A Comparative Study on Photosynthetic Characteristics of Dryopteris fragrans and Associated Plants in Wudalianchi City, Heilongjiang Province, China
Chen Ling-ling, Liang Yan-tao, Wang He-meng, Zhang Tong, Bo Zhi-gang, Zhao Zong-bao, and Chang Ying*
1 College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
2 Life Science Department, Daqing Normal University, Daqing 163712, Heilongjiang, China
Introduction
Dryopteris fragrans(L.) Schott, a deciduous perennial herb used in China for the treatment of skin diseases (Shenet al., 2006), exhibits antibacterial,antioxidant, analgesic, antitumor and immunomodulatory activities.Multiple substances, such as flavonoids,sterols and other medicinal components, have been isolated and characterized from this fern (Fanet al.,2012).Notably,D.fragranshas a very narrow geographic distribution and is limited to Asia, Europe and North America.In China, it is found exclusively in Wudalianchi City, Heilongjiang Province and thrives only in areas within volcanic geological landforms.There it grows in association with several other plant species, mainlySambucus williamsii,Artemisia sacrorum,Chelidonium majus,Sorbaria sorbifolia,Woodsia ilvensis,Potentilla asperrimaandUrtica angustifolia.The unique, but limited, geographic distribution ofD.fragranshas probably played an important role in shaping its physiological and ecological characteristics.
Fern occupies an important place in plant phyletic evolution and uniquely exhibits independent gametophyte and sporophyte life stages.Moreover,D.fragransis a unique type of fern that grows in extreme environment.Therefore, due to its medicinal,nutritional and ornamental value, achieving optimalD.fragransgrowth is currently an important cha-llenge.Because photosynthesis is a very complex process, photosynthetic physiological ecology can systematically address this complexity through experimental indoor control methods, field sampling methods, isotopic techniques and other methods(Knight and Mitchell, 1989; Aguilaret al., 2015).Such studies can elucidate the relationships between ecological factors and multiple plant physiological phenomena.For example, on the one hand, photosynthetic efficiency of plants is influenced by external factors, such as light intensity, temperature and relative atmospheric humidity (Freeland, 1952;Arzariet al., 2005).On the other hand, internal factors, such as leaf size, leaf maturity, chlorophyll content and nitrate-reductase activity also play a role (Spoeher and Mcgee, 1924) (Osterhoutet al.,1919).In spite of this complexity, researchers have successfully employed several endangered plants.For example, researches onTrillium tschonoskiifound that this endangered plant photosynthesis(Liaoet al., 2006) and cannot adapt to humid environments (Macedoet al., 2011; Hanget al., 2008).In this study, various photosynthetic characteristics ofD.fragransand its associated plants were measured and compared, including net photosynthesis rate,chlorophyll content, nitrate reductase activity, light compensation point (LCP) and light saturation point(LSP).The results indicated that coordination exists betweenD.fragransphotosynthetic characteristics and its growth environment.Moreover, these results also served to identify factors underlying the narrow geographic distribution ofD.fragransand provided a theoretical foundation to justify protection of wild resources and facilitate artificial cultivation ofD.fragrans.
Materials and Methods
Natural conditions
The experimental site was located within the mainD.fragransnatural habitat regions (Wudalianchi City,China).This area has a temperate continental monsoon climate, with average temperature of –0.5℃, average annual precipitation of 476 mm and average relative humidity of 69.2%.The frost period typically lasted from early October to early May, with an average annual frost-free period of 121 days.
Plant materials
Healthy representative plants ofD.fragransand its main associated plants, includingSambucus williamsii,Artemisia sacrorum,Chelidonium majus,Sorbaria sorbifolia,Woodsia ilvensis,Potentilla asperrimaandUrtica angustifoliawere chosen for sampling.Leaves, which carried out plants' major photosynthetic function, were typically sampled using two or three leaves per plant (13 cm long, 3.5 cm wide).Leaves with similar spatial orientation and angle were chosen(Caiet al., 2008) with westward posture and 30º dip angle with respect to the ground (Yanget al., 2010; Li,2005).
Diurnal variations of photosynthetic rate (Pn)
On sunny days in mid-July, a LCi portable photosynthesis measurement system (ADC BioScientific,Ltd., UK) was used to measure net photosynthesis rate [Pn, μmol · (m2· s-1)-1] each hour from 6: 00 a.m.to 6: 00 p.m (Jin, 2002).Each measurement was repeated 3 times.
