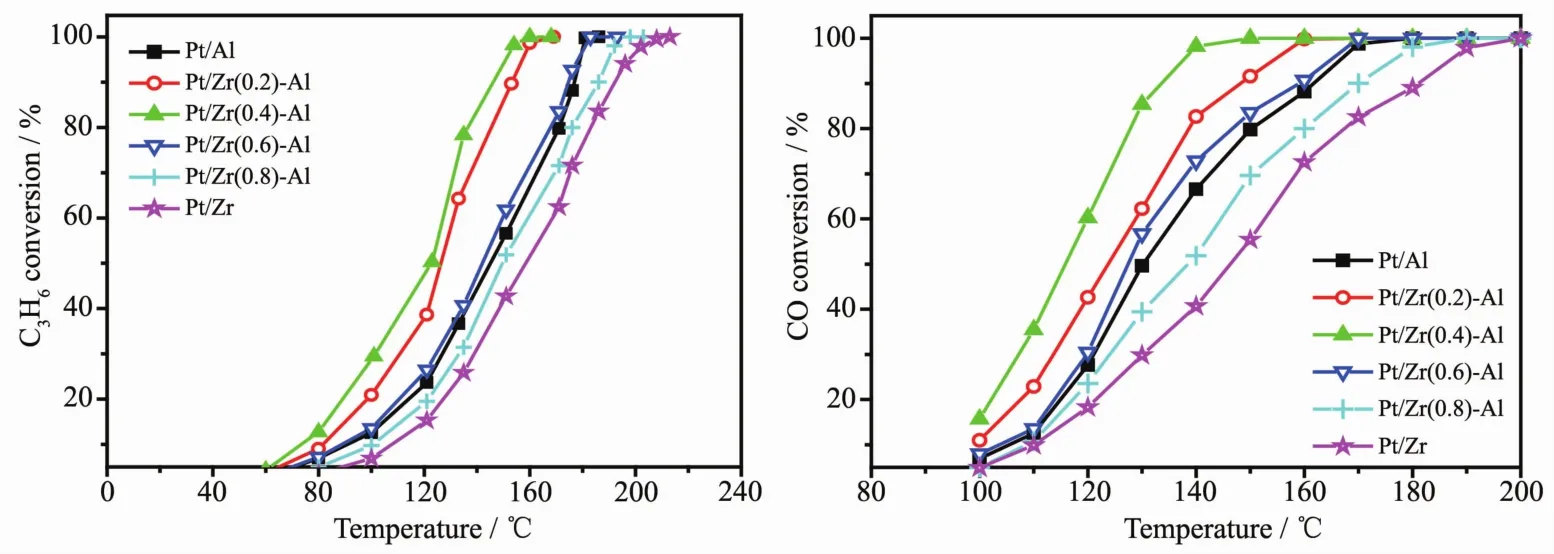
Fig.1 C3H6&CO catalytic oxidation properties over Pt/Al2O3,Pt/ZrO2-Al2O3 and Pt/ZrO2 serial catalysts
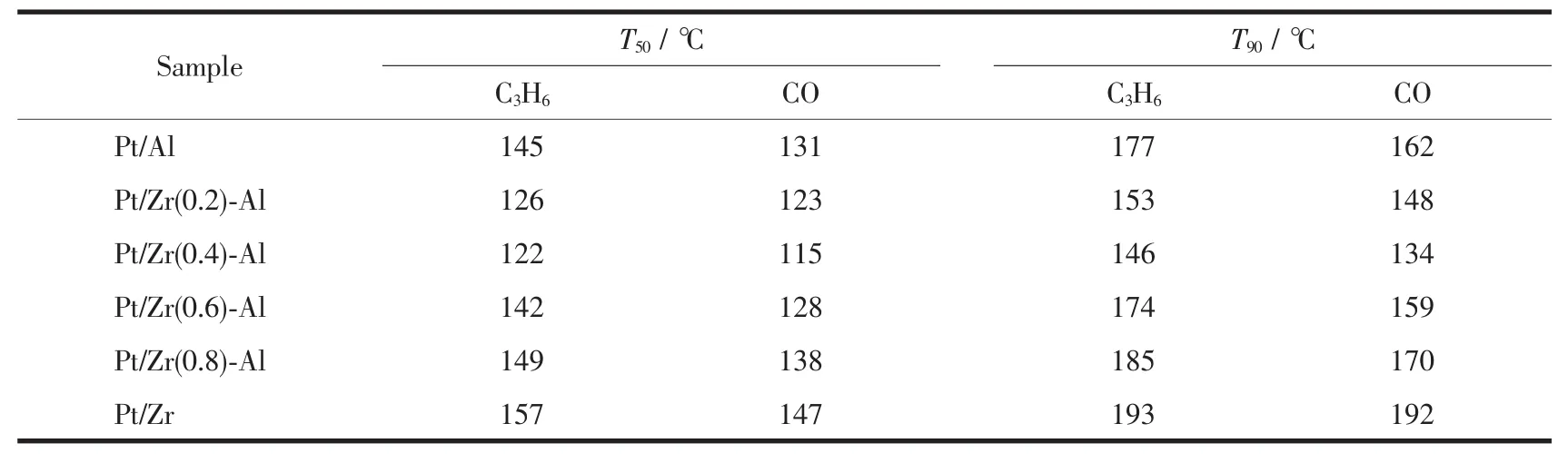
Table 1 T50 and T90 statistics of Pt/Al2O3,Pt/Zr O2-Al2O3 and Pt/Zr O2 serial catalysts
Fig.1 shows the curves of C3H6and CO conversion over Pt/Al2O3,Pt/ZrO2-Al2O3,Pt/ZrO2serial catalysts under different temperatures.Table 1 lists the C3H6and CO temperature of light-off(T50)and the complete conversion temperature(T90)over a series of catalysts.It can be seen from Table 1 that the T50and T90of the serial catalysts are in the order of Pt/Zr(0.4)-Al<Pt/Zr(0.2)-Al<Pt/Zr(0.6)-Al<Pt/Al<Pt/Zr(0.8)-Al<Pt/Zr.With an increased percentage of ZrO2in the carrier,the catalytic activity demonstrates a trend of increasing first and then decreasing.It can be seen that the mass ratio of ZrO2to Al2O3in the carrier has a very important effect on the catalytic oxidation activity of Pt/ZrO2-Al2O3catalyst.
2.2 XRD analysis
In order to understand the effect of the mass ratio of ZrO2to Al2O3on the phase structure of the catalyst,serial catalysts were characterized by XRD.The characterization patterns in Fig.2 exhibit that Pt/Al2O3catalyst shows characteristic diffraction peaks at 2θof 37.4°,46.0°and 66.7°,which can be attributed to the characteristic diffraction peak ofγ-Al2O3[27].For the Pt/ZrO2-Al2O3catalyst with an increased mass percentage of ZrO2in the carrier,the characteristic diffraction peaks ofγ-Al2O3gradually diffuse,and the characteristic diffraction peaks of AlxZr1-xOysolid solution near 2θof 30.5°,35.1°,50.5°and 60.5°gradually increase[28].Pt/ZrO2catalyst exhibits the characteristic diffraction peaks of monoclinic and tetragonal ZrO2near 2θof 24.1°,28.2°,31.5°and 2θof 30.3°,35.0°,50.3°,60.2°,respectively[29].However,Pt/Al2O3,Pt/ZrO2-Al2O3and Pt/ZrO2serial catalysts haven′t showed obvious crystal diffraction peaks of Pt or Pt species,indicating that Pt is highly dispersed in the series of catalysts.
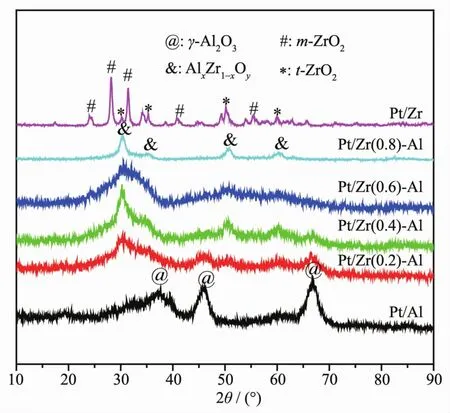
Fig.2 XRD patterns of Pt/Al2O3,Pt/ZrO2-Al2O3 and Pt/ZrO2 serial catalysts
2.3 N2 adsorption-desorption tests
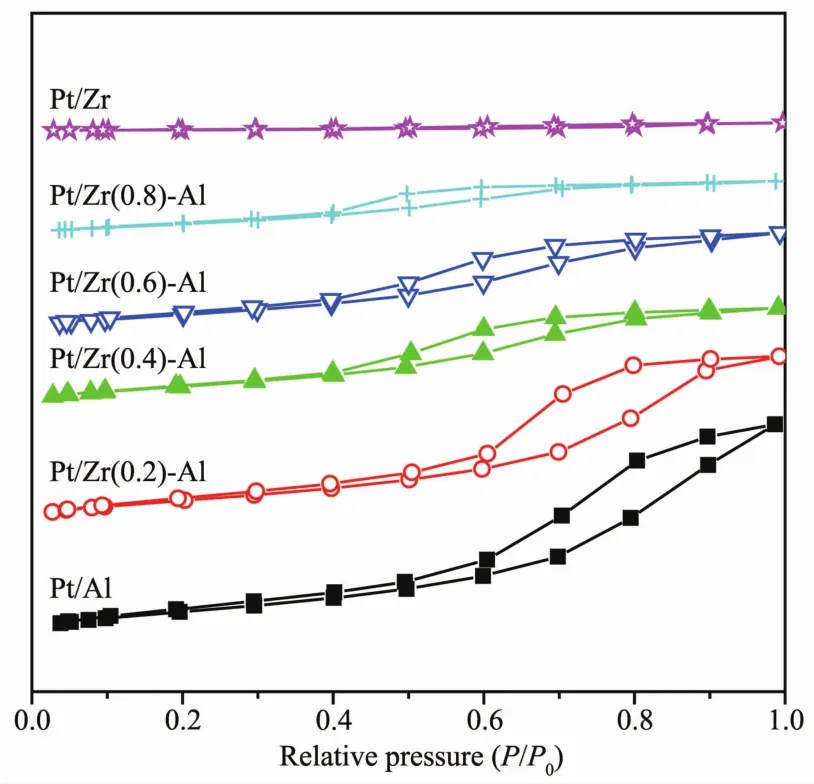
Fig.3 N2 adsorption-desorption isotherm of Pt/Al2O3,Pt/ZrO2-Al2O3 and Pt/ZrO2 serial catalysts
In order to investigate the effect of ZrO2to Al2O3mass ratio on the pore structure and specific surface area of Pt/ZrO2-Al2O3catalyst, N2adsorptiondesorption under low temperature was measured over the serial catalysts.The test results are shown in Fig.3.According to IUPAC classification of adsorption isotherms[30],curves on Pt/Al2O3and Pt/ZrO2-Al2O3catalysts are close to the adsorption isotherms of typeⅣ,while the Pt/ZrO2catalyst shows a typeⅠadsorption isotherms.This means that Pt/Al2O3and Pt/ZrO2-Al2O3catalysts own mesoporous textures,while Pt/ZrO2catalyst is microporous.Pt/Al2O3and Pt/ZrO2-Al2O3catalysts have a significant H2-type hysteresis loop at P/P0of 0.4~1.With increasing of ZrO2content in the catalyst carrier,the distance between the upper and lower closing points of the hysteresis loop is getting closer.This indicates that the pore size distribution of the catalyst becomes more and more narrowed with the increase of the mass ratio of ZrO2to Al2O3in the carrier[31].
Generally,the large specific surface area catalyst can provide more reactive sites during the catalytic reaction.While the catalyst with large pore volume and size can reduce the diffusion resistance to the mass transfer and improve the catalytic conversion efficiency.It can be seen from Table 2 that the specific surface area,pore volume and pore size of the catalyst show a decreasing trend with increasing mass ratio of ZrO2to Al2O3in the carrier.Based on the catalytic activity evaluation data,it is found that the smaller specific surface area,pore volume and pore size may be part of the reasons for a lower catalytic activity of Pt/ZrO2and Pt/Zr(0.8)-Al.