Measurement of light compensation point and light saturation point
CO2concentration was set to 450 µmol · mol-1and the relative humidity to 80% as described previously(Zhanget al., 2010).The saturating light intensity was determined by varying the light intensity until it was no longer a factor limiting the photosynthesis rate.Light compensation point (LCP) was determined using photosynthetically active radiation-net photo-synthetic rate response curves.
Measurement of chlorophyll content
A soaking extraction method was applied to extract chlorophyll using a mixed ethanol-acetone solution.Chlorophyll content was determined using spectrophotometry (Shuet al., 2010).Three individualD.fragransplants growing at the same location were used to measurePnand were chosen to determine chlorophyll a and chlorophyll b contents using the Beer-Lambert law.
Measurement of nitrate reductase activity
The activity of nitrate reductase was measured as previously described (Fresneauet al., 2007; Giaimoet al., 2002).NaNO2was used to generate a standard curve and the activity of nitrate reductase was determined from the curve.
Results
Diurnal variations of Pn in D.fragrans and its main associated plants
白天明又苦笑了一下,他说:“苏石在城里没事,没啥大事,这是他叫我带回来给你的。”他指了指屋檐下的那旅行包,鼓鼓囊囊的。他又说:“我先走了,有什么事你问爸吧,刚才我都跟爸说了。”说着,白天明就跟小偷似的,拎起自己的包,折转屁股溜了。
The diurnal variations in leaf net photosynthetic rate(Pn) forD.fragransand its main associated plants are shown in Fig.1, showing dramatic changes for all the plants studied.Moreover, the diurnalPnprofiles ofD.fragrans,W.ilvensisandU.angustifoliaexhibited unimodal change pro files, whileC.majusandA.gmeliniiexhibited bimodal rate change pro files.The maximal photosynthesis rate (Pmax) forS.williamsiiandP.asperrimawere the highest and were mainly observed at noon or 1: 00 p.m.OnlyC.majus andP.asperrimaexhibited an additionalPmaxpeak around 11: 00 a.m.Pmaxvalues were the lowest inD.fragransandA.gmelinii(Fig.2).
Comparison of monthly variations of chlorophyll content in D.fragrans and its main associated plants
The results demonstrated that leaf chlorophyll content directly correlated with photosynthetic capacity for all the plants grown under similar conditions within a certain range (Figs.3-5).During the growth period from April to October, the chlorophyll was tested content monthly for all the plants, except for a gap in some data for April (Fig.4), when the average temperature was only –11.2℃.Due to the cold temperatures,S.williamsii,S.sorbifoliaandU.angustifolialeaves were not completely grown by this time point and data were not collected.
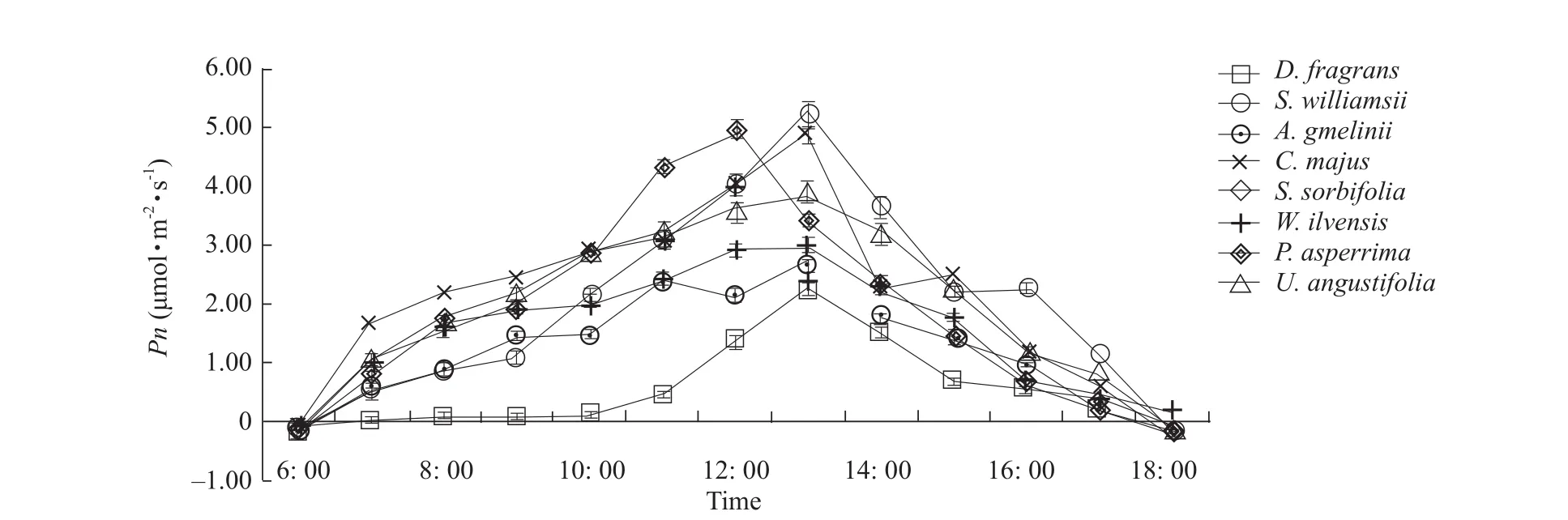
Fig.1 Diurnal variations of Pn
Chlorophyll content changes followed a consistent pattern, with a gradual decrease from May to July,followed by an increase from July to September.In October,C.majus,S.sorbifoliaandU.angustifoliaplants lost their leaves.The chlorophyll a/b ratio changes were quite stable across the entire growing season, with the most values remaining between 1.5 and 3.0 and exhibiting common trends.However, early in the growing season, chlorophyll a/b values were the highest, followed by a slow decline until they reached their lowest values in July and August.Therefore,the chlorophyll content was maximal, the chlorophyll a/b value was the lowest.With leaf aging, the ratio gradually rose again, during spring and autumn, the chlorophyll a/b ratio was larger, favoring absorption of longer light wavelengths.In summer, chlorophyll a/b value was relatively lower, favoring absorption of shorter wavelengths.Therefore, chlorophyll a,chlorophyll b, chlorophyll a+b and chlorophyll a/b each varied significantly for different months.
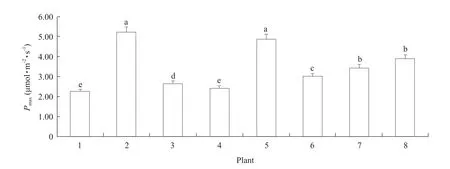
Fig.2 Average diurnal Pmax of D.fragrans and associated plants

Fig.3 Chlorophyll a content of D.fragrans and main associated plants

Fig.4 Chlorophyll b content of D.fragrans and main associated plants
Fig.5 showed that changes in chlorophyll a,chlorophyll b and chlorophyll a+b values exhibited similar trends.However, these values were much higher forA.sacrorumandC.majus, with large variations among different months.In contrast, forP.asperrima,S.williamsii,D.fragrans,S.sorbifolia,W.ilvensisandC.majus, these values were lower,with only minimal variation.InD.fragrans, these values were only a little higher than forC.majusandW.ilvensis.It was well known that chlorophyll a mainly absorbed red light, while chlorophyll b absorbs blue light.Red light absorption forD.fragranswas initially higher than forW.ilvensisandC.majus, while blue light absorption byD.fragranswas still higher than that ofW.ilvensisbut lower than that ofC.majuslater, from August to October.To summarize, plant chlorophyll content had a direct effect on photosynthetic efficiency.Thus, in thisD.fragranscommunity,whenW.ilvensisexhibited higher photosynthetic efficiency,C.majusphotosynthetic efficiency was noticeably lower, whileD.fragransefficiency fell among values for these species.