Table 2 Physical parameters of Pt/Al2O3,Pt/Zr O2-Al2O3 and Pt/Zr O2 serial catalysts
2.4 H 2-TPR analysis

Fig.4 H2-TPR curves of Pt/Al2O3,Pt/ZrO2-Al2O3 and Pt/ZrO2 serial catalysts
In order to study the effect of the mass ratio of ZrO2to Al2O3on the reduction performance of Pt/ZrO2-Al2O3catalyst,the serial catalysts were characterized by H2-TPR.The characterization patterns are shown in Fig.4.It can be seen that there are roughly two reduction peaks in each catalyst,one at low temperature and another at high temperature,which can be attributed to the reduction of the high dispersion of Pt and the reduction of the large Pt particles,respectively[32].With increased percentage of ZrO2in the carrier,the reduction temperature shifts firstly to lower temperature and then to higher temperature,which is consistent with the evolution of catalytic activity.This indicates that the mass ratio of ZrO2to Al2O3in the carrier has an important effect on the interaction strength between Pt and AlxZr1-xOysolid solution in Pt/ZrO2-Al2O3catalyst,which affects the catalytic oxidation activity of the catalyst.
2.5 Pt particle size analysis
To investigate the effect of ZrO2to Al2O3on the Pt particle size in Pt/ZrO2-Al2O3catalyst,the CO in situ DRIFTS technique was used.As show in Fig.5,the peaks near 2 050 cm-1correspond to the linear adsorption of CO on the catalyst,while the peaks near 1 835 cm-1correspond to the bridging adsorption of CO on the catalyst[33].According to previous studies,comparing to the large Pt particle,the Pt atom in each small Pt particle owns less number of Pt-Pt bonds,thus enabling itself to provide more charge density to the 2π*orbital of the adsorbed CO.Therefore,the different intensities between linear adsorption and bridge adsorption in the graph correspond to different sizes of Pt particles in the catalyst.The stronger the linear adsorption,the weaker the bridge adsorption,indicating that the Pt particles in the catalyst are smaller and vice versa.It can be seen from Fig.5 that the Pt/ZrO2-Al2O3catalyst with 40%content of ZrO2exhibits the highest ratio of linear adsorption to bridge adsorption intensity,when compared with Pt/Al2O3and Pt/ZrO2catalysts,indicating that Pt/Zr(0.4)-Al catalyst owns the smallest Pt particle size in all catalysts.

Fig.5 CO in-situ DRIFT spectra of Pt/Al2O3,Pt/ZrO2-Al2O3 and Pt/ZrO2 serial catalysts
The Pt particles in Pt/Al2O3,Pt/ZrO2-Al2O3and Pt/ZrO2catalysts were quantitatively compared.The results are shown in Table 2 using COpulse adsorption technique.The results show that the Pt particle size is in the order of Pt/Zr(0.4)-Al<Pt/Zr(0.2)-Al<Pt/Zr(0.6)-Al<Pt/Al<Pt/Zr(0.8)-Al<Pt/Zr.Pt particle size exhibits the trend of decreasing first and then increasing,when the mass percentage of ZrO2in the support was increased.This shows that the mass ratio of ZrO2to Al2O3is helpful to enhance the interaction between Pt and ZrO2-Al2O3,promote the dispersion of Pt and increase the oxidation activity of Pt/ZrO2-Al2O3under low temperature,which is consistent with the results of H2-TPR and catalytic activity.
3 Conclusions
The influence of phase structure, texture properties,reduction properties and particle size on the catalytic oxidation activity of Pt/ZrO2-Al2O3catalysts were studied.ZrO2-Al2O3composite oxide carrier shows the mesoporous texture and large specific surface area similar to Al2O3,while a new phase of AlxZr1-xOysolid solution could also be formed,which is beneficial to the dispersion of Pt,reducing the diffusion resistance to mass transfer,as well as improving the catalytic conversion efficiency of catalyst.A suitable mass ratio of ZrO2to Al2O3in the carrier is helpful to enhance the interaction between Pt and ZrO2-Al2O3,promote the dispersion of Pt and improve the low temperature oxidation activity of Pt/ZrO2-Al2O3catalyst.The Pt/ZrO2-Al2O3catalyst with40∶60 exhibitsexcellent catalytic oxidation activity.The T50and T90of C3H6and CO oxidation is less than 125 and 150 ℃,respectively,demonstrating the material′s potential to be applied in close coupled catalyst.