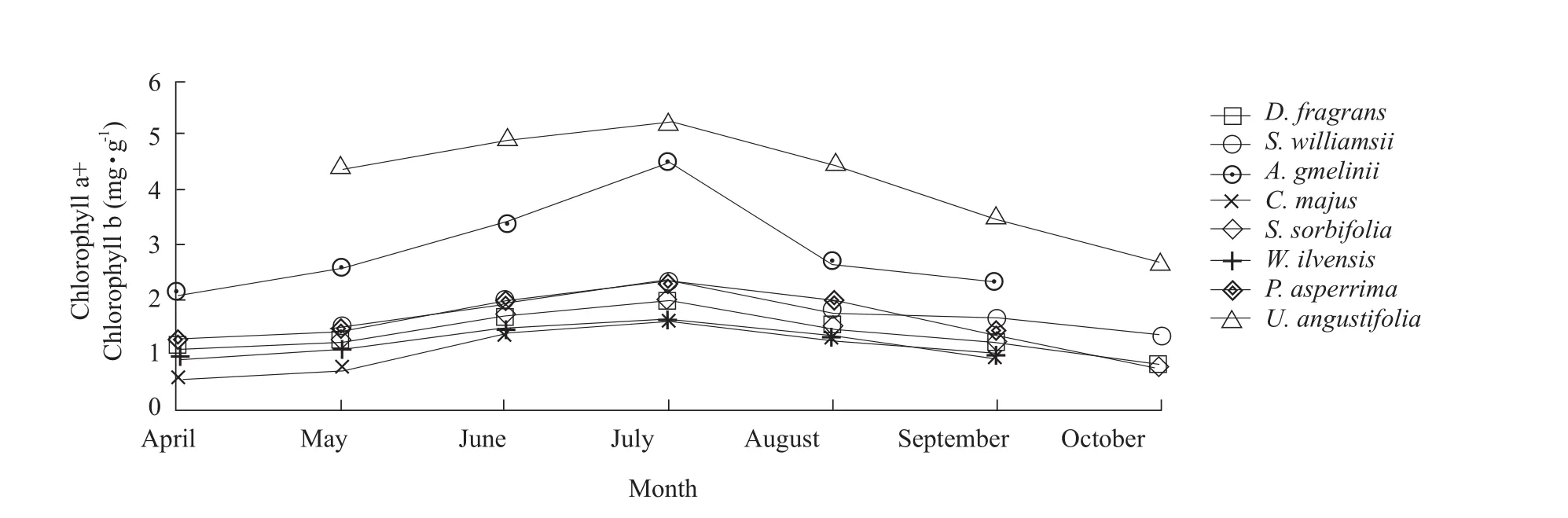
Fig.5 Chlorophyll a+b comparisons for D.fragrans and associated plants for each month
Low chlorophyll a/b values for smaller plants have established that they utilize blue-purple wavelengths more efficiently.Fig.6 showed the highest maximum chlorophyll a/b value forD.fragransfollowed byA.gmelinii,W.ilvensis,C.majus,U.angustifolia,P.asperrima,S.sorbifolia,S.williamsii.These results showed thatD.fragranswas poorly adapted to its environment relative to its associated plants.

Fig.6 Comparison of chlorophyll a/b for each plant species averaged over all the months
Variation in activity of nitrate reductase in D.fragrans and associated plants
As shown in Fig.8, the maximum nitrate reductase activity varied.The highest maximum nitrate reductase activity value was observed forS.williamsii, while the minimum was forA.gmelinii.Notably,the average nitrate reductase activity inD.fragranswas higher than only that ofA.gmelinii.In addition,becausePnshowed a positive correlation with enzyme activity, the relatively lowD.fragransnitrate reductase activity reflected its relatively lower photosynthesis rate and weaker photo-synthesis capacity than its associated plants.
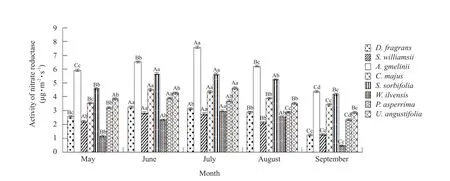
Fig.7 Monthly variation in nitrate reductase activity for all the studied plants
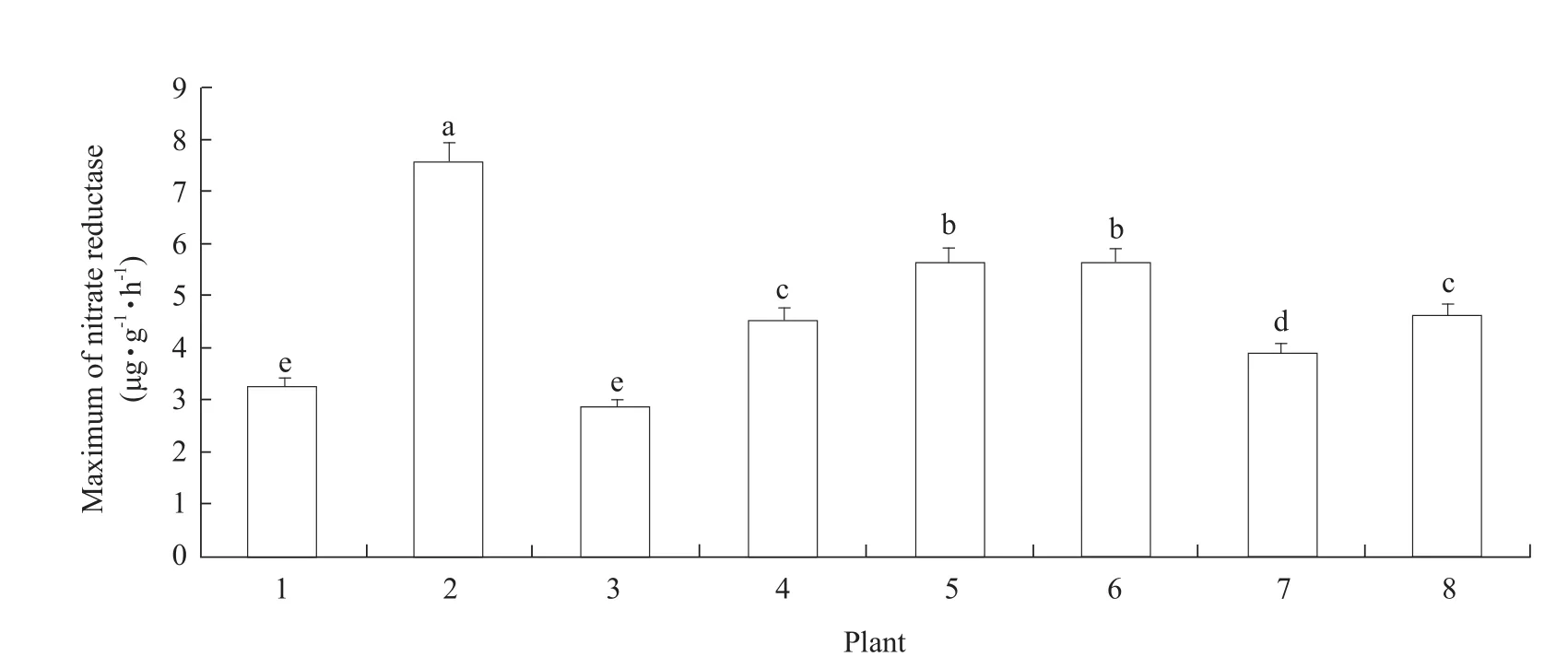
Fig.8 Comparison of maximum nitrate reductase activity in D.fragrans and associated plants
Variation of LCP and LSP in D.fragrans and its main associated plants
A common pattern in LCP seasonal dynamic variation was observed overall (Figs.9 and 10).With the attainment of leaf maturity and increase in chlorophyll content, LCP appeared to decline generally, reaching a minimum in July.Subsequently, with leaf aging and reduction of chlorophyll content, LCP steadily and consistently increased, reaching a peak in September.A low LCP, small canopy density and strong light intensity in the rocky environment ofD.fragranscommunity gave rise to excessive photosynthesis,which greatly impacted growth.Therefore, in these eight species, the chlorophyll content and associated leaf growth both peaked in July, followed by a steady decrease with leaf aging.Generally, LSP could be utilized to measure plant photosynthetic capacity,as a higher LSP correlates with a largerPnvalue.Compared with its associated plants,D.fragransexhibited a relatively low LSP, suggesting a narrower ecological amplitude to light adaptation.