[1]Morgan C.Johnson Matthey Technol.Rev.,2014,58(4):217-220
[2]Heck R M,Farrauto R J,Weidenkaff A.Appl.Catal.,A,2001,221(1):443-457
[3]Gandhi H S,Graham GW,Mccabe R W.J.Catal.,2003,216(1):433-442
[4]KANG Xin-Ting(康新婷),TANG Hui-Ping(汤慧萍),ZHANG Jian(张健),et al.Rare Metal Materials and Engineering(稀有金属材料与工程),2006,35(Suppl.2):442-447
[5]Keav S,Matam SK,Ferri D,et al.Catalysts,2014,4(3):226-255
[6]Wang J H,Chen H,Hu Z C,et al.Catal.Rev.Sci.Eng.,2015,57(1):79-144
[7]Bagwan N,Satpute S.International Journal of Advanced Production and Industrial Engineering,2017,504:22-26
[8]SONG Wei-Cong(宋为聪),SHI Zhong-Hua(史忠华),YAO Yan-Lin(姚艳玲),et al.Chinese J.Inorg.Chem.(无机化学学报),2009,25(5):838-843
[9]Yang C W,Zhang Q,Li J,et al.J.Energy Chem.,2016,25(3):375-380
[10]He J J,Wang C X,Zheng T T,et al.Johnson Matthey Technol.Rev.,2016,60(3):196-203
[11]DU Jun-Chen(杜君臣),CHANG Shi-Ying(常仕英),HUANG Wei-Qiang(黄卫强),et al.Journal of Molecular Catalysis(China)(分子催化),2015,29(5):482-493
[12]Shen M Q,Song L Y,Wang J,et al.Catal.Commun.,2012,22(18):28-33
[13]Zheng T T,He J J,Xia W Z,et al.Catal.Commun.,2015,71:51-55
[14]LI Ning(李凝),LUO Lai-Tao(罗来涛).Chin.J.Catal.(催化学报),2007,28(9):773-778
[15]Dömök M,OszkóA,Baán K,et al.Appl.Catal.,A,2010,383(1):33-42
[16]Souza M M V M,Aranda D A G,Schmal M.J.Catal.,2001,204(2):498-511
[17]Morán-Pineda M,Castillo S,López T,et al.Appl.Catal.,B,1999,21(2):79-88
[18]Long E Y,Wang Y,Zhang X Y,et al.Chin.J.Catal.,2010,31(3):313-316
[19]Wang Y,Tang SY,Long E,et al.Chin.J.Catal.,2011,32(2):303-308
[20]Cooper J,Beecham J.Platinum Met.Rev.,2013,57(4):281-288
[21]Matsouka V,Konsolakis M,Yentekakis IV,et al.Top.Catal.,2011,54(16/17/18):1124-1134
[22]Shi Z H,Gong M C,Chen Y Q.Chin.Chem.Lett.,2006,17(9):1271-1274
[23]Wang G,Meng M,Zha YQ,etal.Fuel,2010,89(9):2244-2251[24]Fang R M,Cui Y J,Chen SJ,et al.Chin.J.Catal.,2015,36(2):229-236
[25]Yang X,Yang L Y,Lin SY,et al.Chin.J.Catal.,2014,35(8):1267-1280
[26]Zhu Z Z,Lu G Z,Guo Y,et al.J.Ind.Eng.Chem.,2012,18(6):2135-2140
[27]DU Jun-Chen(杜君臣),ZHANGAi-Min(张爱敏),MA Jiang-Li(马江丽),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(3):415-420
[28]Wang Y,Xu H D,Shang H Y,et al.J.Energy Chem.,2014,23:461-467
[29]Zhao Y J,Zhou J,Zhang JG,et al.J.Mol.Catal.A:Chem.,2009,309(1):35-39
[30]Sing K SW.Pure Appl.Chem.,1985,57(4):603-619
[31]XU Hui-Yuan(徐慧远),LUO Jing-Jie(罗靖杰),YAN Chun-Rong(严春蓉),et al.Journal of Fuel Chemistry and Technology(燃料化学学报),2012,40(11):1370-1402
[32]YAN Qian-Gu(严前古),GAOLi-Zhen(高丽珍),YUAN Song-Yue(远松月),et al.Chem.J.Chinese Universities(高等学校化学学报),1998,19(8):1300-1303
[33]Bourane A,Olivier Dulaurent A,Bianchi D.Langmuir,2001,17(18):5496-5502
[34]Fanson PT,Delgass W N,Lauterbach J.J.Catal.,2001,204(1):35-52
[35]Avila M S,Vignatti C I,Apesteguía C R,et al.Catal.Lett.,2010,134(1/2):118-123