Fig.9 LCP variation D.fragrans and main associated plants
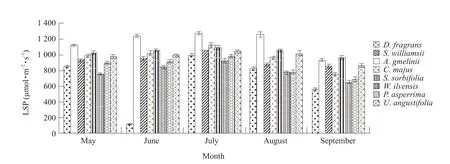
Fig.10 LSP variation in D.fragrans and associated plants
Discussion
The net photosynthetic rate (Pn) in different habitats was a single peak pattern.In the summer morning, leaf photosynthetic rate ofD.fragransand other associated plants increased gradually.With the increased of the height of the sun, maximum value was at 1: 00 p.m.This was due to the high temperature and light in the northeast in summer, but in the morning the temperature was low, with the increased of PAR and temperaturePnwas also rising to and peak at the same time.Then the temperature was higher,the leaf water content was reduced, and the stomata were partially closed, which resulted in the decrease of Ci concentration and the decreased ofPn.Plant net photosynthetic rate determines the level of accumulation of plant photosynthetic products, which can further affect the speed of plant growth (Zhanget al., 2014).D.fragrans niche similarity and niche overlap of this plant were higher, which showed that their niches were more similar (Huanget al.,2013).Previous findings had shown that the growth ofD.fragransresponded to specifically defined environment factors.Here, measurements of photosynthetic rate and other photosynthetic physiological indices demonstrated that these values were not higher forD.fragrans, but were lower among most of its associated plants.For example, a lowerPnvalue reflected a weak photosynthetic capacity forD.fragransrelative to other plants in this community.In addition, the lowPnchanged at noon coupled with a higher light energy utilization rate both suggested thatD.fragranshad a certain resistance to strong light.
Strong light environment was not conducive to the synthesis of chlorophyll and chloroplast development.Chlorophyll content and chlorophyll a/b had a direct effect on the photosynthetic rate.Chlorophyll a/b were small, meant the higher the use of blue violet, the higher ability to adapt to less light environment (Liet al., 2011).When chlorophyll a and chlorophyll b decreased, photosynthetic activity of plants increased.Compared with associated plants, the total chlorophyll content and chlorophyll a to chlorophyll b content ratio inD.fragransremained consistently at a middle level, demonstrating thatD.fragransmight adapt to light, but had weak competitive ability.At the same time, the study found thatD.fragransnitrate reductase activity varied significantly in different seasons, reaching the maximum in July before declining.
The heliophytes had high LCP and LSP; however,the shade plants had low LCP and LSP (Liet al., 2011).In our study, low LCP and LSP values forD.fragranssuggested it had a stronger ability to utilize weak light than its associated plants.Overall, the results of this study linked the narrow geographic distribution ofD.fragransto its growth disadvantage relative to its associated plants.
Conclusions
Photosynthesis is one of the most significant physiological processes underlying plant growth and greatly impacts subsequent plant size and development.D.fragransis mainly distributed in rocks, an inhospitable environment that is neither warm nor damp enough for most plants to thrive.Therefore, during competition within a mixed plant community, the success ofD.fragranspartly depends on its growth speed.BecausePndetermined the rate of plant growth to a certain extent, this factor should play a role.Moreover, because previous researches indicated thatD.fragransgrowth characteristics helped it to adapt to environmental factors, photosynthetic physiological indices and the photosynthetic rate ofD.fragransand its main associated plants were analyzed.The study showed thatD.fragranswere not dominant and exhibit even lower values than for the associated plants.By comparing these photosynthetic characteristics, a potential coordination betweenD.fragransand the growing environment were observed that partly explained the reason behind the narrow geographic distribution ofD.fragrans.Moreover, the information obtained from the analyses should provide a theoretical basis for further resource protection, exploitation and artificial cultivation ofD.fragrans.
Aguilar E, Allende L, Del Toro F J,et al.2015.Effects of elevated CO2and temperature on pathogenicity determinants and virulence of potato virus X/Potyvirus-associated synergism.Molecular Plant-microbe Interactions, 28: 1364-1373.
Arzari R, Tadmor Y, Meir A,et al.2005.Light signaling genes and their manipulation towards modulation of phy-tonutrients content in tomato fruits.Biotech aology Advances, 28: 108-118.
Cai R G, Zhang M, Yin Y P,et al.2008.Photosynthetic characteristics and antioxidative metabolism of flag leaves in responses to nitrogen application during grain filling of field-grow wheat.Agricultural Sciences in China, 7(2): 157-167.
Fan H Q, Shen Z B Chen Y F,et al.2012.Study on antifungal susceptibility of different extract ofDryopteris fragrans.Journal ofChinese Medicinal Materials, 35: 1981-1985.
Freeland R O.1952.Effect of age of leaves upon the rate of photosynthesis in some conifers.Plant Physiology, 27: 685-690.
Fresneau C, Ghashghaie J, Cornicet G,et al.2007.Drought effect on nitrate reductase and sucrose-phosphate synthase activities in wheat (Triticum durumL.): role of leaf internal CO2.Journal of Experimental Botany, 67(5): 2983-2992.
Giaimo J M, Gusev A V, Wasielewski M R,et al.2002.Excited-state symmetry breaking in cofacial and linear dimers of a green perylenediimide chlorophyll analogue leading to ultrafast charge separation.Journal of the American Chemical Society, 124(29): 8530-8531.
Hang G f, Zhai S H, Wen-Hua S U,et al.2008.Effects of light intensity and air temperature on the photosynthesis of Neottopteris Nidus.Journal of Kunming University, 4: 62-63.
Huang Q Y, Lichun H U, Fan R,et al.2013.Characteristics of plant niche on medicinal herbDryopteris fragrans(L.) Schott.Journal of Northeast Agricultural University, 44(7): 143-148.
Jin Z X.2002.The Photosynthetic characteristics of the main species of the Hep-tacodium miconioides community in Tiantai mountain of Zhejiang Province, China.Acta Ecologica Sinica, 1645-1652.
Macedo A F, Leal-Costa M V, Tavares E S,et al.2011.The effect of light quality nn leaf production and development of in avitro-ultured plants ofAlternanthera brasilianaKuntze.Environmental and Experimental Botany, 70: 43-50.
Knight S L, Mitchell C A.1989.Effects of incandescent radiation on photosynthesis, growth rate and yield of 'Waldmann's Green' leaf lettuce.Sci Hortic(Amsterdam), 35: 37-49.
Li F W.2005.Studies on the photosynthetic characterizations and distributions of rear earth elements in fern Dicranopteris dichotoma.Institute of Botany, the Chinese Academy of Sciences, Beijing.
Li L, Li X Y, Lin L S,et al.2011.Comparison of chlorophyll content and fluorescence parameters of six pasture species in two habitats in China.Chinese Journal of Plant Ecology, 35(6): 672-680.
Li Y H, Zhang K M, Hong-Fang Y U,et al.2011.Photosynthetic characteristics of ten cultivars of autumn chrysanthemum (Dendranthema morifolium) and correlation analysis between net photosynthetic rate and some physio cological factors.Journal ofPlaut Resources and Euviroumeut, 21(1): 70-76.
Liao J X, Ge Y, Guan B H,et al.2006.Photosynthetic characteristics and growth ofMosla hangchowensisand M-dianthera under different irradiances.Biol Plantarum, 50: 737-740.
Osterhout W J, Haas A R.1919.The temperature coefficient of photosynthesis.The Journal of General Physiology, 1: 295-298.
Shen Z B, Luo W Y, Yan Y S,et al.2006.Study on terpene ofDryopteris fragransL.Journal of Chinese Medicinal Materials, 29:334-335.
Shu Z Z, Zhang X S, Chen J,et al.2010.The simplification of chlorophyll content measurement.Plant Physiology Communications, 6(4): 399-402.
Spoeher H A, Mcgee J M.1924.Absorption of carbon dioxide the first step in photosynthesis.Science, 59: 513-514.
Yang X Y, Wang X F, Wei M,et al.2010.Changes of nitrate reductase activity in cucumber seedlings in response to nitrate stress.Agricultural Sciences in China, 9(2): 216-222.
Zhang Z W, Zhang B Y, Tong H F,et al.2010.Photosynthetic LCP and LSP of different grapevine cultivars.Journal of Northwest Forestry University, 25(1): 24-29.
Zhang Y Q, Li S W, Wei F U,et al.2014.Effects of nitrogen application on yield, photosynthetic characteristics and water use efficiency of hybrid millet.Journal of Plant Nutrition and Fertilizer, 5:1119-1126.
 Journal of Northeast Agricultural University(English Edition)2018年1期
Journal of Northeast Agricultural University(English Edition)2018年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Effect of Inoculation Rhizobium and Response of Soybean-Rhizobium System to Insoluble Phosphate
- Characteristics and Degradation Mechanism of Fomesafen
- Comparative Study of Proximate, Chemical and Physicochemical Properties of Less Explored Tropical Leafy Vegetables
- Effect of Mineral and Vitamin Supplementation on Performance and Haemotological Values in Broilers
- Comparative Research on Facultative Anaerobic Cellulose Decomposing Bacteria Screened from Soil and Rumen Content and Diet of Dairy Cow
- Pharmacokinetics of Milbemycin Oxime in Dogs Following Its Intravenous and Oral Administration
